Abstract
A dense population of the phototrophic consortium “Pelochromatium roseum” was investigated in the chemocline of a temperate holomictic lake (Lake Dagow, Brandenburg, Germany). Fluorescence in situ hybridization revealed that the brown epibionts of “P. roseum” constituted up to 37% of the total bacterial cell number and up to 88% of all green sulfur bacteria present in the chemocline. Specific amplification of 16S rRNA gene fragments of green sulfur bacteria and denaturing gradient gel electrophoresis fingerprinting yielded a maximum of four different DNA bands depending on the year of study, indicating that the diversity of green sulfur bacteria was low. The 465-bp 16S rRNA gene sequence of the epibiont of “P. roseum” was obtained after sorting of individual consortia by micromanipulation, followed by a highly sensitive PCR. The sequence obtained represents a new phylotype within the radiation of green sulfur bacteria. Maximum light-dependent H14CO3− fixation in the chemocline in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea suggested that there was anaerobic autotrophic growth of the green sulfur bacteria. The metabolism of the epibionts was further studied by determining stable carbon isotope ratios (δ13C) of their specific biomarkers. Analysis of photosynthetic pigments by high-performance liquid chromatography revealed the presence of high concentrations of bacteriochlorophyll (BChl) e and smaller amounts of BChl a and d and chlorophyll a in the chemocline. Unexpectedly, isorenieratene and β-isorenieratene, carotenoids typical of other brown members of the green sulfur bacteria, were absent. Instead, four different esterifying alcohols of BChl e were isolated as biomarkers of green sulfur bacterial epibionts, and their δ13C values were determined. Farnesol, tetradecanol, hexadecanol, and hexadecenol all were significantly enriched in 13C compared to bulk dissolved and particulate organic carbon and compared to the biomarkers of purple sulfur bacteria. The difference between the δ13C values of farnesol, the major esterifying alcohol of BChl e, and CO2 was −7.1%, which provides clear evidence that the mode of growth of the green sulfur bacterial epibionts of “P. roseum” in situ is photoautotrophic.
Phototrophic consortia are unique, highly structured aggregates consisting of colorless bacteria and green sulfur bacteria (34, 37, 41, 44, 52). In motile phototrophic consortia, numerous green sulfur bacteria (so-called epibionts) are attached around a single, flagellated, colorless cell of a member of the β subclass of the Proteobacteria, thereby forming a barrel-shaped highly regular structure (14, 15, 37). Motile phototrophic consortia were first described in the early 20th century (7, 27) and since then have been detected in numerous stratified freshwater lakes and ponds worldwide (40). To date, seven different types of motile phototrophic consortia have been distinguished on the basis of color, size, and shape and on the basis of the number and morphology of the epibionts (41).
Phototrophic consortia can contribute up to two-thirds of the total bacterial biomass in the chemoclines of stagnant water bodies (17) and therefore are significant for the biogeochemical cycles in these environments (40). However, epibionts of motile phototrophic consortia represent unique phylotypes among the green sulfur bacteria (15) and therefore may differ in physiology from their free-living relatives. Intact consortia of the “Chlorochromatium aggregatum” type depend on 2-oxoglutarate for growth and exhibit chemotaxis towards this compound (14). The epibionts are capable of using hydrogen sulfide as an electron donor in the light, as shown by reduction of the alternative electron acceptor carbonyl cyanide m-chlorophenylhydrazone, and therefore are likely to grow lithotrophically within the association (14).
However, in situ measurements for a natural community of the phototrophic consortium “Pelochromatium roseum” revealed that the vertical diffusive flux of hydrogen sulfide was not sufficient to explain the large biomass of consortia present in the chemocline (40). Sulfide could be produced within the phototrophic consortium population, either by the central bacterium in each consortium or by accompanying bacteria outside the consortium (40). However, since the cell yield of green sulfur bacteria is significantly increased by assimilation of organic compounds (36), it is also possible that the epibionts assimilate naturally occurring organic carbon substrates and hence grow mixotrophically in situ. As a prerequisite for understanding the physiological basis of cell-cell interactions and the biogeochemical significance of phototrophic consortia, the carbon metabolism of the epibionts therefore needs to be elucidated.
All known green sulfur bacteria are obligate photolithoautotrophs and employ the reversed citric acid cycle for CO2 fixation (58). Because different enzymes are involved in the primary steps of CO2 fixation, the fractionation of stable carbon isotopes in the cell material of green sulfur bacteria differs significantly from that in other autotrophs, which employ the Calvin cycle (47, 59). In general, the in situ physiology of free-living green sulfur bacteria in terms of carbon metabolism has been studied only once by using the stable carbon isotope approach (16); however, mixed microbial communities were used in this study. In the present study, we selected an ecosystem dominated by the phototrophic consortium “P. roseum” and investigated its in situ carbon metabolism by identifying suitable biomarkers specific for the green sulfur bacterial epibionts and determining their stable carbon isotope ratios (δ13C).
MATERIALS AND METHODS
Study site and sampling procedures.
Lake Dagow (area, 0.24 km2; maximum depth, 9.5 m), is a eutrophic lake located approximately 100 km north of Berlin, Germany (9). Each year, an anoxic, sulfide-containing hypolimnion develops after the onset of summer stratification (May until September). The lake was visited on 15 July 1994, 2 to 9 August 1995, 4 to 15 August 1997, 2 to 7 July 1998, and 19 and 21 July 1999. On these dates, communities of anoxygenic phototrophic bacteria had developed at the oxic-anoxic boundary layer (the chemocline).
Water samples were obtained at a location in the deepest part of the lake by using a bilge pump connected to gas-tight isoversinic tubing. The inlet consisted of two polyvinyl chloride cones that were 1 cm apart (23). This device allowed reproducible sampling of different water layers in 5-cm intervals.
Physical and chemical parameters.
Underwater irradiance was determined with an LI-1000 spherical quantum sensor (wavelength range, 400 to 700 nm; Li Cor, Lincoln, Nebr.) connected to a data logger. Conductivity and temperature were measured with an LF 196 conductivity meter (WTW, Weilheim, Germany), pH was measured with a pH/T electrode connected to a 196T microprocessor pH meter (WTW), and oxygen concentrations were measured with an Oxi 196 oximeter (WTW).
Sulfide contents were determined by the methylene blue method (10). Contact with atmospheric oxygen was avoided, and aliquots of lake water were directly pumped into glass vials containing zinc acetate in order to precipitate and preserve sulfide.
For separation of particulate organic carbon (POC), dissolved organic carbon (DOC), and dissolved inorganic carbon (DIC), chemocline water samples were filtered through precombusted (450°C, 5 h) GF/F glass fiber filters (nominal pore size, 0.7 μm; Whatman, Göttingen, Germany) to collect the POC. The filtrate was used for DOC measurement, and it was collected in acid-washed glass bottles and subsequently lyophilized. POC and DOC samples were stored at −20°C until further processing occurred. After removal of carbonates by acidification with 0.1 M hydrochloric acid, organic carbon concentrations in the samples were determined by combustion by using a Ströhlein UCI Coulomat 702 (JUWE, Korschenbroich, Germany).
Glass serum bottles were used for collection of DIC samples. Prior to sampling, the bottles were cleaned with acid, sealed with butyl rubber stoppers, evacuated, and filled with nitrogen gas. Approximately 20 to 30 ml of a water sample was injected into each bottle, and the bottles were stored at −20°C until processing occurred.
Cell counting.
Numbers of bacteria were determined by epifluorescence microscopy of black polycarbonate membrane filters (pore size, 0.2 μm; Isopore GTBP membranes; Millipore, Eschborn, Germany) after staining with 4′,6-diamidino-2-phenylindole (DAPI) (45). In order to quantify the epibionts of phototrophic consortia, the consortia had to be disaggregated prior to counting. Therefore, glutardialdehyde fixation of the samples was omitted and natural samples were directly stained with DAPI at a final concentration of 0.1 μg ml−1 (63). During deposition on a membrane filter, the phototrophic consortia disassembled, but the epibionts remained arranged in concentric rings around each central bacterium. For samples containing low numbers of phototrophic consortia, a total of 100 microscopic fields were examined. In these cases, the number of consortia (X) per milliliter and the corresponding standard deviation (SD) were calculated from the ratio (p) of microscopic fields containing phototrophic consortia to the total number of fields by employing a most-probable-number formula (8):
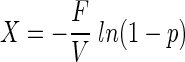 |
(1) |
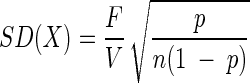 |
(2) |
where F is the ratio of the total filter area to the area of the microscopic field, n is the total number of fields, and V is the volume of sample filtered (in milliliters).
Fluorescence in situ hybridization.
In order to determine the ratio of free-living green sulfur bacteria to green sulfur bacterial epibionts of phototrophic consortia, fluorescence in situ hybridization on black polycarbonate filters (pore size, 0.2 μm; Isopore GTBP membranes; Millipore) was used. The oligodeoxynucleotide probe S-F-GSB-532-a-A-15 (63) labeled with Cy3 (Interactiva Biotechnologie, Ulm, Germany) was employed. Prior to filtration of water samples, each filter was rinsed with 5 ml of acetone (analytical grade) and 5 ml of particle-free distilled water to reduce the background fluorescence. Depending on the density of the phototrophic consortia, 0.4- to 4-ml water samples were filtered, and fluorescence in situ hybridization was carried out as described previously (63). Cells were counterstained with DAPI (final concentration, 0.2 μg · ml−1), and the filters were embedded in antifading mounting fluid (10 ml of glycerol, 10 ml of 10× SET [1.5 M NaCl, 10 mM EDTA, 200 mM Tris-HCl; pH 7.8]) for microscopic examination. Epifluorescence microscopy was performed with a Zeiss Axiolab epifluorescence microscope equipped with a no. 1 filter set for DAPI and an HQ-Cy3 filter set (AHF Analysentechnik, Tübingen, Germany) for Cy3 epifluorescence.
16S rRNA gene sequences of epibionts.
Phototrophic consortia were separated from the chemocline microbial community by using a micromanipulator and an inverted microscope (13, 14). Batches of 5 to 40 consortia or 50 ng of genomic DNA extracted from the complete microbial community was used to amplify 16S rRNA gene fragments; primers GC 357f and GSB 840r and the PCR conditions described previously (32) were used. Water samples were concentrated by centrifugation, and genomic DNA was extracted from pellets as described previously (39). Amplification products were separated on the basis of melting behavior by denaturing gradient gel electrophoresis (DGGE) by using a gradient consisting of 35 to 70% denaturing agents (31). The resulting DNA fragments were excised from the gel, recovered by electroelution, reamplified, and sequenced as described previously (32).
Phylogenetic analysis of 16S rRNA gene sequences of epibionts and environmental sequences was performed by using the ARB phylogeny package (28). The program Fast Aligner V1.03 was used for alignment of all complete 16S rRNA gene sequences of green sulfur bacteria available through the National Center for Biotechnology Information website (1). The sequence of Chloroherpeton thalassium ATCC 35110 was used as the outgroup. The alignment was manually corrected based on secondary structure information, and a phylogenetic tree was constructed by using the maximum-likelihood program DNA_ML. Partial 16S rRNA gene sequences of epibionts were then inserted into the phylogenetic tree without changing the tree topology by employing the Parsimony Interactive tool.
Photosynthetic CO2 assimilation.
Photosynthetic CO2 fixation was measured by using an incubation rack which allowed placement of incubation tubes 5 cm apart (vertical distance) but prevented shading of tubes by other tubes (35). The light and dark incubation tubes were filled with water samples from the corresponding depths. Subsequently, 3-(3,4-dichlorophenyl)-1,1-dimethylurea was added to a final concentration of 20 μM to inhibit oxygenic photosynthesis, and each tube was spiked with 150 kBq of an NaH14CO3 solution. Incubation lasted for 6 h. Immediately after the incubation rack was retrieved, 1 ml of each sample was pipetted into 10 ml of scintillation solution (Aquasafe 300; Canberra-Packard, Dreieich, Germany) for determination of the total radioactivity. After this, samples were filtered through 0.2-μm-pore-size membrane filters (cellulose nitrate; diameter, 25 mm; Schleicher and Schuell, Dassel, Germany), and the filters were placed in glass vials containing 5 drops of 1 M HCl. After 30 min, the vials were opened in a fume hood, the filters were dried in air, and 10 ml of scintillation solution was added to each vial. All samples were counted with a Packard 1600TR scintillation counter. To calculate photosynthetic rates, the CO2 assimilation values of dark controls were subtracted.
Analysis of photosynthetic pigments.
In 1994 to 1998, photosynthetic pigments were quantified after collection of particulate matter on 0.2-μm-pore-size polycarbonate filters and extraction in 99.5% acetone. Bacteriochlorophyll (BChl) a was quantified as described by Steenbergen et al. (60). Dichromatic equations (38) were used for quantification of BChl d and e since some samples also contained chlorophyll (Chl) a.
A detailed analysis of the composition of BChl homologs and different carotenoids was performed with chemocline samples obtained in 1999. Pigments were analyzed by high-performance liquid chromatography (HPLC) (Sykam, Fürstenfeldbruck, Germany) after extraction in methanol by using the method of Borrego and Garcia-Gil (6) as modified by Glaeser et al. (18) and a Nova-Pack C18 HPLC column (4.6 by 250 mm; 4-μm mesh; Waters, Milford, Mass.) together with a C18 ODS-2 guard column (4 by 40 mm; 4-μm mesh; Waters). Absorption spectra were recorded between 300 and 800 nm with a diode array spectrophotometer (TIDAS NMC 301; J&M, Aalen, Germany), and BChl e concentrations were calculated by using the molar extinction coefficient determined recently (5). For identification and calibration of the separated pigments, we employed extracts of pure cultures of brown- and green-colored green sulfur bacteria.
For identification of different BChl homologs, mass spectra were recorded with an HPLC-mass spectrometry (MS) system (Thermo Separation Products, San Jose, Calif.) (18). The HPLC was coupled via a flow splitter (split ratio, 1:4, with the larger flow feeding the mass spectrometer) to an ion trap mass spectrometer (Finnigan LCQ; Thermoquest-Finnigan, San Jose, Calif.) equipped with an atmospheric pressure chemical ionization source and to a UV2000 UV/visible light detector (Thermo Separation Products). Mass spectra were measured in the positive ion mode. The following atmospheric pressure chemical ionization conditions were used: source current, 5 μA; vaporizer temperature, 450°C; and temperature of the transfer capillary, 250°C. MS-MS experiments were done in the dependent scan mode. For these experiments, helium was used as the collision gas (relative collision energy, 35%). The UV/VIS detector was operated at a fixed wavelength of 450 nm.
δ13C.
Large quantities of photosynthetic pigments were required to determine stable carbon isotope fractionation values of individual BChl homologs or carotenoids. Particulate matter from 20 liters of chemocline water was harvested by centrifugation (15 min at 13,500 × g at 4°C), and the pellets were pooled, lyophilized and stored at −80°C until extraction. Photosynthetic pigments were recovered by consecutive extraction (15 min at 4°C with sonication) with methanol, methanol-dichloromethane, and dichloromethane (18). Supernatants were pooled, concentrated with a rotary evaporator, transferred to brown glass tubes, dried at room temperature in a stream of nitrogen gas, and stored at −80°C until they were used. All extraction steps were performed in dim light, and pesticide residue analysis-grade solvents were used.
Prior to analysis, carotenoids in the bulk extracts were completely hydrogenated by a 1-h pretreatment in ethyl acetate by using hydrogen gas as the electron donor and platinum oxide (PtO2) as the catalyst. A few drops of glacial acetic acid were added to the solvent to facilitate saturation of double bounds. The PtO2 was removed by centrifugation and subsequent filtration of the solvent through extracted cotton wool.
Solutions of the esterifying alcohols in dichloromethane were then transesterified into their trimethylsilyl (TMS) ester derivatives in dichloromethane after addition of N-methyl-N-trimethylsilyl-trifluoroacetamide (CS Chromatographie Service, Langerwehe, Germany) and incubation at 70°C for 1 h. The TMS esters were first identified by injection of 1- to 2-μl samples into a gas chromatograph (GC)-flame ionization detector system (HP 5890 Series II GC; Hewlett-Packard, Waldbronn, Germany) equipped with a DB-5 fused silica column (30 m by 0.25 mm; film thickness, 0.1 μm; J&W, Folsom, Calif.) and a temperature-programmable injector (KAS 3; Gerstel, Mühlheim an der Ruhr, Germany). The temperature of the GC oven was programmed to increase from 60°C (1 min isothermal) to 305°C (50 min isothermal) at a rate of 3°C · min−1. The final temperature of the GC oven was set at 320°C to measure hydrogenated carotenoids. Helium was used as the carrier gas at a linear flux of 22.7 cm · s−1.
GC-MS and GC-isotope ratio monitoring (irm) MS were performed as described above by using a DB-5MS column for GC-MS and a DB-5HT column for GC-irm MS. Mass spectra were obtained with a Finnigan MAT SSQ 710B mass spectrometer (Finnigan-Thermoquest, San Jose, Calif.) operated at 70 eV with a scan range of m/z 50 to 650 and a scan rate of 1 scan · s−1. δ13C values were determined with an irm MS system equipped with a Cu-Ni-Pt oxidation reactor by using O2 for in-line oxidation of the alcohols to CO2 at 940°C, a Cu reduction reactor operated at 600°C, and an irm mass spectrometer (MAT 252; Finnigan-Thermoquest). Water was trapped in line by using a Nafion membrane. Ratios of m/z 45, 46, and 47 were monitored, and δ13C values were calibrated with a CO2 standard at the beginning and end of each sample. Squalane was used as the internal standard. In all cases duplicate samples were analyzed, and δ13C values (per mille) were calculated as follows (51):
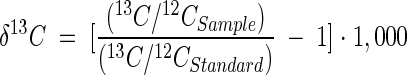 |
(3) |
All values given below are relative to the δ13C of Pee Dee belemnite (51). The δ13C values of fatty alcohols and fatty acids were corrected for the isotopic contribution of the TMS group by using the following equation (46):
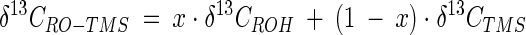 |
(4) |
where x is the ratio of the number of carbon atoms present in the free alcohol to the number of carbon atoms present in its TMS derivative, δ13CROH is the δ13C of the free alcohol, δ13CRO-TMS is the δ13C of the TMS derivative of the alcohol, and δ13CTMS is the δ13C of the TMS group as determined by measurement of the farnesol contents before and after derivatization. The δ13CTMS was −38.9%.
δ13C values of DIC (δ13CDIC) were determined from CO2 contents of water samples in glass serum bottles obtained by acidification with phosphoric acid. Subsamples were retrieved from the headspace with a gas-tight syringe and injected directly into the GC-irm MS system. δ13C values of CO2 (δ13CCO2) were calculated from δ13CDIC values and in situ temperatures (TK) (in Kelvins) (48, 49) as follows:
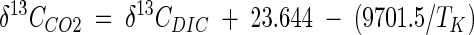 |
(5) |
POC and DOC samples were combusted in a Carlo Erba EA 1108 elementary analyzer coupled to the irm MS system. Organic carbon was combusted in line at 1,040°C in a Cr2O3-Co2O3-Ag oxidation reactor. Inorganic carbonates were removed by acidification with 0.1 M hydrochloric acid prior to measurement, and water was trapped by using MgClO4.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences determined in the present study have been deposited in the GenBank database under accession numbers AJ006182 to AJ006184 (DGGE bands 2 to 4), AJ272090 (DGGE band 8), AJ 272094 (DGGE band 7), and AY 247957 to AY247959 (DGGE bands 5, 6, and 1).
RESULTS
Physicochemical conditions.
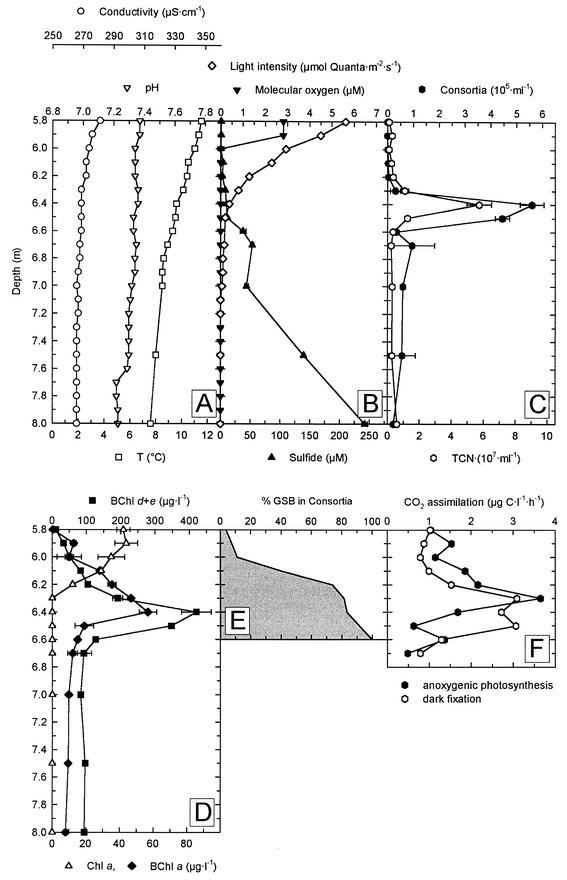
Light microscopic investigation of samples from the chemocline of Lake Dagow revealed a dense assemblage of phototrophic bacteria and the numerical dominance of the brown-colored phototrophic consortium designated “P. roseum” (Fig. 1). In order to obtain initial information about the potential in situ physiology of phototrophic consortia, the physicochemical conditions in the natural habitat were investigated. The vertical gradients of temperature and conductivity indicated that there was stable stratification of the water column below a depth of 4 m in all years (Fig. 2). On all sampling dates, steep opposing vertical gradients of molecular oxygen and sulfide concentrations were found between depths of 5.5 and 8.0 m (Fig. 2B and 3A). In this depth interval, the water temperature ranged from 12 to 7°C and the pH ranged from 7.3 to 7.4, whereas the conductivity remained constant (Fig. 2A). The light intensity was between 0.4 and 1.1 μmol of quanta · m−2 · s−1, and molecular oxygen was absent at the depth at which phototrophic consortia were most abundant (Fig. 2B and C and 3A). A comparison of five consecutive years showed that the environmental parameters for the depth at which the maximum abundance of phototrophic consortia occurred were different in the different years that samples were obtained (Table 1). However, the sulfide concentrations and light intensities consistently were very low in the layer in which phototrophic consortia occurred.
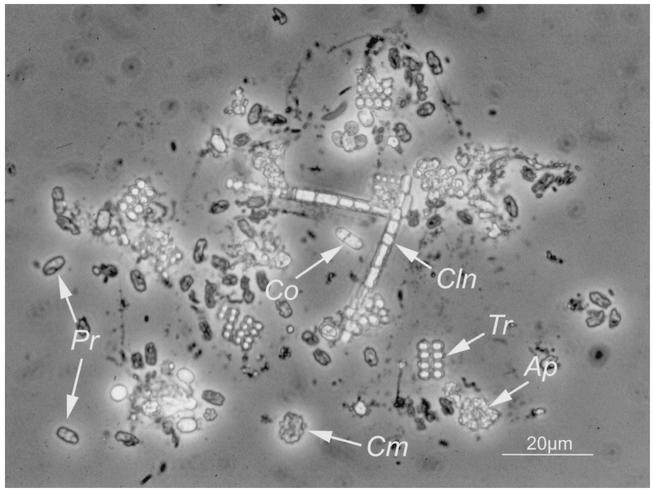
FIG. 1.
Phase-contrast microphotograph of the bacterial community present at a depth of 6.4 m of in Lake Dagow on 4 August 1998. Ap, Amoebobacter purpureus-like cells; Cln, Chloronema-like cells; Cm, “C. magnum”; Co, Chromatium okenii-like cells; Pr, “P. roseum”; Tr, Thiopedia rosea-like cells. With bright-field microscopy, “P. roseum” has a distinct brown color.
FIG. 2.
Physical, chemical, and biological parameters in the chemocline of Lake Dagow on 4 July 1998. (A) Conductivity, temperature, and pH. (B) Concentrations of molecular oxygen and sulfide. (C) Numbers of phototrophic consortia and total cell number (TCN). (D) Concentrations of BChl a, BChl d and e, and Chl a. (E) Fraction of green sulfur bacteria (GSB) associated with phototrophic consortia as determined by fluorescence in situ hybridization. (F) CO2 assimilation rates as determined by incubation with NaH14CO3.
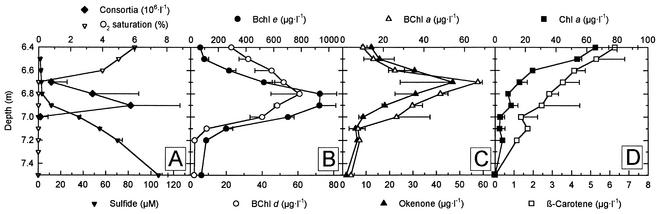
FIG. 3.
Composition of phototrophic bacteria in the Lake Dagow chemocline on 19 July 1999 as determined by HPLC analyses. (A) Numbers of phototrophic consortia, molecular oxygen saturation (expressed as a percentage of air saturation), and concentrations of sulfide. (B to D) Photosynthetic pigments of green sulfur bacteria (BChl d and BChl e) (B), purple sulfur bacteria (BChl a and okenone) (C), and cyanobacteria and algae (Chl a and β-carotene) (D).
TABLE 1.
Levels of photosynthetic consortia and environmental conditions in the biomass over 5 consecutive years maximum
| Dates | Depth of maximum abundance of “P. roseum” (m) | Maximum no. of “P. roseum” (cells/ml) | Relative abundance of epibionts (% of total cell no.) | Relative abundance of epibionts (% of green sulfur bacteria) | Light intensity (μmol of quanta · m−2 · s−1) | Concn (μg · liter−1) of:
|
Sulfid concn | ||
|---|---|---|---|---|---|---|---|---|---|
| BChl d + BChl e | BChl a | Chl a | |||||||
| 2 to 9 August 1995a | 7.7 | (3.2 ± 1.5) × 105 | 19.1 | NDb | 0.42 | 342 | 13.7 | 0 | 18 |
| 2 to 11 August 1996a | 7.7 | 5.8 × 104 | 6.4 | ND | 0.06 | 493 | 29.4 | 22.2 | 219 |
| 4 to 15 August 1997 | 6.6 | (7.8 ± 0.8) × 104 | 1.7 | ND | 1.1 | 292 | 13.7 | 0 | 25 |
| 2 to 7 July 1998 | 6.4 | (5.6 ± 0.5) × 105 | 16.4 | 84 | 0.4 | 420 | 56.9 | 0 | 10 |
| 19 and 20 July 1999 | 6.8 | (3.4 ± 2.9) × 106 | 37.2 | 88 | 0.7 | 1,002 | 50 | 0 | 3 |
Values from a previous study (41).
ND, not determined.
Pigment composition of the chemocline community of phototrophic bacteria.
The maximum numbers of brown-colored phototrophic consortia coincided with the maximum concentrations of BChl d and e in the chemocline (Fig. 2C and D and 3A and B). Only low numbers of the green-colored “Chlorochromatium magnum” (15) and “C. aggregatum” consortia were present (Fig. 1). Occasionally, green- and brown-colored cells resembling Pelodictyon clathratiforme and Pelodictyon phaeoclathratiforme, respectively, were observed (not visible in Fig. 1). In addition, purple-colored cells with intracellular sulfur globules were detected. In terms of cell morphology and size, the capacity to form cell aggregates, and the presence of gas vacuoles, these cells closely resembled Amoebobacter purpureus, Thiopedia rosea, and Chromatium okenii (Fig. 1). In addition, green-colored filaments containing gas vacuoles resembling the green filamentous bacterium Chloronema sp. were observed in some years.
BChl d and e were the predominant photosynthetic pigments in the chemocline in five consecutive years (Table 1 and Fig. 2D and 3B to D). On average, the concentration of BChl d and e exceeded the concentration of BChl a by 1 order of magnitude and the concentration of Chl a by 2 orders of magnitude (Table 1). A more detailed analysis of the photosynthetic pigments in July 1999 by HPLC and HPLC-MS (Table 2) revealed the dominance of BChl e-containing green sulfur bacteria in the chemocline. BChl e was present at concentrations as high as 934 μg · liter−1 at the depth at which the maximum abundance of phototrophic consortia occurred. The BChl e concentrations exceeded the concentrations of BChl a and d by about 20-fold and the concentrations of Chl a by about 100-fold (Fig. 3B to D). BChl c was never detected in the chemocline.
TABLE 2.
Identification of BChls and Chl a by MS-MS fragmentation
| HPLC peak | Retention time (min) | Mass (m/z)
|
Identification
|
||
|---|---|---|---|---|---|
| [M+H]+a | Fragments | [8, 12] BChl substituentsb | Esterifying alcohol | ||
| 1 | 28.4 | 821 | 803, 617, 599 | [E, E] BChl eF | Farnesol (222)d |
| 2 | 29.5 | 835 | 817, 631, 613 | [P, E] BChl eF | Farnesol (222) |
| 2sc | 29.5 | 793 | 589 | [E, E] BChl dF | Farnesol (222) |
| 3 | 30.5 | 849 | 831, 645, 627 | [I, E] BChl eF | Farnesol (222) |
| 4 | 34.2 | 839 | 821, 631, 613 | [E, E] BChl eHen | Hexadecenol (240) |
| 4 | 34.2 | 813 | 795, 599 | [E, E] BChl eT | Tetradecanol (214) |
| 5 | 35.0 | 853 | 835, 631, 613 | [P, E] BChl eHen | Hexadecenol (240) |
| 5 | 35.0 | 827 | 809, 613 | [P, E] BChl eT | Tetradecanol (214) |
| 6 | 36.3 | 811 | 589 | [E, E] BChl dHen | Hexadecenol (240) |
| 7 | 35.8 | 867 | 849, 645, 627 | [I, E] BChl eHen | Hexadecenol (240) |
| 7 | 35.8 | 841 | 823, 7 | [I, E] BChl eT | Tetradecanol (214) |
| 9 | 37.1 | 841 | 823, 617, 599 | [E, E] BChl eHan | Hexadecanol (242) |
| 10 | 37.8 | 855 | 837, 631, 613 | [P, E] BChl eHan | Hexadecanol (242) |
| 11 | 38.1 | 813 | 589 | [E, E] BChl dHan | Hexadecanol (242) |
| 12 | 38.5 | 869 | 851, 645, 627 | [I, E] BChl eHan | Hexadecanol (242) |
| 12 | 38.5 | 911 | 853, 633 | BChl aP | Phytol (296) |
| 13 | 38.8 | 839 | 589 | [E, E] BChl dOen | Octadecanol (268) |
| 14 | 39.4 | 867 | 589 | [E, E] BChl dp | Phytol (296) |
| 17 | 43.0 | 893 | 615 | Chl aP | Phytol (296) |
[M + H]+, quasi-molecular ion.
E, ethyl; I, isobutyl; M, methyl; P, n-propyl.
s, shoulder.
The numbers in parentheses are m/z values.
The carotenoids isorenieratene and β-isorenieratene are specific for BChl e-containing green sulfur bacteria. Even at the maximum biomass of “P. roseum,” however, the concentrations of both carotenoids were below the detection limit (2 μg · liter−1). The only carotenoids present at considerable levels in the chemocline were okenone and β-carotene. The concentration of okenone was as high as 47 μg · liter−1, and the vertical distribution of this compound was similar to that of BChl a (Fig. 3C). The vertical distribution of β-carotene clearly correlated with the distribution of Chl a (Fig. 3D). Traces of additional yellow carotenoids were detected in the chemocline, but these compounds were not characterized further.
Phylogenetic composition of green sulfur bacteria in the chemocline.
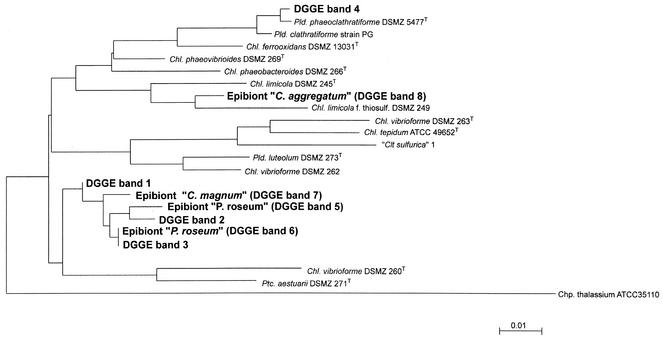
The green sulfur bacteria present in the chemocline of Lake Dagow were characterized further by using a culture-independent molecular approach. A 465-bp fragment of the 16S rRNA gene was amplified from genomic DNA by a PCR method specific for green sulfur bacteria, and the DNA fragments were separated by DGGE on the basis of their different melting behaviors. Up to four different DNA fingerprints were detected in the chemocline microbial community (Fig. 4). Two of the 16S rRNA gene fragments had the same melting behavior as the fragments obtained from intact “P. roseum” consortia separated manually by micromanipulation. In contrast, 16S rRNA gene fragments of the green-colored “C. aggregatum” and “C. magnum” consortia were not detected in the natural community (Fig. 4).
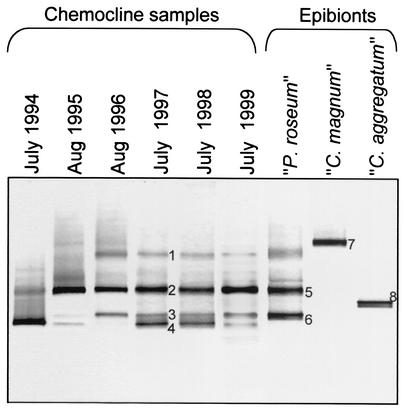
FIG. 4.
16S rRNA gene fingerprints of green sulfur bacteria from the chemocline microbial community in Lake Dagow for six consecutive years compared to fingerprints of isolated consortia. The latter were generated by using 10 isolated intact consortia (central bacterium plus attached epibionts) of “C. aggregatum,” 10 consortia of “P. roseum,” and five consortia of “C. magnum.” A negative image of an ethidium bromide-stained DGGE gel is shown. The numbers of DNA bands correspond to sequences in the phylogenetic tree in Fig. 5.
The 16S rRNA gene fragments of the phototrophic consortia and four DNA bands from the natural community that exhibited different melting behaviors (indicated by numbers in Fig. 4) were excised and sequenced. Phylogenetic analysis confirmed that one of the two 16S rRNA gene sequences from the epibionts of “P. roseum” (Fig. 4, band 6) was indeed identical to a sequence (band 3) from the natural community (Fig. 5). However, the second fingerprint of “P. roseum” (Fig. 4, band 5) and the corresponding DNA band from the natural community (band 2) contained two different 16S rRNA gene sequences (Fig. 5).
FIG. 5.
Phylogenetic position of the “P. roseum” epibiont in the radiation of green sulfur bacteria. A phylogenetic tree was constructed by the maximum-likelihood method from full-length sequences of the most closely related green sulfur bacteria. The partial 16S rRNA gene sequences were then inserted by using a maximum-parsimony tool as implemented in the ARB phylogeny package. The DNA sequence of the epibiont of “P. roseum” in band 6 is identical to the DNA sequence in band 3 from the entire chemocline microbial community (compare Fig. 4). Bar = 0.01 fixed point mutation per base. Pld., Pelodictyon; Chl., Chlorobium; Clt., Clathrochloris; Ptc., Prosthecochloris; Chp., Chloroherpeton.
The combined evidence from pigment determination and molecular fingerprinting analyses indicated that the consortium “P. roseum” dominates the phototrophic community in Lake Dagow. Whole-cell in situ hybridization was used to quantify the abundance of “P. roseum” epibionts in comparison to the abundance of other green sulfur bacteria in the chemocline.
Numerical dominance of epibiotic green sulfur bacteria.
The fraction of green sulfur bacteria associated with phototrophic consortia was determined in 1998 and 1999. Fluorescence in situ hybridization with a probe specific for green sulfur bacteria (GSB-532) revealed that in July 1998, 84% of all green sulfur bacteria at the depth at which the maximum biomass occurred (6.4 m) (Fig. 2) was associated with phototrophic consortia (Fig. 2E and Table 1). In the following year, 88.5% of all green sulfur bacteria were epibionts (Table 1). Evidently, epibionts of “P. roseum” are the dominant anoxygenic phototrophs in the chemocline of Lake Dagow.
Based on a value of 20 epibionts per consortium (40), epibionts of “P. roseum” represented a significant fraction of the total cell counts and contributed up to 37% of all cells at the depth at which the maximum abundance of phototrophic consortia occurred (Table 1).
Photosynthetic CO2 assimilation.
As a first assessment of the in situ physiology of the phototrophic epibionts, the vertical distribution of anoxygenic NaH14CO3 assimilation was measured across the chemocline. Oxygenic photosynthesis was inhibited by the addition of 3-(3,4-dichlorophenyl)-1,1-dimethylurea. The maximum level of of anoxygenic photosynthesis was 3.7 μg of C · liter−1 · h−1 and was detected at the top of the layer of phototrophic consortia at a depth of 6.3 m (Fig. 2F) and a light intensity of 0.8 μmol of quanta · m−2 · s−1. The maximum values of dark CO2 fixation were detected between 6.3 and 6.5 m and were as high as 3.1 μg of C · liter−1 · h−1 (Fig. 2F). These comparatively high values for dark CO2 fixation were probably caused by chemolithoautotrophic bacteria or by anaplerotic CO2 fixation reactions of chemoorganoheterotrophic bacteria or both.
Stable carbon isotope fractionation of biomarkers and bulk carbon fractions.
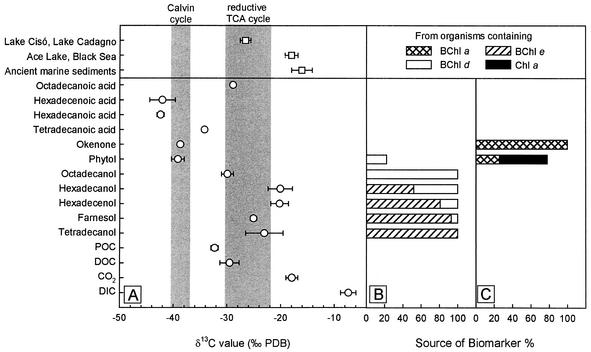
The carbon metabolism of the epibionts was investigated further by using the δ13C values of the biomarkers. The carotenoids isorenieratene and β-isorenieratene occur exclusively in BChl e-containing strains of green sulfur bacteria (12) and a few streptomycete species (25) and are therefore the most commonly used biomarkers of green sulfur bacteria. In contrast to all other known brown-colored green sulfur bacteria, however, isorenieratene and β-isorenieratene were absent in “P. roseum.” Accordingly, we used esterifying alcohols of BChl e as specific biomarkers and compared their δ13C values to those of the esterifying alcohols of other BChls and Chl a and to those of fatty acids, carotenoids, POC, DOC, and DIC.
Esterifying alcohols of BChls and Chl a were first identified by mass spectra derived from HPLC-MS-MS analysis and are listed in Table 2. Minor BChl e homologs containing dodecanol and pentadecenol were detected but not analyzed further, because the amounts of esterifying alcohols obtained were far too small to permit determination of δ13C values. Farnesol, hexadecanol, and hexadecenol occurred in BChl d, as well as in BChl e, whereas phytol was found in BChl a and BChl d, as well as in Chl a (Table 2). In order to identify suitable biomarkers for the BChl e-containing epibionts, the relative abundance of each esterifying alcohol in BChl a, d, and e homologs and Chl a was calculated based on the pigment composition determined by HPLC. BChl e homologs containing tetradecanol and hexadecenol coeluted. In these cases the relative contributions of different BChls to esterifying alcohols were calculated from the relative intensities of the quasi-molecular ions generated from the corresponding BChl homologs during MS. This calculation revealed that one-third of BChl e in the three HPLC peaks was esterified to tetradecanol.
According to our calculations, 100% of the tetradecanol, 92.9% of the farnesol, 80.7% of the hexadecenol, and 51.8% of the hexadecanol originated from BChl e (Fig. 6B). Consequently, tetradecanol, farnesol, and hexadecenol are suitable biomarkers for brown-colored green sulfur bacteria in Lake Dagow and, due to the strong numerical dominance of phototrophic consortia, also for epibionts of “P. roseum.” The remaining amounts of the esterifying alcohols indicated above most likely originated from additional green sulfur bacteria present in the chemocline, mostly the epibionts of “C. magnum” and the free-living organism P. clathratiforme, which contain BChl d homologs with the same HPLC elution pattern (6). Both bacteria most likely were also sources of octadecanol in July 1999.
FIG. 6.
δ13C values and origin of biomarkers of phototrophic bacteria isolated from a depth of 6.8 m in Lake Dagow on 19 July 1999. (A) δ13C values of specific biomarkers. Symbols: ○, δ13C values from the chemocline of Lake Dagow; □, average of δ13C values from previously published papers (for Lake Cisó and Lake Cadagno, isorenieratene from sediments [20, 50]; for Ace Lake and the Black Sea, isorenieratene, chlorobactene, and farnesane from sediments [22, 54, 57]; for ancient sediments, isorenieratene isolated from rocks [19, 21, 42, 55, 56]). The shaded areas indicate the range of Δδ13C values for the biomass of green and purple sulfur bacteria for the chemocline of Lake Dagow as calculated from previously published data (29, 47, 59). TCA, tricarboxylic acid. (B) Source of specific biomarkers derived from CO2 fixation by the reversed citric acid cycle. (C) Source of specific biomarkers derived from CO2 fixation via the Calvin cycle.
Farnesol, the major esterifying alcohol of BChl e, was depleted in 13C compared to CO2 (difference between δ13C values [Δδ13C] = −7.1‰) and enriched in 13C compared to DOC (Δδ13C = 11.6‰) and POC (Δδ13C = 14.4‰) (Fig. 6A). Compared to farnesol, straight-chain esterifying alcohols of BChl e were less depleted in 13C (average Δδ13C = −3.2‰). Okenone originated exclusively from BChl a-containing bacteria, and phytol originated from organisms containing BChl a, Bchl d, and Chl a (Fig. 6C). The Δδ13C values of phytol and okenone were −21.3 and −20.8‰, respectively.
In order to account for vertical differences in the stable carbon fractionation of DIC, δ13CDIC values were determined from 1997 to 1999 for 10-cm intervals across the chemocline and converted to δ13CCO2 values. However, the differences in δ13CCO2 values for different depths were not significant. For example, on 19 July 1999, δ13CCO2 was −17.3‰ ± 1.5‰ at 6.7 m and −18.4‰ ± 1.3‰ at 6.9 m. Similar δ13CCO2 values were obtained for chemocline samples in 1997 and 1998. However, larger differences in δ13CCO2 values were detected within the epilimnion (−12.6‰ ± 0.36‰ at 6.1 m and −15.1‰ ± 0.6‰ at 6.5 m) and between the epilimnion and hypolimnion (−21.2‰ ± 0.6‰ at 7.0 m) in August 1997.
DISCUSSION
Numerical significance and unique characteristics of the epibionts.
Combined with the microscopic evidence, our pigment analyses and molecular ecology approaches revealed that epibionts of “P. roseum” represent the numerically dominant green sulfur bacteria in the chemocline of Lake Dagow. First, the detailed analysis of photosynthetic pigments demonstrated the predominance of BChl e-containing green sulfur bacteria over the green sulfur bacteria containing BChl d or other phototrophs containing BChl a or Chl a. Second, a low diversity of green sulfur bacteria was detected by DGGE fingerprinting in the chemocline of Lake Dagow, and in 1996 to 1999 the 16S rRNA gene sequence of a major band from chemocline samples was identical to one sequence from “P. roseum.” Third, fluorescence in situ hybridization showed that the major fraction (84 to 88%) of green sulfur bacterial cells was associated with intact phototrophic “P. roseum” consortia. Because some of the epibionts were dislodged from phototrophic consortia during filtration of the fluorescence in situ hybridization samples, this fraction actually represents a minimum estimate.
Besides the distinct phylogenetic position of the epibionts and the fact that they are never found in the free-living state, the most obvious physiological characteristic of “P. roseum” is the lack of isorenieratene and β-isorenieratene. Previously, these two carotenoids had been assumed to represent the typical biomarkers of brown-colored green sulfur bacteria (12). Our data confirm recent analyses of the pigments of epibionts of “P. roseum” obtained from a North American lake (Echo Lake) (18). These epibionts, although clearly phylogenetically different from those in Lake Dagow, also lacked isorenieratene and β-isorenieratene. In addition, the epibionts had a very low overall carotenoid content. The molar ratio of total carotenoids to BChl e in Lake Dagow was 5 × 10−3, and the ratio in the Echo Lake enrichment of “P. roseum” was 25 × 10−3 (18). In contrast, free-living green sulfur bacteria have molar ratios of total carotenoids to BChl e of 0.14 to 0.38 depending on the growth conditions (18). Taken together, these data indicate that a low overall carotenoid content and a lack of isorenieratene and β-isorenieratene are common features of brown-colored epibionts of phototrophic consortia, which obviously employ BChl e as the only light-harvesting pigment. These features clearly contradict the hypothesis that carotenoids are essential for light harvesting by green sulfur bacteria (30, 33, 62). Correspondingly, cells in laboratory cultures of Chlorobium phaeobacteroides CL1401, whose carotenoid content was artificially depleted by 85% by using 2-hydroxybiphenyl as an inhibitor of carotenoid synthesis, did not have an altered growth rate compared to the growth rate of noninhibited cells (2).
In conclusion, epibionts of “P. roseum” have distinct characteristics, and therefore, their carbon metabolism potentially could differ from that of their free-living counterparts. Due to their numerical dominance, the consortia in Lake Dagow represent a model population that is well suited for culture-independent physiological studies.
Interpretation of δ13C values.
So far, stable carbon isotope discrimination studies of green sulfur bacteria have been done only with pure cultures (47, 58, 59). In one case, bulk microbial biomass was used to determine δ13C values (16), but there was not a concomitant determination of δ13CCO2 values, so that the carbon metabolism in situ could not be evaluated. Molecular markers of green sulfur bacteria are frequently used in geochemistry for reconstruction of past environmental conditions (19, 21, 24, 42, 43, 53, 55, 57). However, the carbon metabolism of an extant natural community of green sulfur bacteria has never been investigated based on stable carbon isotope fractionation of specific biomarkers.
Theoretically, there could have been unknown farnesol esters other than those of BChls in our samples, and they could have led to false δ13C values. However, this appeared to be highly unlikely under the specific conditions observed in the chemocline of Lake Dagow. Samples were collected by filtration; hence, the farnesol originated almost exclusively from microbial cells and, to a much lower extent, from particulate matter. Given the high specific BChl e content of green sulfur bacteria (100 μg of BChl e · mg of protein−1 [33]) and the dominance of farnesyl esters among the BChl e homologs (18; this study), the cellular content of farnesol is ∼1% of the cellular dry weight. Even if all accompanying cells were archaea and thus contained especially high amounts of isoprenoid lipids (26), only small amounts of farnesol derived from sources other than BChl e would be expected, as demonstrated by the following calculation. Of the total lipids present in archaea (accounting for 2 to 6% of the cellular dry weight [26]), only a small fraction of nonpolar lipids (7 to 30%, corresponding to 0.1 to 1.8% of the cellular dry weight [26]) contains traces of farnesol (≤2% of the nonpolar lipids [61]). Consequently, the maximum amount of farnesol which could originate from archaeal lipids is 0.036% of the cellular dry weight. Considering that in the chemocline of Lake Dagow the number of epibionts almost equals the number of all other accompanying prokaryotes, the amount of farnesol derived from BChl e even under the unrealistic assumption that archaea are dominant would therefore surpass the amount of archaeal farnesol by a factor of at least 21. We concluded that in the chemocline of Lake Dagow, farnesol is a valid biomarker for green sulfur bacterial epibionts of phototrophic consortia.
Since the biochemical building block in carotenoids and farnesol is isoprene, very similar δ13C values are observed for these two biomarkers in cultures of Chlorobium limicola (64). Consequently, our δ13C values for isoprenoid alcohols can be compared to the carbon fractionation values of green sulfur bacterial carotenoids from other environments. The low δ13C values of the biomarkers of “P. roseum” are very similar to those of green sulfur bacteria in sediments of Lake Cadagno (50) and Lake Cisó (20) (Fig. 6). The low δ13C values have been explained by internal recycling of 13C-depleted bicarbonate (3, 4, 20). Indeed, the δ13CCO2 values in the chemocline of Lake Dagow were very low and compared well with values from other holomictic temperate (66) and meromictic (11) lakes. In contrast, the 13C in isorenieratene isolated from sediments of low-productivity environments like Ace Lake, Antarctica (53), or the oligotrophic Black Sea (22, 57) is much less depleted (Fig. 6A).
The difference between the δ13C values of biomarkers and the δ13C values of ambient CO2 (Δδ13C) can be used to elucidate the carbon source used by epibionts of “P. roseum.” In the chemocline of Lake Dagow, the Δδ13C of farnesol was −7.1‰. In photoautotrophically grown cells of green sulfur bacteria, the 13C in isoprenoid biomarkers is enriched by 2 to 3‰ compared to the 13C in the total biomass (64). Accounting for this difference, the Δδ13C for the epibiont biomass was −9.6‰ ± 2.7‰ (Table 3). An almost identical Δδ13C has been reported for photoautotrophically grown cultures of green sulfur bacteria (−10.1‰ after correction of δ13CCO2 values [equation 5] [57]). In a similar manner, Δδ13C values were calculated for purple sulfur bacteria in the chemocline of Lake Dagow and compared to previously published data (29, 59). The results indicate that bacteria belonging to this group also grow photoautotrophically (Table 3).
TABLE 3.
Biomass Δδ13C values: comparison of values for “P. roseum” epibionts and previously published values for green and purple sulfur bacteria
| Organisms | Δδ13C (‰ vs PDB) | Origin of sample | Reference(s) |
|---|---|---|---|
| “P. roseum” epibontsa | −9.6 ± 1a,b | Lake Dagow, Germany | This study |
| Green sulfur bacteria | −10.1 (−12.2)b,c | Pure cultures | 56 |
| Purple sulfur bacteria | −20.3 | Lake Dagow, Germany | This study |
| Purple sulfur bacteria | −19.8 ± 0.6 | Pure cultures | 29, 47, 56 |
| Thermochromatium tepidum | −21.6 | Roland's Well, Yellowstone National Park, Wyoming, United States | 29 |
Although Δδ13C values for biomass or biomarkers from natural populations of green sulfur bacteria are not available, photoautotrophic growth of green sulfur bacterial populations in other ecosystems can be inferred from the difference between δ13C values for isoprenoid biomarkers for green sulfur bacteria (GSB) and purple sulfur bacteria (PSB):
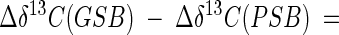 |
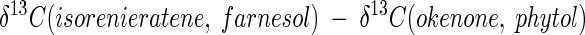 |
(6) |
Based on our data, green sulfur bacteria, as well as purple sulfur bacteria, grow photoautotrophically in Lake Dagow. The 13C in farnesol in Lake Dagow was enriched by 13.7‰ compared to the 13C in okenone and by 14.1‰ compared to the 13C in phytol (Fig. 6). Similar differences between Δδ13C(GSB) and Δδ13C(PSB) were observed for okenone and isorenieratene in the sediments of Lake Cisó (15‰) (20) and Lake Cadagno (18‰) (50). Together with the fact that the absolute δ13C values were also similar in the two lakes mentioned above and Lake Dagow, these data indicate that photoautotrophic growth of free-living green sulfur bacteria occurs in the three ecosystems.
In situ physiology of epibionts of “P. roseum.”
The major goal of the present study was to gain further insight into the interactions between the partner cells of phototrophic consortia under natural conditions as they occur. The central bacterium of the phototrophic consortia phylogenetically belongs to the β subclass of the Proteobacteria (15) and, in contrast to previous speculation (44, 52), is therefore unlikely to reduce sulfate or sulfur. This conclusion is corroborated by the experimental finding that epibionts of phototrophic consortia exhibit electron transfer only in the presence of external sulfide (14). Since the epibionts do not appear to depend on internal sulfide generation, the selective advantage of consortium formation could be related to carbon metabolism. Because the biomass of green sulfur bacteria increases significantly in the presence of organic carbon substrates (36), it appeared to be feasible that epibionts grow mixotrophically or even heterotrophically by taking advantage of organic carbon substrates excreted by the central chemotrophic bacterium.
However, the Δδ13C values determined in the present study strongly indicate that the epibionts of “P. roseum” grow photoautotrophically under in situ conditions instead of utilizing significant amounts of organic carbon substrates. Direct assimilation of the organic carbon substrates would be expected to result in lower Δδ13C values for the epibionts, since the 13C in DIC and POC in the chemocline of Lake Dagow was highly depleted (Fig. 6). Although it is unlikely, the only alternative explanation of the observed Δδ13C values is that a major fraction of the cellular carbon of the epibionts is derived from organic carbon substrates which (i) are significantly less depleted in 13C than the bulk DOC, (ii) represent only a small fraction of the total DOC, and (iii) have a δ13C value which upon assimilation and transformation to lipids results in values precisely like the values in autotrophically grown green sulfur bacteria.
Utilization of organic carbon compounds excreted by the central bacterium also appears to be unlikely, since the stable carbon isotopic signature of organic carbon does not change significantly (i.e., it changes by ∼1‰) during chemoorganoheterotrophic metabolism (16), so that transfer of organic carbon from the central bacterium to the epibionts would yield Δδ13C values lower than those observed. From our results we concluded that assimilation of external or internal organic carbon compounds does not provide a selective advantage to the symbiotic green sulfur bacteria in phototrophic consortia.
Since the epibionts of “P. roseum” grow photoautotrophically in situ, an estimate of their maximum growth rate could be obtained based on the peak carbon assimilation rate and the corresponding biomass of epibionts. This analysis yielded a growth rate of the epibionts of 0.045 day−1, corresponding to a doubling time of 15.3 days at the in situ light flux, 0.8 μmol of quanta · m−2 · s−1. For comparison, the growth rates of green sulfur bacteria in pure cultures even at similar low light intensities range from 0.17 to 0.07 day−1 (33, 36), and the growth rates of intact “C. aggregatum” consortia reach 0.188 day−1 (14). Obviously, growth of the epibionts in situ is limited by factors other than light intensity. The maximum in situ growth rate of free-living phototrophic sulfur bacteria (0.063 day−1 [65]) is comparable to that of the epibionts of “P. roseum” in Lake Dagow. Therefore, epibionts in the associated state are expected to be able to compete with, but not outcompete, free-living green and purple sulfur bacteria in their natural habitat.
Our detailed analysis revealed that the carbon metabolism of epibionts of phototrophic consortia in situ is similar to that of their free-living counterparts. Evidently, there is a selective advantage other than internal recycling of carbon or sulfur which maintains epibionts in the associated state and which causes the dominance of green sulfur bacterial epibionts of “P. roseum” over free-living green sulfur bacteria in the natural habitat. Whereas green sulfur bacteria (with the exception of the gliding organism C. thalassium) are not motile, the epibionts of phototrophic consortia like “P. roseum” are carried by the motile central bacterium and hence become motile in the associated state (40). Based on the data obtained in the present study, it appears likely that the acquisition of motility makes epibionts of phototrophic consortia more competitive in heterogeneous aquatic environments, where they can exploit local accumulations of compounds (e.g., sulfide) much faster than their free-living relatives that are not motile.
Acknowledgments
We are indebted to J. Rullkötter (Institute for Chemistry and Biology of the Marine Environment, University of Oldenburg) for providing MS facilities, to K. Mangelsdorff for GC-MS measurements, and to U. Güntner for irm MS measurements. H. Rütters and T. Möhring helped with the HPLC-MS. We thank M. Segl (University of Bremen) for determining δ13CCO2 values. L. Bañeras (Institute of Aquatic Ecology, University of Girona, Girona, Spain) provided helpful technical advice concerning pigment separation by HPLC. We are also indebted to H.-D. Babenzien (Leibniz Institute for Freshwater Ecology and Inland Fisheries, Neuglobsow, Germany) for generously letting us use laboratory facilities during field studies.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arellano, J. B., C. M. Borrego, A. Martínez-Planells, and L. J. Garcia-Gil. 2001. Effect of carotenoid deficiency on cells and chlorosomes of Chlorobium phaeobacteroides. Arch. Microbiol. 175:226-233. [DOI] [PubMed] [Google Scholar]
- 3.Behrens, A., P. Schaeffer, S. Bernasconi, and P. Albrecht. 2000. Mono- and bicyclic squalene derivatives as potential proxies for anaerobic photosynthesis in lacustrine sulfur-rich sediments. Geochim. Cosmochim. Acta 64:3327-3336. [Google Scholar]
- 4.Berry, J. A., J. Throughton, and O. E. Bjørkman. 1974. Carbon isotope fractionation in open and closed systems. Carnegie Inst. Wash. Year Book 73:785-790. [Google Scholar]
- 5.Borrego, C. M., J. B. Arellano, C. A. Abella, T. Gillbro, and L. J. Garcia-Gil. 1999. The molar extinction coefficient of bacteriochlorophyll e and the pigment stoichiometry in Chlorobium phaeobacteroides. Photosynth. Res. 60:257-264. [Google Scholar]
- 6.Borrego, C. M., and L. J. Garcia-Gil. 1994. Separation of bacteriochlorophyll homologs from green phototrophic sulfur bacteria by reversed-phase HPLC. Photosynth. Res. 41:157-163. [DOI] [PubMed] [Google Scholar]
- 7.Buder, J. 1914. Chlorobium mirabile. Ber. Dtsch. Bot. Ges. 31:80-97. [Google Scholar]
- 8.Button, D. K., F. Schut, P. Quang, R. Martin, and B. R. Robertson. 1993. Viability and isolation of marine bacteria by dilute culture: theory, procedures, and initial results. Appl. Environ. Microbiol. 59:881-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caspar, S. J. (ed.). 1985. Lake Stechlin—a temperate oligotrophic lake. Dr. W. Junk Publishers, Dordrecht, The Netherlands.
- 10.Cline, J. D. 1969. Spectrophotometric determination of hydrogen sulfide in natural water. Limnol. Oceanogr. 14:454-458. [Google Scholar]
- 11.Deevey, S. D., N Nakai, and M. Stuiver. 1963. Fractionation of sulfur and carbon isotopes in a meromictic lake. Science 139:407-408. [DOI] [PubMed] [Google Scholar]
- 12.de Wit, R., and P. Caumette. 1995. An overview of the brown-colored isorenieratene containing green sulfur bacteria (Chlorobiaceae), p. 908-909. In J. O. Grimalt and C. Dorronsoro (ed.), Organic geochemistry: developments and applications to energy, climate, environment and human history. A.I.G.O.A., Donostia-San Sebastian, Spain.
- 13.Fröhlich, J., and H. König. 2000. New techniques for isolation of single prokaryotic cells. FEMS Microbiol. Rev. 24:567-572. [DOI] [PubMed] [Google Scholar]
- 14.Fröstl, J., and J. Overmann. 1998. Physiology and tactic response of “Chlorochromatium aggregatum.” Arch. Microbiol. 169:129-135. [DOI] [PubMed] [Google Scholar]
- 15.Fröstl, J., and J. Overmann. 2000. Phylogenetic affiliation of the bacteria that constitute phototrophic consortia. Arch. Microbiol. 174:50-58. [DOI] [PubMed] [Google Scholar]
- 16.Fry, B. 1986. Sources of carbon and sulfur nutrition for consumers in three meromictic lakes of New York State. Linmnol. Oceanogr. 31:79-88. [DOI] [PubMed] [Google Scholar]
- 17.Gasol, J. M., K. Jürgens, R. Massana, J. I. Calderón-Paz, and C. Pedrós-Alió. 1995. Mass development of Daphnia pulex in a sulfide-rich pond (Lake Cisó). Arch. Microbiol. 132:279-296. [Google Scholar]
- 18.Glaeser, J., L. Bañeras, H. Rütters, and J. Overmann. 2001. Novel bacteriochlorophyll e structures and species-specific variability of pigment composition in green sulfur bacteria. Arch. Microbiol. 177:476-485. [DOI] [PubMed] [Google Scholar]
- 19.Grice, K., R. Gibbison, J. E. Atkinson, L. Schwark, C. B. Eckhardt, and J. R. Maxwell. 1996. Maleimides (1H-pyrolle-2,5-diones) as molecular indicators of anoxygenic photosynthesis in ancient water columns. Geochim. Cosmochim. Acta 60:3913-3924. [Google Scholar]
- 20.Hartgers, W. H., S. Schouten, J. F. Lopez, J. S. Sinninghe Damsté, and J. O. Grimalt. 2000. 13C-contents of sedimentary bacterial lipids in a shallow sulfidic monomictic lake (Lake Cisó, Spain). Org. Geochem. 31:777-786. [Google Scholar]
- 21.Hartgers, W. H., J. S. Sinninghe Damsté, A. G. Requejo, J. Allan, J. M. Hayes, U. Ling, T.-M. Xie, J. Primack, and J. W. de Leeuw. 1993. A molecular and carbon isotopic study towards the origin and diagenetic fate of diaromatic carotenoids. Org. Geochem. 22:703-725. [DOI] [PubMed] [Google Scholar]
- 22.Huang, Y., K. H. Freeman, R. T. Wilkin, M. A. Arthur, and A. D. Jones. 2000. Black Sea chemocline oscillations during the Holocene: molecular and isotopic studies of marginal sediments. Org. Geochem. 31:1525-1531. [Google Scholar]
- 23.Jørgensen, B. B., J. G. Kuenen, and Y. Cohen. 1979. Microbial transformation of sulfur compounds in a stratified lake (Solar Lake, Sinai). Limnol. Oceanogr. 24:799-822. [Google Scholar]
- 24.Koopmanns, M. P., J. Köster, H. M. E. van Kaam, F. Kenig, S. Schouten, A. H. Hartgers, J. W. de Leeuw, and J. S. Sinninghe Damsté. 1996. Diagenetic and catagenetic products of isorenieratene: molecular indicators for photic zone anoxia. Geochim. Cosmochim. Acta 60:4467-4496. [Google Scholar]
- 25.Krügel, H., P. Krubasik, K. Weber, H. P. Saluz, and G. Sandmann. 1999. Functional analysis of genes from Streptomyces griseus involved in the synthesis of isorenieratene, a carotenoid with aromatic end groups, revealed a novel type of carotenoid desaturase. Biochim. Biophys. Acta 1439:57-64. [DOI] [PubMed] [Google Scholar]
- 26.Langworthy, T. A. 1985. Lipids of archaebacteria, p. 459-497. In C. R. Woese and R. S. Wolfe (ed.), The bacteria, vol. 8. Academic Press, New York, N.Y.
- 27.Lauterborn, R. 1906. Zur Kenntnis der sapropelischen Flora. Allg. Bot. 2:196-197. [Google Scholar]
- 28.Ludwig, W., O. Strunk, S. Klugbauer, N. Klugbauer, M. Weizenegger, J. Neumaier, M. Bachleitner, and K. H. Schleifer. 1998. Bacterial phylogeny based on comparative sequence analysis. Electrophoresis 19:554-568. [DOI] [PubMed] [Google Scholar]
- 29.Madigan, M. T., R. Takigiku, R. G. Lee, H. Gest, and J. M. Hayes. 1989. Carbon isotope fractionation by thermophilic phototrophic sulfur bacteria: evidence for autotrophic growth in natural populations. Appl. Environ. Microbiol. 55:639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montesinos, E., R. Guerrero, C. Abella, and I. Esteve. 1983. Ecology and physiology of the competition for light between Chlorobium limicola and Chlorobium phaeobacteroides in natural habitats. Appl. Environ. Microbiol. 46:1007-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overmann, J., M. J. L. Coolen, and C. Tuschak. 1999. Specific detection of different phylogenetic groups of chemocline bacteria based on PCR and denaturing gradient gel electrophoresis of 16S rRNA gene fragments. Arch. Microbiol. 172:83-94. [DOI] [PubMed] [Google Scholar]
- 33.Overmann, J., H. Cypionka, and N. Pfennig. 1992. An extremly low-light-adapted phototrophic sulfur bacterium from the Black Sea. Limnol. Oceanogr. 32:150-155. [Google Scholar]
- 34.Overmann, J., and F. Garcia-Pichel. September 2002, revision date. The phototrophic way of life. In M. Dworkin et al. (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed. [Online.] Springer, New York, N.Y. http://ep.springer-ny.com:6336/contents/.
- 35.Overmann, J., J. T. Beatty, K. J. Hall, N. Pfennig, and T. G. Northcote. 1991. Characterization of a dense, purple sulfur bacterial layer in a meromictic salt lake. Limnol. Oceanogr. 36:846-859. [Google Scholar]
- 36.Overmann, J., and N. Pfennig. 1989. Pelodictyon phaeoclathratiforme sp. nov., a new brown-colored member of the Chlorobiaceae forming net-like colonies. Arch. Microbiol. 152:401-406. [Google Scholar]
- 37.Overmann, J., and K. Schubert. 2002. Phototrophic consortia: model systems for symbiotic interrelations between prokaryotes. Arch. Microbiol. 177:201-208. [DOI] [PubMed] [Google Scholar]
- 38.Overmann, J., and M. M. Tilzer. 1998. Control of primary productivity and the significance of photosynthetic bacteria in a meromictic kettle lake, Mittlerer Buchensee, West Germany. Aquat. Sci. 51:261-278. [Google Scholar]
- 39.Overmann, J., and C. Tuschak. 1997. Phylogeny and molecular fingerprinting of green sulfur bacteria. Arch. Microbiol. 167:302-309. [DOI] [PubMed] [Google Scholar]
- 40.Overmann, J., C. Tuschak, J. M. Fröstl, and H. Sass. 1998. The ecological niche of the consortium “Pelochromatium roseum.” Arch. Microbiol. 169:120-128. [DOI] [PubMed] [Google Scholar]
- 41.Overmann, J., and H. van Gemerden. 2000. Microbial interactions involving sulfur bacteria: implications for the ecology and evolution of bacterial communities. FEMS Microbiol. Rev. 24:591-599. [DOI] [PubMed] [Google Scholar]
- 42.Pancost, R. D., K. H. Freeman, M. E. Patzkowsky, D. A. Wavrek, and J. W. Collister. 1998. Molecular indicators of redox and marine photoautotroph composition in the late Middle Ordovician of Iowa, USA. Org. Geochem. 29:1649-1662. [Google Scholar]
- 43.Passier, H. F., H.-J. Bosch, L. J. L. Nijenhuis, M. E. Böttcher, A. Leenders, J. S., Sinninghe Damsté, G. J. de Lange, and J. W. de Leeuw. 1999. Sulphidic Mediterranean surface waters during Pliocene sapropel formation. Nature 397:146-149. [Google Scholar]
- 44.Pfennig, N. 1980. Syntrophic mixed cultures and symbiotic consortia with phototrophic bacteria: a review, p. 127-131. In G. Gottschalk et al. (ed.) Anaerobes and anaerobic infections. Gustav Fischer Verlag, Stuttgart, Germany.
- 45.Porter, K. G., and Y. S. Feig. 1980. Use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25:943-948. [Google Scholar]
- 46.Putschew, A., B. M. Scholz-Böttcher, and J. Rullkötter. 1996. Early diagenesis of organic matter and related sulfur incorporation in surface sediments of meromictic Lake Cadagno in the Swiss Alps. Org. Geochem. 25:379-390. [Google Scholar]
- 47.Quandt, L., G. Gottschalk, H. Zielger, and W. Stichler. 1977. Isotope discrimination by photosynthetic bacteria. FEMS Microbiol. Lett. 1:125-128. [Google Scholar]
- 48.Rau, G. 1978. Carbon-13 depletion in a subalpine lake: carbon flow implications. Science 201:901-902. [DOI] [PubMed] [Google Scholar]
- 49.Rost, R., I. Zondervan, and U. Riebesell. 2002. Light-dependent carbon isotope fractionation in the coccolithophorid Emiliania huxleyi. Limnol. Oceanogr. 47:120-128. [Google Scholar]
- 50.Schaeffer, P., P. Adam, P. Wehrung, and P. Albrecht. 1997. Novel aromatic carotenoid derivatives from sulfur photosynthetic bacteria in sediments. Tetrahedron Lett. 48:8413-8416. [Google Scholar]
- 51.Schidlowski, M. 1988. A 3,800-million-year isotopic record of life from carbon in sedimentary rocks. Nature 333:313-318. [Google Scholar]
- 52.Schink, B. 1991. Syntrophism among prokaryotes, p. 276-299. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes. Springer Verlag, New York, N.Y.
- 53.Schouten, S., W. I. C. Rupstra, M. Kok, E. C. Hopmans, R. E. Summons, J. K. Volkman, and J. S. Sinninghe Damsté. 2001. Molecular organic tracers of biogeochemical processes in a saline meromictic lake (Ace Lake). Geochim. Cosmochim. Acta 65:1629-1640. [Google Scholar]
- 54.Schouten, S., H. M. E. van Kaam-Peters, M. Schoell, and J. Sinninghe Damsté. S. 2000. Effects of an oceanic anoxic event on Early Toarcian carbon. Am. J. Sci. 300:1-22. [Google Scholar]
- 55.Sinninghe Damsté, J. S., and J. Köster. 1998. A euxinic southern North Atlantic Ocean during the Cenomanian/Turonian oceanic anoxic event. Earth Planet. Sci. Lett. 158:165-173. [Google Scholar]
- 56.Sinninghe Damsté, J. S., S. Schouten, and A. C. T. van Duin. 2001. Isorenieratene derivatives in sediments: possible controls on their distribution. Geochim. Cosmochim. Acta 65:1557-1571. [Google Scholar]
- 57.Sinninghe Damsté, J. S., S. G. Wakeham, M. E. L. Kohnen, J. M. Hayes, and J. W. de Leeuw. 1993. A 6,000-year sedimentary molecular record of chemocline excursion in the Black Sea. Nature 362:827-829. [DOI] [PubMed] [Google Scholar]
- 58.Sirevåg, R. 1995. Carbon metabolism in green bacteria, p. 871-883. In R. E. Blankenship, M. T. Madigan, and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Advances in photosynthesis, vol. II. Kluwer Academic Publishers, Dordrecht, The Netherlands,
- 59.Sirevåg, R., B. B. Buchanan, J. A. Berry, and J. H. Troughton. 1977. Mechanisms of CO2 fixation in bacterial photosynthesis studied by the carbon isotope fractionation technique. Arch. Microbiol. 112:35-38. [DOI] [PubMed] [Google Scholar]
- 60.Steenbergen, C. L. M., and H. J. Korthals. 1982. Distribution of phototrophic microorganisms in the anaerobic and microaerophilic strata of Lake Vechten (The Netherlands). Pigment analysis and the role in primary production. Limnol. Oceanogr. 27:883-895. [Google Scholar]
- 61.Tornabene, T. G., T. A. Langworthy, G. Holzer, and J. Oró. 1979. Squalenes, phytanes and other isoprenoids as major neutral lipids of methanogenic and thermoacidophilic “Archaebacteria.” J. Mol. Evol. 13:73-83. [DOI] [PubMed] [Google Scholar]
- 62.Trüper, H.-G., and S. Genovese. 1968. Characterization of photosynthetic sulfur bacteria causing red water in Lake Faro (Messina, Sizily). Limnol. Oceanogr. 13:225-232. [Google Scholar]
- 63.Tuschak, C., J. Glaeser, and J. Overmann. 1999. Specific detection of green sulfur bacteria by in situ-hybridization with a fluorescently labeled oligonucleotide probe. Arch. Microbiol. 171:265-272. [DOI] [PubMed] [Google Scholar]
- 64.van der Meer, M. T. J., S. Schouten, and J. S. Sinninghe Damsté. 1998. The effect of the reversed tricarboxylic acid cycle on the 13C contents of bacterial lipids. Org. Geochem. 28:527-533. [Google Scholar]
- 65.van Gemerden, H., and J. Mas. 1995. Ecology of phototrophic sulfur bacteria, p. 49-85. In M. T. Madigan and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 66.Wachniew, P., and K. Rózanski. 1997. Carbon budget of a mid-latitude, groundwater-controlled lake: isotopic evidence for the importance of dissolved inorganic carbon recycling. Geochim. Cosmochim. Acta 61:2453-2465. [Google Scholar]