Abstract
Free-living nematodes are known to ingest food-borne pathogens and may serve as vectors to contaminate preharvest fruits and vegetables. Caenorhabditis elegans was selected as a model to study the effectiveness of sanitizers in killing Salmonella enterica serotype Poona ingested by free-living nematodes. Aqueous suspensions of adult worms that had fed on S. enterica serotype Poona were treated with produce sanitizers. Treatment with 20 μg of free chlorine/ml significantly (α = 0.05) reduced the population of S. enterica serotype Poona compared to results for treating worms with water (control). However, there was no significant difference in the number of S. enterica serotype Poona cells surviving treatments with 20 to 500 μg of chlorine/ml, suggesting that reductions caused by treatment with 20 μg of chlorine/ml resulted from inactivation of S. enterica serotype Poona on the surface of C. elegans but not cells protected by the worm cuticle after ingestion. Treatment with Sanova (850 or 1,200 μg/ml), an acidified sodium chlorite sanitizer, caused reductions of 5.74 and 6.34 log10 CFU/worm, respectively, compared to reductions from treating worms with water. Treatment with 20 or 40 μg of Tsunami 200/ml, a peroxyacetic acid-based sanitizer, resulted in reductions of 4.83 and 5.34 log10 CFU/worm, respectively, compared to numbers detected on or in worms treated with water. Among the organic acids evaluated at a concentration of 2%, acetic acid was the least effective in killing S. enterica serotype Poona and lactic acid was the most effective. Treatment with up to 500 μg of chlorine/ml, 1% hydrogen peroxide, 2,550 μg of Sanova/ml, 40 μg of Tsunami 200/ml, or 2% acetic, citric, or lactic acid had no effect on the viability or reproductive behavior of C. elegans. Treatments were also applied to cantaloupe rind and lettuce inoculated with S. enterica serotype Poona or C. elegans that had ingested S. enterica serotype Poona. Protection of ingested S. enterica serotype Poona against sanitizers applied to cantaloupe was not evident; however, ingestion afforded protection of the pathogen on lettuce. These results indicate that S. enterica serotype Poona ingested by C. elegans may be protected against treatment with chlorine and other sanitizers, although the basis for this protection remains unclear.
An increase in the number of outbreaks of infections associated with consuming fresh produce in recent years is thought to have been caused in part by changes in agronomic, harvesting, processing, and consumption practices and patterns (5, 7, 14, 22, 23). Fruits and vegetables can become contaminated with human pathogenic microorganisms before and during harvesting, during transport, processing, distribution, and marketing, and at sites of preparation in food service and home settings. Inadequate surface washing and improper storage of produce can lead to growth of pathogenic microorganisms (4, 5, 23, 28).
Salmonellosis is among the most frequently reported causes of food-borne gastroenteritis in the United States (9, 20, 22). Although outbreaks of salmonellosis associated with produce are less frequent than outbreaks linked to foods of animal origin, a diverse range of fruits and vegetables, including cantaloupe, watermelon, lettuce, tomatoes, and seed sprouts, have been implicated (5, 22). Several outbreaks of salmonellosis have been associated with consumption of cantaloupe. In 1991, more than 400 cases of S. enterica serotype Poona infection were linked to cantaloupe (10). More recently, cantaloupe was implicated in outbreaks of S. enterica serotype Saphra (21) and S. enterica serotype Poona (13) infections. Salmonella may survive on preharvest-contaminated cantaloupe rind during subsequent postharvest handling and preparation for consumption (31).
The rind of cantaloupes acts as a physical barrier, preventing or greatly minimizing penetration of microorganisms into the interior. However, mechanical damage to the cantaloupe during harvesting and washing and during subsequent handling and preparation for consumption may compromise the surface integrity, allowing Salmonella to enter tissues and grow to high numbers if held for sufficient time at nonrefrigeration temperatures. Alternatively, Salmonella present on the surface of cantaloupes can be transferred to the internal tissues during slicing (31).
Washing the surface of raw fruits and vegetables with tap water is recommended as a means for consumers to remove soil and some of the microorganisms it may contain, but this method should not be relied upon to disinfect the surface (4, 8, 31). The effectiveness of washing produce with a sanitizer for the purpose of killing or removing microorganisms is dependent on the chemical properties of the sanitizer, contact time, and temperature (4, 30); types of microorganisms present and their attachment mechanisms (27); and physical characteristics of fruits and vegetables (6). Treatment with chlorinated water, various organic acids, and other sanitizers, followed by washing with potable water, reduces microbial populations on fruits and vegetables (4). Hydrogen peroxide has been suggested as an alternative to chlorine for disinfecting fresh produce and appears to reduce microbial populations without leaving significant residues (26).
Caenorhabditis elegans, a free-living bacterivorous nematode that has been used as a model organism for studying host-pathogen interactions (16, 17), is also representative of an important group of soil nematodes. The nematode feeds primarily on bacteria, and the adult worm lives for 2 weeks or longer under optimal environmental conditions (36). C. elegans obtains needed nutrients in soil by taking in water containing suspended bacteria, other microorganisms, and other particles through its oral opening (stoma) and then spitting out the water while retaining nutrients (3, 18). Bacterivorous nematodes may defecate 30 to 60% of ingested cells in a viable form (12).
Studies have indicated that C. elegans and perhaps other free-living nematodes may serve as carriers or vectors of human enteric pathogens (11, 29, 32, 33). Rude et al. (25) recovered nematode eggs or larvae from eight salad vegetables; Salmonella was also detected on 8% of the samples. We have observed that C. elegans is attracted to several strains of Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes. S. enterica serotype Typhimurium can grow and establish a persistent infection in the intestine of C. elegans (1). Chang et al. (11) reported that Salmonella and Shigella ingested by two free-living nematodes, Cheilobus quadrilabiatus and Diplogaster nudicapitatus, are protected against inactivation by chlorinated water. These pathogens survived in the worm's gut for up to 4 days.
The objective of the study reported here was to determine the effectiveness of sanitizers used to decontaminate the surfaces of raw fruits and vegetables in killing S. enterica serotype Poona that had been ingested by C. elegans. Lethality of sanitizers to S. enterica serotype Poona internalized by C. elegans was tested by using suspensions of worms in water that were then dried on the surfaces of cantaloupe rinds and lettuce leaves.
MATERIALS AND METHODS
Nematode used.
A free-living, bacterivorous nematode, C. elegans (N2, wild-type strain), was used in all experiments. The worms were cultured on K agar (pH 6.5), which contains (per liter of deionized water) potassium chloride (2.36 g), sodium chloride (3.0 g), Bacto Peptone (2.5 g; BBL/Difco, Sparks, Md.), and agar (17.0 g) (34). The basal medium was sterilized by autoclaving at 121°C for 15 min, cooled to 47 to 50°C, and supplemented with a solution of (per liter of deionized water) 95% cholesterol (1.0 g; Aldrich, Milwaukee, Wis.), calcium chloride (11.1 g), and magnesium sulfate (24.7 g). The solution was then poured into plastic petri dishes (100 mm in diameter). E. coli OP50, a strain not pathogenic to humans, was cultured at 37°C for 24 h in OP50 broth, which contains (per liter of deionized water) sodium chloride (5.0 g) and Bacto Peptone (10.0 g). K agar was surface inoculated with 0.1 ml of a 24-h OP50 broth culture of E. coli OP50 and incubated at 37°C for 24 h to establish confluent growth.
Bacterial strain and growth conditions.
S. enterica serotype Poona, isolated from a patient in an outbreak associated with consumption of cantaloupe (10), was cultured in tryptic soy broth (pH 7.3; BBL/Difco) supplemented with 50 μg of nalidixic acid (Sigma Chemical Co., St. Louis, Mo.)/ml (TSBN) at 37°C for 24 h. At least two consecutive 24-h loop transfers to 10 ml of TSBN were made before it was used as a nutrient source for C. elegans or applied to cantaloupe rind and lettuce leaves.
Culturing C. elegans for sanitizer treatments.
The surface of K agar plates containing 500 to 1,000 eggs, along with adult worms, was washed with 5.0 ml of K medium (broth) (pH 4.8), which contains the same ingredients as K agar except that agar and peptone are omitted (35). Eggs and worms suspended in K medium were collected by centrifugation (500 × g, 2 min, 21°C), and the supernatants were decanted. The pellet was resuspended in 10 ml of 0.013 M NaOH (pH 12.1) solution containing 1% NaOCl and incubated under constant agitation at 22°C for 15 min. This treatment kills worms in all life stages except that of eggs. To collect viable eggs, the suspension was centrifuged (500 × g, 2 min, 21°C), followed by removal of the supernatant. The pellet was resuspended in 10 ml of K medium and the suspension was centrifuged (500 × g, 2 min, 21°C). After the final wash, all but ca. 0.5 ml of the supernatant was removed from the tube. One milliliter of K medium was added, followed by depositing 0.1 ml of egg suspension on the surface of a K agar plate on which a lawn of E. coli OP50 had grown. The number of adult worms that developed at 21°C was monitored by using a stereomicroscope. Worms on plates incubated at 21°C for 3 days were used in sanitization experiments.
Preparation of sanitizer solutions.
Seven chemical solutions used or having potential for use as produce sanitizers were evaluated for their effectiveness in killing S. enterica serotype Poona ingested by C. elegans. Hydrogen peroxide (VWR, West Chester, Pa.) was tested at concentrations of 0.5, 1.0, and 2.0%. Chlorine (20, 50, 100, 200, and 500 μg/ml) solutions were prepared by combining sodium hypochlorite (Aldrich Chemical Co., Inc., Milwaukee, Wis.) with 0.05 M potassium phosphate buffer (pH 6.8). The concentration of free chlorine was measured by using an amperometric titrator (Hach, Loveland, Colo.). Sanova, a sodium chlorite solution (Alcide Corporation, Redmond, Wash.), at 850, 1,200, and 2,550 μg/ml; Tsunami 200 (Ecolab, St. Paul, Minn.) at 20 and 40 μg/ml; and hydrogen peroxide, acetic acid, citric acid, and lactic acid, each at 0.5, 1.0, and 2.0%, were evaluated. Sterile deionized water was used as a control. All chemical treatment solutions were used within 30 min of preparation.
Ingestion of S. enterica serotype Poona by C. elegans.
S. enterica serotype Poona was streaked onto the surface of tryptic soy agar (BBL/Difco) supplemented with nalidixic acid (50 μg/ml, pH 7.3) (TSAN) and incubated at 37°C for 24 h. Adult C. elegans worms grown on K agar inoculated with E. coli OP50 were removed by applying 10 ml of K medium to each plate and gently rubbing the surface with a sterile bent glass rod. The suspension was transferred to a sterile 15-ml tube and centrifuged (500 × g, 2 min, 21°C). The worms were resuspended in 10 ml of K medium and centrifuged again to reduce the number of E. coli OP50 organisms on the worm cuticle. The supernatant was removed, and the pellet was resuspended in 1.0 ml of K medium at 21°C. The worms were allowed to settle to the bottom of the tube for 5 min. A suspension (20 μl) containing 20 to 30 adult worms was deposited onto the surface of TSAN on which 24-h colonies of S. enterica serotype Poona had formed. The worms were allowed to ingest S. enterica serotype Poona for 3 h at 22°C before being used as inocula for testing the lethality of sanitizer solutions, either in aqueous suspension or on the surface of cantaloupe rind or lettuce leaves.
Treatment of C. elegans with sanitizers after ingestion of S. enterica serotype Poona.
By using a sterile 32-gauge platinum wire attached to the tip of a Pasteur pipette, 10 worms that had fed on S. enterica serotype Poona for 3 h were transferred from the TSAN plates to 20 μl of sterile deionized water on the tip of a sterile spoonula and deposited in 2 ml of chemical treatment solution in a 50-ml centrifuge tube. After 5 min at 21°C, all but ca. 0.5 ml of the treatment solution or water containing the worms was removed and 4 ml of Dey-Engley (DE) neutralizing broth (pH 7.6 to 7.8; BBL/Difco) was added. The worms in the suspension were sonicated (Sonicate 450, Danbury, Conn.) by using a duty cycle of 25% for 25 s at 21°C to rupture the cuticle of C. elegans and to release ingested Salmonella. Undiluted samples (0.25 ml in quadruplicate and 0.1 ml in duplicate) of sonicate and duplicate 0.1-ml quantities of suspensions diluted in 0.1% peptone water were surface plated on TSAN to determine populations of S. enterica serotype Poona. Plates were incubated at 37°C for 24 h before colonies were counted. Presumptive S. enterica serotype Poona colonies were picked and confirmed by using Salmonella latex agglutination (Oxoid, Basingstoke, United Kingdom) and API20 biochemical assays (bioMérieux Vitek, Hazelwood, Mo.).
Effect of chemical sanitizers on the behavior of C. elegans.
Worms that had ingested S. enterica serotype Poona and that had been treated with the chemical sanitizers but not sonicated were placed back onto a 24-h lawn of E. coli OP50 on K agar to determine if treatment had an adverse effect on viability and reproduction. After a 5-min treatment with test chemicals followed by neutralization with DE broth, the mixture of chemical solution and DE broth was decanted, leaving the treated worms in ca. 0.5 ml in the tube. Worms were placed on the surface of K agar containing a lawn of E. coli OP50. Plates were incubated at 21°C for up to 4 days, and worms were observed for characteristic movement and reproductive behavior.
Inoculation of cantaloupe and lettuce with C. elegans that had ingested S. enterica serotype Poona.
The effectiveness of sanitizers in killing S. enterica serotype Poona ingested by C. elegans and deposited on the surface of cantaloupe rind and lettuce leaves was investigated. Western-type cantaloupes (Cumumis melo L. var. reticulatus Naud.) were purchased from a supermarket in Griffin, Ga. A sterile stainless steel template (3.8 by 3.8 cm) was placed on the surface of the rind, and pieces (2 to 4 mm deep) were removed with a sanitized scalpel. Worms that had fed on 24-h colonies of S. enterica serotype Poona grown on TSAN were prepared as described above. A 20-μl K medium suspension containing 10 worms was placed on each piece of cantaloupe rind and was allowed to dry for 1 h at 37°C or 1 h at 37°C followed by 24 h of drying at 4°C before being treated with test chemicals.
Iceberg lettuce (Lactuca sativa L.) was also purchased from a supermarket in Griffin, Ga. The core and three outer leaves were removed from the lettuce heads and were discarded. A sterile stainless steel template (3.8 by 3.8 cm) was placed on the surface of inner leaves. Pieces of leaves were made by cutting around the perimeter of the template with a sanitized scalpel. The procedure for inoculating lettuce with C. elegans that had ingested S. enterica serotype Poona was the same as that described for cantaloupe rind, except inoculum was dried for 1 or 24 h at 37°C.
Inoculation of cantaloupe and lettuce with S. enterica serotype Poona not ingested by C. elegans.
Experiments were also done to determine the efficacy of sanitizers in killing S. enterica serotype Poona inoculated onto the surface of cantaloupe rind or lettuce leaves. A 24-h TSBN culture of S. enterica serotype Poona grown at 37°C was loop transferred three times before being streaked onto TSAN and incubated for 24 h at 37°C. TSAN plates containing colonies of S. enterica serotype Poona were flooded with 12 ml of 0.05 M potassium phosphate buffer (pH 6.8), and cells were suspended with a sterile bent glass rod. Ten milliliters of the suspension was centrifuged (2,000 × g, 15 min, 22°C), and the pellet was resuspended in 10 ml of 0.1% peptone. The optical density at 610 nm of the suspension was adjusted to 1.27 before depositing 20 μl of solution on each piece of cantaloupe, drying them for 1 h at 37°C or 1 h at 37°C followed by 24 h at 4°C, and treatment with test chemicals. Each piece of lettuce leaf was inoculated with 20 μl of suspension, followed by drying for 1 or 24 h at 37°C.
Treatment of cantaloupe and lettuce inoculated with S. enterica serotype Poona or C. elegans that had ingested S. enterica serotype Poona.
Each piece of cantaloupe rind or lettuce leaf inoculated with S. enterica serotype Poona or C. elegans that had ingested S. enterica serotype Poona was placed in a 50-ml test tube. Ten milliliters of chlorinated water (50 and 200 μg/ml), Sanova (850 and 1,200 μg/ml), or Tsunami 200 (20 and 40 μg/ml) was added, and the mixture was agitated for 3 min at 22°C on a platform shaker set at 150 rpm. Ten milliliters of double-strength DE broth was added, and the mixture was homogenized (Polytron PCU11; Brinkmann Instruments, Westbury, N.Y.) at medium speed for 30 s. The homogenate was then sonicated by using a duty cycle of 25% for 25 s. Undiluted samples (0.25 ml in quadruplicate and 0.1 ml in duplicate) of sonicate and duplicate 0.1-ml quantities of suspensions diluted in 0.1% peptone water were surface plated on TSAN and XLD agar (BBL/Difco). Plates were incubated for 24 h at 37°C. Twenty milliliters of double-strength lactose broth (BBL/Difco) supplemented with nalidixic acid (50 μg/ml) (LBN) was added, and the remaining homogenate or sonicate was incubated at 37°for 24 h. If no presumptive Salmonella colonies formed on TSAN or XLD agar on which samples of cantaloupe or lettuce inoculated with S. enterica serotype Poona or C. elegans that had ingested S. enterica serotype Poona had been spread, enriched LBN (1 ml) was inoculated into 10 ml of selenite cystine and was incubated at 37°C for 24 h, followed by being streaked onto bismuth sulfite agar (pH 7.7; BBL/Difco) supplemented with nalidixic acid (50 μg/ml) (BSAN) and incubated at 37°C for 24 h. Presumptive colonies of S. enterica serotype Poona that formed on BSAN were selected and confirmed by latex agglutination and biochemical tests as described above.
Statistical analysis.
Each experiment was replicated at least three times. Each replicate consisted of an aqueous suspension of test organism or three pieces of inoculated cantaloupe rind or lettuce leaf. Data were subjected to the Statistical Analysis System (SAS Institute, Cary, N.C.) for analysis of variance and Duncan's multiple range tests to determine significant differences (α ≤ 0.05) between mean values.
RESULTS AND DISCUSSION
pH of treatment solutions.
Ranges in pH values for chemical sanitizer solutions were the following: chlorine, pH 6.82 to 6.95; hydrogen peroxide, pH 5.06 to 5.43; Sanova, pH 2.58 to 2.62; Tsunami 200, pH 3.66 to 3.85; acetic acid, pH 2.66 to 2.98; citric acid, pH 2.50 to 2.83; and lactic acid, pH 2.16 to 2.52 (Table 1). After the addition of DE broth to neutralize active compounds and/or pH of the treatment solutions, the pH of the mixture ranged from 6.00 (2% lactic acid) to 7.78 (water control). The pH of neutralized chemical solutions would not be expected to adversely affect the viability of S. enterica serotype Poona or C. elegans. C. elegans is tolerant of pH environments in the range of 3.2 to 11.2 (15).
TABLE 1.
pH of chemical sanitizer solutions before and after adding DE neutralizing broth
| Sanitizer | Concn in:
|
pH
|
||
|---|---|---|---|---|
| μg/ml | % | Before adding DEa | After adding DEb | |
| Chlorine | 0 | 5.30 | 7.78 | |
| 20 | 6.82 | 7.70 | ||
| 50 | 6.85 | 7.17 | ||
| 100 | 6.86 | 7.78 | ||
| 200 | 6.89 | 7.79 | ||
| 500 | 6.95 | 7.81 | ||
| Sanova | 0 | 5.30 | 7.78 | |
| 850 | 2.58 | 7.10 | ||
| 1,200 | 2.60 | 7.04 | ||
| 2,500 | 2.62 | 6.71 | ||
| Tsunami 200 | 0 | 5.30 | 7.78 | |
| 20 | 3.85 | 8.18 | ||
| 40 | 3.66 | 8.07 | ||
| Hydrogen peroxide | 0 | 5.30 | 7.78 | |
| 0.5 | 5.22 | 8.55 | ||
| 1.0 | 5.06 | 8.71 | ||
| 2.0 | 5.43 | 8.54 | ||
| Acetic acid | 0 | 5.30 | 7.78 | |
| 0.5 | 2.98 | 7.08 | ||
| 1.0 | 2.80 | 6.39 | ||
| 2.0 | 2.66 | 5.03 | ||
| Citric acid | 0 | 5.30 | 7.78 | |
| 0.5 | 2.83 | 7.95 | ||
| 1.0 | 2.69 | 7.76 | ||
| 2.0 | 2.50 | 7.31 | ||
| Lactic acid | 0 | 5.30 | 7.78 | |
| 0.5 | 2.52 | 7.42 | ||
| 1.0 | 2.16 | 7.12 | ||
| 2.0 | 2.16 | 6.00 | ||
pH of chemical treatment solutions (2 ml) containing C. elegans (10 worms) that fed on S. enterica serotype Poona. Treatment was applied for 5 min at 22°C, followed by the addition of 4 ml of DE neutralizing broth.
pH of mixtures of chemical treatment solutions and DE broth.
Efficacy of sanitizers in killing S. enterica serotype Poona in aqueous suspension.
Treatment of S. enterica serotype Poona in aqueous solutions of sanitizers for 5 min at 21°C resulted in the elimination of all viable cells. Initial populations ranged from 7.42 to 7.80 log10 CFU/ml.
Efficacy of sanitizers in killing S. enterica serotype Poona ingested by C. elegans.
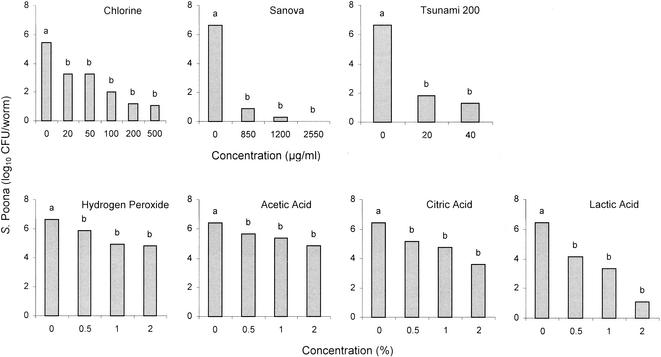
C. elegans worms that had fed on S. enterica serotype Poona were suspended in aqueous chemical treatment solutions for 5 min at 21°C and then were analyzed for populations of the pathogen that survived. Treatment of worms with 20 μg of free chlorine/ml resulted in a significant (α ≤ 0.05) reduction (2.19 log10 CFU/worm) in population compared to results for treatment with water (Fig . 1). Treatment with 500 μg of chlorine/ml caused the largest reduction (4.37 log10 CFU/worm) compared to results for treatment with water; however, there was no significant difference in the number of S. enterica serotype Poona cells surviving treatments with 20, 50, 100, 200, or 500 μg of chlorine/ml.
FIG. 1.
Populations of S. enterica serotype Poona (S. Poona) recovered from C. elegans that had fed on the pathogen, followed by treatment with chemical sanitizers. Within each sanitizer, bars noted with a different letter are significantly different (α = 0.05).
A similar trend occurred when worms which had ingested S. enterica serotype Poona were treated with other chemical sanitizers, i.e., the lowest concentration tested caused a significant (α ≤ 0.05) reduction in population of S. enterica serotype Poona versus that resulting from treatment with water, but reductions were not significantly increased by treatment with higher concentrations (Fig. 1). Treatment with 2% hydrogen peroxide reduced the S. enterica serotype Poona population by 1.41 log10 CFU/worm versus that for treatment with water. Sanova was the most effective sanitizer in reducing the populations of S. enterica serotype Poona. Treatment with 850 and 1,200 μg of Sanova/ml caused reductions of 5.74 and 6.34 log10 CFU/worm, respectively, compared to population reduction by treatment with water. S. enterica serotype Poona was not detected (< 2 CFU/worm) in worms treated with 2,550 μg of Sanova/ml.
Treatment with 20 and 40 μg of Tsunami 200/ml, a peroxyacetic acid-based sanitizer, resulted in reductions of 4.83 and 5.34 log10 CFU/worm, respectively, compared to numbers detected on worms washed with water. Among the organic acids evaluated, at a concentration of 2% acetic acid was least effective in killing S. enterica serotype Poona (1.61 log10 CFU/worm reduction compared to the number surviving in or on worms treated with water) and lactic acid was most effective (5.32 log10 CFU/worm reduction). Treatment with 2% citric acid resulted in a reduction of 2.82 log10 CFU/worm compared to the number detected on or in worms washed with water. Significant reductions in Salmonella resulting from treatment of worms with the lowest concentrations of sanitizers may reflect lethality to cells on the surface of worms. Treatment with higher concentrations failed to cause additional significant reductions. This was attributed to the inability of active forms of chemicals to penetrate the worm's cuticle and reach the ingested cells.
Effects of sanitizers on viability and reproductive behavior of C. elegans.
Experiments were done to determine if treatment with chemical sanitizers causes lethality or changes in reproductive behavior of C. elegans that had ingested S. enterica serotype Poona. Treated worms were placed on K agar containing a lawn of E. coli OP50, incubated at 21°C, and observed for 4 days. Viability and reproductive behavior was not affected by treatment with water. Worms placed onto K agar containing a lawn of E. coli OP50 produced eggs within 24 h, and the eggs developed into larval stages. Treatment of worms with up to 500 μg of chlorine/ml was not lethal and did not affect the ability of C. elegans to produce eggs. Treatment with hydrogen peroxide at concentrations of 0.5 or 1% was not lethal to C. elegans; the numbers of worms increased during the 4-day observation period. Treatment with 2% hydrogen peroxide, however, resulted in the death of some of the worms within 1 day. Eggs were produced by some of the worms before death. These eggs hatched and developed into larvae that showed no signs of being affected by the 2% hydrogen peroxide treatment that killed the parent worms. The pH of 2% hydrogen peroxide (7.43) was higher than that of the other solutions of test chemicals but probably did not contribute to lethality. Regardless of concentration of chemicals in the treatment solution, Sanova, Tsunami 200, and acetic, citric, and lactic acids did not appear to have an effect on the viability or reproductive behavior of C. elegans. These observations demonstrate the ability of C. elegans to survive exposure to highly acidic environments.
Efficacy of sanitizers in killing S. enterica serotype Poona ingested by C. elegans and inoculated onto cantaloupe.
The effectiveness of chemical treatments in killing S. enterica serotype Poona that had been ingested by C. elegans and then inoculated onto the surface of cantaloupe rind was determined. Based on the extent of lethality of sanitizers to S. enterica serotype Poona internalized in C. elegans suspended in aqueous chemical solutions (Fig. 1), chlorine, Sanova, and Tsunami 200 were selected for evaluation. Overall, treatment with Sanova or Tsunami resulted in the greatest reductions in population of S. enterica serotype Poona; chlorine was evaluated because it is widely used as a produce sanitizer and could serve as a standard against which to compare the efficacy of other sanitizers.
Salmonella was not detected on the surface of uninoculated cantaloupe. The number of S. enterica serotype Poona in the inoculum (5.82 log10 CFU/worm) applied to cantaloupe rind was not significantly (α ≤ 0.05) reduced during drying for 1 h (Table 2). Drying inoculum for 25 h, however, did cause a significant reduction in population compared to drying for 1 h. Populations of S. enterica serotype Poona recovered from rind on which inocula had dried for 1 h followed by treatment with water or all concentrations of test chemicals were not significantly different (α ≤ 0.05). With the exception of treatment with 50 μg of chlorine/ml, significant reductions in populations of S. enterica serotype Poona on rind on which inoculum was dried for 25 h were caused by treatment with all concentrations of test chemicals compared to the amount of reduction caused by the water control. The highest reduction in the number of S. enterica serotype Poona cells was achieved by treatment with 1,200 μg of Sanova/ml.
TABLE 2.
Populations of S. enterica serotype Poona recovered from cantaloupe rind inoculated with C. elegans that had ingested the pathogen and then had been dried for 1 h at 37°C or 1 h at 37°C followed by 24 h at 4°C before treatment
| Treatment | Concn of chemical (μg/ml) | Population size (log10 CFU/worm)a and amt of population reduction atb:
|
|||
|---|---|---|---|---|---|
| 1 h
|
25 h
|
||||
| Population size | Reduction | Population size | Reduction | ||
| Water (control) | 0 | A 5.71 A | B 5.05 A | ||
| Chlorine | 50 | A 5.34 A | 0.37 | A 4.88 A | 0.17 |
| 200 | A 4.54 A | 1.17 | B 3.43 B | 1.62 | |
| Sanova | 850 | A 2.67 A | 3.04 | A 2.47 B | 2.58 |
| 1,200 | A 2.17 A | 3.54 | A 1.66 B | 3.34 | |
| Tsunami 200 | 20 | A 4.32 A | 1.39 | B 2.70 B | 2.35 |
| 40 | A 2.92 A | 2.79 | B 1.94 B | 3.11 | |
The population of S. enterica serotype Poona was 5.82 log10 CFU/worm before inoculating cantaloupe. Values in the same column followed by the same letter are not significantly different (α = 0.05). Values in the same row preceded by the same letter are not significantly different (α = 0.05).
Within the drying time, reduction (log10 CFU/worm) compared to results for treating cantaloupe with water.
Efficacy of sanitizers in killing S. enterica serotype Poona ingested by C. elegans and inoculated onto lettuce.
The effectiveness of chlorine, Sanova, and Tsunami 200 in killing S. enterica serotype Poona that had been ingested by C. elegans and inoculated onto the surface of lettuce leaves was determined. Salmonella was not detected in uninoculated lettuce. A drying time of 1 h between application of C. elegans to lettuce and treatment with water did not cause a significant (α = 0.05) reduction in the population of S. enterica serotype Poona applied to the lettuce (6.55 log10 CFU/worm); however, the population was significantly reduced during drying for 24 h. Regardless of the drying conditions (1 or 24 h at 37°C) after inoculating lettuce, the number of S. enterica serotype Poona cells recovered (6.13 log10 CFU/worm and 5.66 log10 CFU/worm, respectively) after treatment with water was not significantly different (α ≤ 0.05) from the number in the inoculum (6.55 log10 CFU/worm) (Table 3). Within the drying time, some of the chemical treatments resulted in significant (α ≤ 0.05) reductions in population of S. enterica serotype Poona; however, reductions did not exceed 2.65 log10 CFU/worm compared to that obtained with water treatment.
TABLE 3.
Populations of S. enterica serotype Poona recovered from lettuce inoculated with C. elegans that had ingested the pathogen and then dried for 1 or 24 h at 37°C before treatment
| Treatment | Concn of chemical (μg/ml) | Population size (log10 CFU/worm)a and amt of population reduction atb:
|
|||
|---|---|---|---|---|---|
| 1 h
|
24 h
|
||||
| Population size | Reduction | Population size | Reduction | ||
| Water (control) | 0 | A 6.13 A | A 5.66 A | ||
| Chlorine | 50 | A 6.09 A | 0.04 | A 5.59 AB | 0.07 |
| 200 | A 5.39 B | 0.74 | A 5.09 AB | 0.57 | |
| Sanova | 850 | A 4.36 B | 1.77 | B 3.14 B | 2.52 |
| 1,200 | A 3.48 B | 2.65 | A 3.94 B | 1.72 | |
| Tsunami 200 | 20 | A 5.55 B | 0.58 | A 5.09 AB | 0.57 |
| 40 | A 5.33 B | 0.80 | B 4.08 B | 1.58 | |
The population of S. enterica serotype Poona was 6.55 log10 CFU/worm before inoculating lettuce. Values in the same column followed by the same letter are not significantly different (α = 0.05). Values in the same row preceded by the same letter are not significantly different (α = 0.05).
Within the drying time, reduction (log10 CFU/worm) compared to results for treating lettuce with water.
Efficacy of sanitizers in killing S. enterica serotype Poona inoculated onto cantaloupe.
The efficacy of sanitizers in killing S. enterica serotype Poona not ingested by C. elegans but inoculated onto the surface of cantaloupe rind was determined. The population of S. enterica serotype Poona in 20 μl of inoculum (7.79 log10 CFU) was significantly reduced (α ≤ 0.05) on rind during the 1- or 25-h drying periods (Table 4). The number of S. enterica serotype Poona recovered from rind treated with water was not significantly affected by drying time. Treatment with Sanova (1,200 μl/ml) or Tsunami (20 or 40 μg/ml) resulted in significant (α ≤ 0.05) reductions in populations of S. enterica serotype Poona on cantaloupe rind. Park and Beuchat (24) evaluated the efficacy of sanitizers in killing or removing Salmonella inoculated onto the surface of whole cantaloupes. Their data also show that Sanova and Tsunami are superior to chlorine (200 μg/ml) in reducing populations of the pathogen.
TABLE 4.
Populations of S. enterica serotype Poona recovered from cantaloupe rind inoculated with the pathogen and then dried for 1 h at 37°C or 1 h at 37°C followed by 24 h at 4°C before treatment with chemical sanitizers
| Treatment | Concn of chemical (μg/ml) | Population size (log10 CFU/piece of cantaloupe rind)a and amt of population reduction atb:
|
|||
|---|---|---|---|---|---|
| 1 h
|
25 h
|
||||
| Population size | Reduction | Population size | Reduction | ||
| Water (control) | 0 | A 6.74 A | A 6.47 A | ||
| Chlorine | 50 | A 6.39 B | 0.35 | A 6.19 AB | 0.55 |
| 200 | A 6.03 BC | 0.71 | A 6.14 AB | 0.33 | |
| Sanova | 850 | A 4.27 C | 2.47 | A 5.49 AB | 0.98 |
| 1,200 | A 5.14 C | 1.60 | A 5.61 B | 0.86 | |
| Tsunami 200 | 20 | A 4.53 C | 2.21 | A 5.23 B | 1.24 |
| 40 | A 4.24 C | 2.50 | A 4.23 B | 2.24 | |
The population of S. enterica serotype Poona was 7.79 log10 CFU/20 μl of inoculum deposited on each piece of cantaloupe rind. Values in the same column followed by the same letter are not significantly different (α = 0.05). Values in the same row preceded by the same letter are not significantly different (α = 0.05).
Within the drying time, reduction (log10 CFU/piece of cantaloupe rind) compared to results for treating cantaloupe with water.
Within a given drying time and chemical treatment, reduction in populations of S. enterica serotype Poona that was ingested by C. elegans before inoculating cantaloupe (Table 2) was generally higher than reduction on cantaloupe inoculated with free S. enterica serotype Poona (Table 4). This suggests that a higher percentage of ingested cells, compared to free S. enterica serotype Poona cells, was sensitized to sanitizers, perhaps as a result of exposure to grinding and digestive enzymes in the gut of C. elegans. Sanitizers would need to reach the site of S. enterica serotype Poona, however, to cause lethality. These observations suggest that protection of S. enterica serotype Poona against sanitizers is not afforded by C. elegans dried on the surface of cantaloupe.
Efficacy of sanitizers in killing of S. enterica serotype Poona inoculated onto lettuce.
The efficacy of sanitizers in killing S. enterica serotype Poona not ingested by C. elegans but inoculated onto the surface of lettuce was determined. Shown in Table 5 are populations of S. enterica serotype Poona recovered from lettuce treated with water and chemical sanitizers. The inoculum (20 μl) contained 7.56 log10 CFU. After drying for 1 h at 37°C, the population of S. enterica serotype Poona (7.18 log10 CFU/g) recovered from lettuce by washing with water was not significantly reduced; however, the population (6.45 log10 CFU/g) detected on lettuce dried for 24 h after inoculation was significantly smaller than that recovered from lettuce treated with water. There was a significant (α ≤ 0.05) reduction in the number of S. enterica serotype Poona cells recovered from lettuce dried for 24 h and washed with water compared to that of lettuce dried for 1 h. On balance, however, populations of S. enterica serotype Poona recovered from lettuce treated with sanitizers were not significantly affected by drying time.
TABLE 5.
Populations of S. enterica serotype Poona recovered from lettuce inoculated with the pathogen followed by drying for 1 or 24 h at 37°C before treatment with chemical sanitizers
| Treatment | Concn of chemical (μg/ml) | Population size (log10 CFU/piece of lettuce)a and amt of population reduction atb:
|
|||
|---|---|---|---|---|---|
| 1 h
|
25 h
|
||||
| Population size | Reduction | Population size | Reduction | ||
| Water (control) | 0 | A 7.18 A | B 6.45 A | ||
| Chlorine | 50 | A 6.44 B | 0.74 | A 6.35 A | 0.10 |
| 200 | A 5.99 B | 1.19 | B 5.57 B | 0.88 | |
| Sanova | 850 | B 4.67 B | 2.51 | A 5.15 B | 1.30 |
| 1,200 | A 4.34 B | 2.84 | A 4.19 B | 2.26 | |
| Tsunami 200 | 20 | A 5.44 B | 1.70 | A 5.05 B | 1.40 |
| 40 | A 5.07 B | 2.11 | A 5.01 B | 1.44 | |
The population of S. enterica serotype Poona was 7.86 log10 CFU/20 μl of inoculum deposited on each piece of lettuce. Values in the same column followed by the same letter are not significantly different (α = 0.05). Values in the same row preceded by the same letter are not significantly different (α = 0.05).
Within the drying time, reduction (log10 CFU/piece of lettuce) compared to results for treating lettuce with water.
With the exception of lettuce treated with 50 μg of chlorine/ml after drying the inoculum for 24 h, there was a significant decrease (α ≤ 0.05) in the number of S. enterica serotype Poona cells recovered from lettuce treated with all concentrations of chemical sanitizers compared to the number recovered from lettuce treated with water (control). Within the drying time there were no significant differences in the number of S. enterica serotype Poona cells recovered from lettuce treated with all other concentrations of sanitizers. Treatment with Sanova (1,200 μg/ml) caused the largest reductions in S. enterica serotype Poona populations (2.84 log10 CFU/g, 1 h drying time, and 2.26 log10 CFU/g, 24 h drying time). Treatment of lettuce with 50 μg of chlorine/ml was the least effective in reducing the S. enterica serotype Poona population at both drying times.
Within a given drying time and chemical treatment, reduction in the number of S. enterica serotype Poona ingested by C. elegans and inoculated onto lettuce (Table 3) was generally lower than the reduction on lettuce inoculated with free S. enterica serotype Poona (Table 5). This is contrary to observations made on the behavior of S. enterica serotype Poona on cantaloupe rind and would provide evidence that C. elegans protects S. enterica serotype Poona against inactivation by sanitizers. Comparison of the behavior of S. enterica serotype Poona on cantaloupe and lettuce, however, should be limited to results from samples on which the inoculum was dried for 1 h. Subsequent drying for 24 h was at 4°C for cantaloupe and 37°C for lettuce, making comparison of these reductions caused by chemical treatments difficult. The extent of drying of inoculum for 1 h may have been different on cantaloupe and lettuce, however, resulting in differences in sensitivity or accessibility of S. enterica serotype Poona to sanitizers.
Other work has shown that C. elegans is attracted to avirulant strains of S. enterica serotype Typhimurium, Listeria welshimeri, and Bacillus cereus (2). We have observed that attraction of C. elegans to colonies of S. enterica serotype Poona was stronger than to other S. enterica serotypes (Montevideo, Michigan, Enteritidis, Muenchen, Baildon, and Stanley). The ranges in percentage of worms attracted to colonies of the eight serotypes at 21°C were 34 to 65%, 46 to 79%, 63 to 84%, and 65 to 87% within 5, 10, 15, and 20 min, respectively, after depositing worms on K agar. The highest percentage (87%) of worms migrated to S. enterica serotype Poona within 20 min. The life cycle of C. elegans was completed on K agar plates inoculated with each of the eight serotypes of Salmonella. In the study reported here, high numbers of S. enterica serotype Poona were recovered from the interior of C. elegans that had fed on the pathogen. Sonication effectively ruptured the worm's cuticle, releasing S. enterica serotype Poona and enabling a composite measurement of both interior and surface populations. The number of cells recovered would depend on the number ingested as well as the extent of digestion by worms. Enzymes produced internally by C. elegans can affect the survival of bacteria during passage through the alimentary canal (17). Chang et al. (11) determined the number of Salmonella, Shigella, and viruses ingested by two free-living nematodes, D. nudicapitatus and C. quadrilabiatus. They observed that 5 to 16% of cells remained viable in the worms for up to 24 h and survived routine treatment with chlorine at concentrations used for drinking water. Treatment of another free-living nematode, Pristionchus lheritieri, that had ingested S. enterica serotype Typhi and S. enterica serotype Wichita with chlorinated water (10 μg/ml) was not effective in killing these pathogens (29).
Free-living nematodes have been reported to release viable bacteria by defecating (19). This may be a mechanism by which nematodes could act as vectors to transport bacteria in soil onto the surface of produce. Our study provides evidence that a free-living nematode can harbor S. enterica serotype Poona and supports observations by others that bacteria ingested by C. elegans are protected against treatment with chlorine. The higher lethality of Sanova and Tsunami to S. enterica serotype Poona compared to that of chlorine reveals the availability of alternative sanitizers to treat produce that may harbor free-living nematodes that have ingested S. enterica serotype Poona and, perhaps, other pathogens. Results also show the general ineffectiveness of chemical sanitizers in eliminating Salmonella on cantaloupe and lettuce, regardless of whether or not the pathogen is ingested in C. elegans. Additional experiments are needed to better define the extent of protection of Salmonella ingested by C. elgans and other free-living nematodes against the potential lethal affects of produce sanitizers.
Acknowledgments
The nematode strain used in this work was provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources.
REFERENCES
- 1.Aballay, A., P. Yorgey, and F. M. Ausubel. 2000. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr. Biol. 10:1539-1542. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, G. L., K. N. Caldwell, L. R. Beuchat, and P. L. Williams. Association of a free-living nematode, Caenohrabditis elegans, with surrogates of foodborne pathogenic bacteria. J. Food Prot., in press. [DOI] [PubMed]
- 3.Avery, L., and J. H. Thomas. 1997. Feeding and defecation, p. 679-716. In D. L. Riddle, T. Blumenthal, B. J. Meyer, and J. R. Priess (ed.), C. elegans II. Cold Spring Harbor Laboratory Press, Plainview, N.Y. [PubMed]
- 4.Beuchat, L. R. 1998. Surface decontamination of fruits and vegetables eaten raw: a review. WHO document no. FSF/FOS/98.2. World Health Organization, Geneva, Switzerland.
- 5.Beuchat, L. R. 2002. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 4:413-423. [DOI] [PubMed] [Google Scholar]
- 6.Beuchat, L. R., J. M. Farber, E. H. Garrett, L. H. Harris, M. E. Parish, T. V. Suslow, and F. F. Busta. 2001. Standardization of a method to determine the efficacy of sanitizers in inactivating human pathogenic microorganisms on raw fruits and vegetables. J. Food Prot. 64:1079-1084. [DOI] [PubMed] [Google Scholar]
- 7.Beuchat, L. R., and J. H. Ryu. 1997. Produce handling and processing practices. Emerg. Infect. Dis. 3:459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beuchat, L. R., B. V. Nail, B. B. Adler, and M. R. S. Clavero. 1998. Efficacy of spray application of chlorinated water in killing pathogenic bacteria on raw apples, tomatoes, and lettuce. J. Food Prot. 61:1305-1311. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1990. Foodborne disease outbreaks, 5-year summary, 1983-1987. CDC surveillance summaries, March, 1990. Morb. Mortal. Wkly. Rep. 39(SS-1):15-57. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 1991. Multistate outbreak of Salmonella Poona infections—United States and Canada, 1991. Morb. Mortal. Wkly. Rep. 40:549-552. [PubMed] [Google Scholar]
- 11.Chang, S. L., G. B. Berg, N. A. Clarke, and P. W. Kabler. 1960. Survival, and protection against chlorination, of human enteric pathogens in free-living nematodes isolated from water supplies. Am. Soc. Trop. Med. Hyg. 9:136-142. [DOI] [PubMed] [Google Scholar]
- 12.Chantanao, A., and H. J. Jensen. 1969. Saprozoic nematodes as carriers and disseminators of plant pathogenic bacteria. J. Nematol. 1:216-218. [PMC free article] [PubMed] [Google Scholar]
- 13.Food and Drug Administration. 2000. Produce safety at retail: safe handling practices for melons. Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, College Park, Md.
- 14.Hedberg, C. W., K. L. MacDonald, and M. T. Osterholm. 1994. Changing epidemiology of food-borne disease: a Minnesota perspective. Clin. Infect. Dis. 18:671-682. [DOI] [PubMed] [Google Scholar]
- 15.Khanna, N., C. Cressman III, C. Tatara, and P. Williams. 1997. Tolerance of the nematode Caenorhabditis elegans to pH, salinity, and hardness in acquatic medium. Arch. Environ. Contam. Toxicol. 32:110-114. [DOI] [PubMed] [Google Scholar]
- 16.Kurz, C. L., and J. J. Ewbank. 2000. Caenorhabditis elegans for the study of host-pathogen interactions. Trends Microbiol. 8:142-144. [DOI] [PubMed] [Google Scholar]
- 17.Labrousse, A., S. Chauvet, C. Couillault, C. L. Kurz, and J. J. Ewbank. 2000. Caenorhabditis elegans is a model host for Salmonella typhimurium. Curr. Microbiol. 23:1543-1545. [DOI] [PubMed] [Google Scholar]
- 18.Lee, D. L. 1965. The physiology of nematodes. W. H. Freeman and Co., New York, N.Y.
- 19.Lupi, E., V. Ricci, and D. Burrini. 1995. Recovery of bacteria in nematodes isolated from a drinking water supply. J. Water Supply Res. Technol. AQUA 44:212-218. [Google Scholar]
- 20.Mead, P. S., L. Slutsker, V. Diety, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohle-Boetani, J. C., R. Reporter, S. Benson Werner, S. Abbott, J. Farrar, S. H. Waterman, and D. J. Virgia. 1999. An outbreak of Salmonella serogroup Saphra due to cantaloupes from Mexico. J. Infect. Dis. 180:1361-1364. [DOI] [PubMed] [Google Scholar]
- 22.National Advisory Committee on Microbiological Criteria for Foods. 1999. Microbiological safety evaluations and recommendations on fresh produce. Food Control 10:321-347. [Google Scholar]
- 23.Nguyen-The, C., and F. Carlin. 2000. Fresh and processed vegetables, p. 620-684. In B. M. Lund, T. C. Baird-Parker, and G. G. Gould (ed.), The microbiological safety on quality of food, vol. 1. Aspen Publishers, Inc., Gaithersburg, Md.
- 24.Park, C. M., and L. R. Beuchat. 1999. Evaluation of sanitizers for killing Escherichia coli O157:H7, Salmonella and naturally occurring microorganisms on cantaloupes, honeydew melons, and asparagus. Dairy Food Environ. Sanitation 19:842-849. [Google Scholar]
- 25.Rude, R. R., G. J. Jackson, J. W. Bier, T. K. Sawyer, and N. G. Risty. 1984. Survey of fresh vegetables for nematodes, amoebae, and Salmonella. J. Assoc. Off. Anal. Chem. 67:613-615. [PubMed] [Google Scholar]
- 26.Sapers, G. M., and G. F. Simmons. 1998. Hydrogen peroxide disinfection of minimally processed fruits and vegetables. Food Technol. 52:48-52. [Google Scholar]
- 27.Sapers, G. M., R. L. Miller, M. Jantschke, and A. M. Mattrazzo. 2000. Factors limiting the efficacy of hydrogen peroxide washes for decontamination of apples containing Escherichia coli. J. Food Sci. 65:529-532. [Google Scholar]
- 28.Sewell, A. M., and J. M. Farber. 2001. Foodborne outbreaks in Canada linked to produce. A review. J. Food Prot. 64:1863-1877. [DOI] [PubMed] [Google Scholar]
- 29.Smerda, S. M., H. J. Jensen, and A. W. Anderson. 1970. Escape of salmonellae from chlorination during ingestion by Pristionchus iheritieri (Nematoda: Diplogasterinae). J. Nematol. 3:201-204. [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi, K., and J. F. Frank. 2001. Quantitative determination of the role of lettuce leaf structures in protecting Escherichia coli O157:H7 from chlorine disinfection. J. Food Prot. 64:147-151. [DOI] [PubMed] [Google Scholar]
- 31.Ukuku, D. O., and G. M. Sapers. 2001. Effect of sanitizer treatments on Salmonella Stanley attached to the surface of cantaloupe and cell transfer to fresh-cut tissues during cutting practices. J. Food Prot. 64:1286-1291. [DOI] [PubMed] [Google Scholar]
- 32.Walters, J. V., and R. R. Holcomb. 1967. Isolation of an enteric pathogen from sewage-borne nematodes. Nematologia 13:155. [Google Scholar]
- 33.Wasilewska, L., and J. M. Webster. 1975. Free-living nematodes as disease factors of man and his crops. Int. J. Environ. Stud. 7:201-204. [Google Scholar]
- 34.Williams, P., and D. Dusenbery. 1988. Using the nematode, Caenorhabditis elegans, to predict mammalian acute lethality to metallic salts. Toxicol. Ind. Health 4:469-478. [DOI] [PubMed] [Google Scholar]
- 35.Williams, P., and D. Dusenbery. 1990. Acute toxicity testing using the nematode, Caenorhabditis elegans. Environ. Toxicol. Chem. 9:1285-1290. [Google Scholar]
- 36.Wood, W. B. (ed.). 1988. Introduction to C. elegans, p. 1-16. In The nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Plainview, N.Y.