Abstract
The properties of the bactericidal action of silver zeolite as affected by inorganic salts and ion chelators were similar to those of silver nitrate. The results suggest that the contact of the bacterial cell with silver zeolite, the consequent transfer of silver ion to the cell, and the generation of reactive oxygen species in the cell are involved in the bactericidal activity of silver zeolite.
The bactericidal activity of silver ion has been known since ancient times, and its spectrum is rather broad (5, 38). Silver ion reacts with the thiol group in vital enzymes and inactivates them (10, 24, 34) or interacts with DNA (8), resulting in marked enhancement of pyrimidine dimerization by photodynamic reaction and possible prevention of DNA replication (11, 31, 34). Structural changes in the cell envelope and the presence of some small electron-dense granules formed with silver and sulfur have also been demonstrated in bacterial cells (8).
In Japan, ceramics containing silver ion, such as silver zeolite and silver zirconium phosphate, are of interest for manufacturers aiming to apply antimicrobial compounds to their products (5, 16, 17, 18, 21, 26, 29, 30). In silver zeolite, alkaline or alkaline earth metal ion complexed with crystal aluminosilicate is partially replaced with silver ion by the ion-exchange method. Since these antimicrobial ceramics are being believed to have low toxicity for humans and the activity of the antimicrobial compound is durable (14, 21, 26, 32), it is being extensively used for food preservation, disinfection of medical supplies, and decontamination of surfaces of materials such as toys, kitchen wares, and medical supplies and equipment (14, 26, 32). Silver ion strongly interacts with the ceramics matrix and is minimally released from the matrix in deionized water.
Two mechanisms are proposed for the bactericidal action of silver zeolite. One is the action of silver ion itself released from zeolite (16) and the other is that of reactive oxygen species generated from silver in the matrix (21, 29, 30). While oxygen has been reported to be necessary for the bactericidal activity of silver zeolite by some researchers (17), silver zeolite has also been reported to be effective on oral bacteria under anaerobic conditions by other investigators (18).
In this study, we examined the bactericidal activity of silver zeolite against Escherichia coli cells in the presence of a variety of substances and in anaerobiosis, comparing its properties with those of silver nitrate.
E. coli strain OW6 (Pro−) (20), strain CSH7 (lacY rspL thi) (28), and its catalase-deficient mutant UM1 (katE katG lacY rspL thi) (25) were used in this study. Cells were grown to mid-log phase at an optical density at 650nm of approximately 0.3 at 37°C in M9 medium (36) supplemented with 0.2% glucose. If necessary, 0.1% vitamin-free Casamino Acids (EM9) or 1 mg of thiamine hydrochloride per liter (TM9) was added to the medium for the cultivation of strain OW6 or other strains, respectively (20). Cells were collected by centrifugation (6,890 × g, 5 min, 4°C) and washed with 20 mM HEPES-NaOH buffer (pH 7.0), 20 mM potassium phosphate buffer (pH 7.0), or deionized water (pH 6.7). Cells were resuspended in the same fresh solution at a final optical density at 650 nm of 0.06 (approximately 2 × 107 cells per ml), and then either the suspension of silver zeolite (Zeomic AG10D, containing 2.5% silver; Shinanen Co., Tokyo, Japan) at a final density ranging from 10 to 100 mg/liter or the solution of silver nitrate (Wako Pure Chemical Ind., Osaka, Japan) at a final concentration ranging from 0.5 to 5.0 μM was added. The suspension was incubated at 37°C for the treatment. To stop the reaction, sodium sulfide was added at a final concentration of 0.01% to the suspension and the mixture was kept on ice for 10 min. For anaerobic treatment, the suspension was bubbled with a mixture of 90% nitrogen, 5% hydrogen, and 5% carbon dioxide (26). Air was used for the controls. In the experiment for cell separation from silver zeolite, 2 ml of silver zeolite (20 mg/liter) suspended in 20 mM potassium phosphate buffer (pH 7.0) was poured into a dialysis membrane (2.4-nm average pore size; Viskase), and the membrane was then immersed in 18 ml of fresh buffer. After incubation for 20 h at 37°C, cells (final concentration, 2.2 × 107/ml) were suspended in the buffer outside the membrane. Viable cells were enumerated by the colony count method (20), using either EM9 or TM9 agar plates containing 0.1% sodium pyruvate. The silver ion content was measured with a polarized Zeeman atomic absorption spectrophotometer (Z-5000; Hitachi, Ltd., Tokyo, Japan). For the content of free silver in the solution, the suspension was centrifuged (6,890 × g, 5 min, 4°C) and the resultant supernatant was filtrated with a polycarbonate membrane filter (pore size, 0.1 μm; Advantec). To the filtrate, nitric acid solution was added at a final concentration of 0.1 N. For the measurement of total content of silver in the cell, cells washed twice with deionized water were incubated in 8 N nitric acid at 80°C for 1 h.
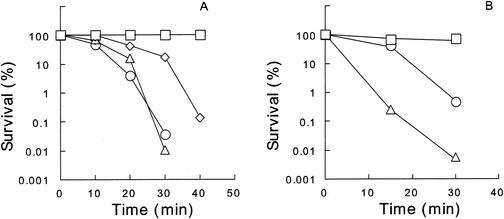
Silver zeolite at 100 mg/ml remarkably reduced the number of viable E. coli OW6 cells in 20 mM potassium phosphate buffer at pH 7.0 and 37°C (Fig. 1A). The survival curves did not follow first-order kinetics. A similar bactericidal activity of silver zeolite was also observed with 20 mM HEPES-NaOH buffer at pH 7.0. The activity of silver zeolite was more marked at a higher temperature in the range of 0 to 42°C and at a higher pH in the range of 6.5 to 8.5 (data not shown), as has already been reported (16). Bactericidal activity of silver nitrate was examined in 20 mM HEPES-NaOH buffer (pH 7.0), because its activity was suppressed in potassium phosphate buffer (data not shown). HEPES-NaOH buffer was used for silver nitrate in further experiments. The bactericidal activity of silver nitrate at 1 μM was comparable to that of silver zeolite at 100 mg/liter (Table 1 and Fig. 1).
FIG. 1.
Bactericidal activities of silver zeolite and silver nitrate at 37°C. (A) Cells were treated with silver zeolite at 0 (□), 10 (⋄), 30 (▵), and 100 (○) mg/ml in 20 mM potassium phosphate buffer (pH 7.0). (B) Cells were treated with silver nitrate at 0.5 (□), 1.0 (○), and 2.0 (▵) mg/ml in 20 mM HEPES-NaOH buffer (pH 7.0).
TABLE 1.
Comparison of the inhibitory effects of several substances on the bactericidal activities of silver zeolite and silver nitratea
| Substance | Concn | Survival (%)
|
|
|---|---|---|---|
| Silver zeolite (100 mg/ml) | Silver nitrate (1 μM) | ||
| None | 0.20 | 0.45 | |
| —b | 0.31 | ND | |
| l-Cysteine | 1 mM | 46 | ND |
| 5 mM | 120 | 92 | |
| l-Histidine | 5 mM | 0.20 | 77 |
| 50 mM | 81 | ND | |
| Bovine serum albumin | 0.01% | 33 | ND |
| Yeast extract | 0.1% | 64 | ND |
| NaCl | 100 mM | 25 | ND |
| MgSO4 | 1 mM | 32 | 46 |
| MnSO4 | 1 mM | 0.75 | 2.6 |
| 10 mM | 3.9 | ND | |
| FeSO4 | 1 mM | <0.001 | 6.3 |
| 1 mMb | 69 | ND | |
| EDTA | 5 mMb | 3.0 | ND |
| 2,2′-Dipyridyl | 1 mM | <0.001 | <0.001 |
| o-Phenanthroline | 1 mM | <0.001 | <0.001 |
Cells were treated in HEPES-NaOH buffer (pH 7.0) containing the indicated substances at 37°C for 30 min. Values are averages of results in two independent experiments. ND, not determined.
Cells were treated in potassium phosphate buffer (pH 7.0).
The effects of various substances on the antimicrobial action of silver zeolite and silver nitrate were compared (Table 1 and data not shown). The activity of silver zeolite was inhibited by the addition of l-cysteine, l-methionine, l-histidine, l-tryptophan, bovine serum albumin, and yeast extract but not by glycine, l-alanine, l-leucine, or l-phenylalanine. The presence of l-cysteine indicated a strong inhibition of the bactericidal action of silver zeolite at relatively low concentrations. Sodium chloride at 100 mM substantially inhibited its activity, but neither sodium acetate nor sodium sulfate did. Gupta et al., however, reported a synergistic bactericidal effect against a silver-resistant E. coli strain between silver ion and highly concentrated chloride ions and suggested the formation of soluble AgCl2− and AgCl3− (13) as the reason for increased activity. In our study, no such synergistic action of chloride was observed. Magnesium sulfate at 1 mM and manganese sulfate at 10 mM inhibited the bactericidal activity strongly and slightly, respectively. Ferrous sulfate at 1 mM strongly inhibited the antimicrobial activity in 20 mM potassium phosphate buffer (pH 7.0), whereas it stimulated it in 20 mM HEPES-NaOH buffer (pH 7.0). EDTA at 5 mM weakly suppressed the action of silver zeolite. The ferrous ion chelators o-phenanthroline and 2,2′-dipyridyl strongly enhanced the antimicrobial activity.
l-Cysteine, l-histidine, and manganese, magnesium, and ferrous ions inhibited the activity of silver nitrate. 2,2′-Dipyridyl, as in the case of silver zeolite, strongly enhanced bactericidal activity, whereas o-phenanthroline did not. The fact that the inhibitory properties of substances tested were similar between silver zeolite and silver nitrate, except in a few cases, suggests that silver ion bound to zeolite matrix is involved in the bactericidal action of silver zeolite. It has been reported that the bactericidal action of silver nitrate was inhibited by chloride, phosphate, and sulfide ions, some proteins, and several amino acids, including cysteine (33, 34), and similar effects of these compounds on the action of mercury ion have been observed (6, 7).
The amounts of silver ion released from the zeolite matrix after incubation for 30 min in 20 mM HEPES-NaOH buffer and phosphate buffer at pH 7.0 were approximately 100 and 50 μg/liter, respectively. Silver ion at 100 μg/liter (approximately 0.93 μM) possesses a detectable bactericidal activity, according to our analysis. However, no detectable activity was observed in the supernatant obtained by centrifugation after incubation of silver zeolite suspension at 37°C for 1 h in the absence of bacterial cells. It also should be noted that in deionized water, no detectable amount of silver was released, although the bactericidal activity is similarly high. This suggests that in water, silver ion may be released from zeolite only when bacterial cells are present. Further, when cells were partitioned from silver zeolite (100 mg/ml of total volume) by a dialysis membrane during the incubation period, the viability after 30 min of treatment was about 3 log cycles higher than that of cells treated without partitioning (data not shown). These results suggest that silver zeolite needs to come into contact with cells to exert its bactericidal action.
When silver nitrate (1 μM) was added to the cell suspension at 37°C in HEPES-NaOH buffer, silver ion was transferred to cells and its amount was up to approximately 7.2 fg per cell after 30 min. The presence of 1 mM manganese or magnesium ions was found to reduce the level of silver transferred by approximately 30 and 45%, respectively, suggesting that the inhibitory effect of these divalent cations results from the reduction of accumulation of silver in the cell. A similar action was reported for zinc ion, which inhibits the uptake of cadmium ion by E. coli cells (22). The manganese uptake through MntH-dependent manganese transport system is inhibited by the presence of nickel, copper, and zinc ions (19). It remains unclear why ferrous ion and its chelators have different effects on the bactericidal activity of silver zeolite. Several complicated actions of ferrous ion on cells, including its role for oxidative stress, may be involved in the survival of E. coli cells treated with silver zeolite (1, 3, 4, 12, 23, 35, 38).
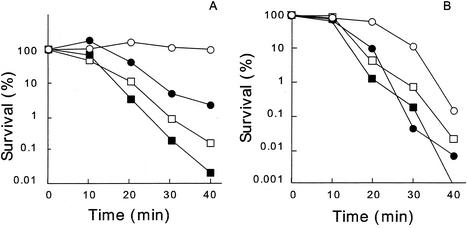
The bactericidal activities of silver zeolite and silver nitrate were examined under anaerobic condition (Fig. 2A). Many more cells were viable under anaerobic conditions than in the presence of oxygen. In addition, the reactive-oxygen scavengers sodium pyruvate (0.1%) and mannitol (5 mM) substantially inhibited the activity of silver zeolite at 10 mg/ml during incubation in 20 mM potassium phosphate buffer for 30 min at 37°C. We also examined how a catalase-deficient strain responds to silver zeolite. Strain UM1 (katE katG) was found to be more sensitive to both silver zeolite and silver nitrate than its parent strain CSH7 (Fig. 2B). The outbreak of oxidative stress caused by toxic heavy metal is proposed for cadmium ion (2, 9, 15, 37). Silver ion is known to inhibit especially thiol group-containing enzymes (34), such as NADH dehydrogenase II in the respiratory system, which is supposed to be a candidate for the site of the production of reactive oxygen species in vivo (27).
FIG. 2.
Bactericidal activities of silver zeolite and silver nitrate in anaerobiosis (A) and on a catalase-deficient strain of E. coli (B). (A) OW6 cells were treated at 37°C with silver zeolite at 10 mg/ml in 20 mM potassium phosphate buffer (circles) or silver nitrate at 1 μM in 20 mM HEPES-NaOH buffer (squares) anaerobically (open symbols) or aerobically (closed symbols). (B) Cells of the wild-type strain UM1 (closed symbols) and a catalase-deficient strain CSH7 (open symbols) were treated similarly but aerobically with silver zeolite (circles) and silver nitrate (squares).
In this study, it was concluded that silver ion plays an important role for the bactericidal action of silver zeolite. It is proposed that two possible successive processes may be involved in the action of silver zeolite. First, bacterial cells that make contact with silver zeolite take in silver ion, which inhibits several functions in the cell and consequently damages cells. The second is the generation of reactive oxygen species, which are produced possibly through the inhibition of a respiratory enzyme(s) by silver ion and attack the cell itself. Although we have not yet succeeded in measuring the amount of silver ion transferred from silver zeolite into cells, its measurement is a prerequisite for clarification of the mode of action of silver zeolite.
Acknowledgments
We are grateful to Chizu Murai and Takako Hara for their assistance in portions of the experiments. We also thank Norihide Amano, Suntory Co., Ltd., for helpful support.
REFERENCES
- 1.Benov, L., and I. Fridovich. 1998. Growth in iron-enriched medium partially compensates Escherichia coli for the lack of manganese and iron superoxide dismutase. J. Biol. Chem. 273:10313-10316. [DOI] [PubMed] [Google Scholar]
- 2.Blom, A., W. Harder, and A. Matin. 1992. Unique and overlapping pollutant stress proteins of Escherichia coli. Appl. Environ. Microbiol. 58:331-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun, M., S. Bungert, and T. Friedrich. 1998. Characterization of the overproduced NADH dehydrogenase fragment of the NADH:ubiquinone oxidoreductase (complex I) from Escherichia coli. Biochemistry 37:1861-1867. [DOI] [PubMed] [Google Scholar]
- 4.Chepuri, V. L. Lemieux, D. C. Au, and R. B. Gennis. 1990. The sequence of the cyo operon indicates substantial structural similarities between the cytochrome o ubiquinol oxidase of Escherichia coli and the aa3-type family of cytochrome c oxidases. J. Biol. Chem. 265:11185-11192. [PubMed] [Google Scholar]
- 5.Clement, J. L., and P. S. Jarrett. 1994. Antimicrobial silver. Metal-Based Drugs 1:467-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, Y. E., and G. Stotzky. 1989. Factors affecting the toxicity of heavy metals to microbes, p. 31-90. In T. J. Beveridge and R. J. Doyle (ed.), Metal ions and bacteria. John Wiley and Sons, Inc., New York, N.Y.
- 7.Farrell, R. E., J. J. Germida, and P. M. Huang. 1990. Biotoxicity of mercury as influenced by mercury(II) speciation. Appl. Environ. Microbiol. 56:3006-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng, Q. L., J. Wu, G. Q. Chen, F. Z. Cui, T. N. Kim, and J. O. Kim. 2000. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 52:662-668. [DOI] [PubMed] [Google Scholar]
- 9.Ferianc, P., A. Farewell, and T. Nystrom. 1998. The cadmium-stress stimulon of Escherichia coli K-12. Microbiology 144:1045-1050. [DOI] [PubMed] [Google Scholar]
- 10.Flemming, C. A., F. G. Ferris, T. J. Beveridge, and G. W. Bailey. 1990. Remobilization of toxic heavy metals absorbed to wall-clay composites. Appl. Environ. Microbiol. 56:3191-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox, C. L., and S. M. Modak. 1974. Mechanisms of silver sulphadiazine action on burn wound infections. Antimicrob. Agents Chemother. 5:528-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green, G. N., H. Fang, R. J. Lin, G. Newton, M. Mather, C. D. Georgiou, and R. B. Gennis. 1988. The nucleotide sequence of the cyd locus encoding the two subunits of the cytochrome d terminal oxidase complex of Escherichia coli. J. Biol. Chem. 263:13138-13143. [PubMed] [Google Scholar]
- 13.Gupta, A., M. Maynes, and S. Silver. 1998. Effects of halides on plasmid-mediated silver resistance in Escherichia coli. Appl. Environ. Microbiol. 64:5042-5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotta, M., H. Nakajima, K. Yamamoto, and M. Aono. 1998. Antibacterial temporary filling materials: the effect of adding various ratios of Ag-Zn-zeolite. J. Oral Rehabil. 25:485-489. [DOI] [PubMed] [Google Scholar]
- 15.Hultberg, M. 1998. Rhizobacterial glutathione levels as affected by starvation and cadmium exposure. Curr. Microbiol. 37:301-305. [DOI] [PubMed] [Google Scholar]
- 16.Im, K., Y. Takasaki, A. Endo, and M. Kuriyama. 1996. Antibacterial activity of A-type zeolite supporting silver ions in deionized distilled water. J. Antibacterial Antifungal Agents 24:269-274. [Google Scholar]
- 17.Inoue, Y., M. Hoshino, H. Takahashi, T. Noguchi, T. Murata, Y. Kanzaki, H. Hamashima, and M. Sasatsu. 2002. Bactericidal activity of Ag-zeolite mediated by reactive oxygen species under aerated condition. J. Inorg. Biochem. 92:37-42. [DOI] [PubMed] [Google Scholar]
- 18.Kawahara, K., K. Tsuruda, M. Morishita, and M. Uchida. 2000. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic condition. Dent. Mater. 16:452-455. [DOI] [PubMed] [Google Scholar]
- 19.Kehres, D. G., M. L. Zaharik, B. B. Finlay, and M. E. Maguire. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 36:1085-1100. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa, M., Y. Matsumura, and T. Tsuchido. 2000. Small heat shock proteins, IbpA and IbpB, are involved in resistances to heat and superoxide stresses in Escherichia coli. FEMS Microbiol. Lett. 184:165-171. [DOI] [PubMed] [Google Scholar]
- 21.Kourai, H., Y. Manabe, and Y. Yamada. 1994. Mode of bactericidal action of zirconium phosphate ceramics containing silver ions in the crystal structure. J. Antibact. Antifung. Agents 22:595-601. [Google Scholar]
- 22.Laddaga, R. S., and S. Silver. 1985. Cadmium uptake in Escherichia coli K-12. J. Bacteriol. 162:1100-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leif, H., V. D. Sled, T. Ohnishi, H. Weiss, and T. Friedrich. 1995. Isolation and characterization of the proton-translocating NADH: ubiquinone oxidoreductase from Escherichia coli. Eur. J. Biochem. 230:538-548. [DOI] [PubMed] [Google Scholar]
- 24.Liau, S. Y., D. C. Read, W. J. Pugh, J. R. Furr, and A. D. Russell. 1997. Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Lett. Appl. Microbiol. 25:279-283. [DOI] [PubMed] [Google Scholar]
- 25.Loewen, P. C. 1984. Isolation of catalase-deficient Escherichia coli mutants and genetic mapping of katE, a locus that affects catalase activity. J. Bacteriol. 157:622-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuura, T., Y. Abe, Y. Sato, K. Okamoto, M. Ueshige, and Y Akagawa. 1997. Prolonged antimicrobial effect of tissue conditioners containing silver-zeolite. J. Dent. 25:373-377. [DOI] [PubMed] [Google Scholar]
- 27.Messner, K. R., and J. A. Imlay. 1999. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J. Biol. Chem. 274:10119-10128. [DOI] [PubMed] [Google Scholar]
- 28.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 29.Miyoshi, H., H. Kourai, T. Maeda, and T. Yoshida. 1998. Role of Cl− adsorbed on silver-loaded zirconium phosphate for the photooxidation of OH− to OH·. J. Photochem. Photobiol. A 113:243-250. [Google Scholar]
- 30.Miyoshi, H., H. Kourai, and T. Maeda. 1998. Light-induced formation of 2,5-dihydroxy-p-benzoquinone from hydroquinone in photoirradiated silver-loaded zirconium phosphate suspension. J. Chem. Soc. Faraday Trans. 94:283-287. [Google Scholar]
- 31.Modak, S. M., and C. R. Fox, Jr. 1973. Binding of silver sulfadiazine to the cellular components of Pseudomonas aeruginosa. Biochem. Pharmacol. 22:2391-2404. [DOI] [PubMed] [Google Scholar]
- 32.Nikawa, H., T. Yamamoto, T. Hamada, M. B. Rahardjo, H. Murata, and S. Nakanoda. 1997. Antifungal effect of zeolite-incorporated tissue conditioner against Candida albicans growth and/or acid production. J. Oral Rehabil. 24:350-357. [DOI] [PubMed] [Google Scholar]
- 33.Richards R. M. 1981. Antimicrobial action of silver nitrate. Microbios 31:83-91. [PubMed] [Google Scholar]
- 34.Russell, A. D., and W. B. Hugo. 1994. Antimicrobial activity and action of silver. Prog. Med. Chem. 31:351-370. [DOI] [PubMed] [Google Scholar]
- 35.Touati, D., M. Jacques, B. Tardat, L. Bouchard, and S. Despied. 1995. Lethal oxidative damage and mutagenesis are generated by iron in fur mutants of Escherichia coli: protective role of superoxide dismutase. J. Bacteriol. 177:2305-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuchido, T., I. Aoki, and M. Takano. 1989. Interaction of the fluorescent dye 1-N-phenylnaphthylamine with Escherichia coli cells during heat stress and recovery from heat stress. J. Gen. Microbiol. 135:1941-1947. [DOI] [PubMed] [Google Scholar]
- 37.VanBogelen, R. A., P. M. Kelley, and F. C. Neidhardt. 1987. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J. Bacteriol. 169:26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young, J. G., B. L. Rogers, H. D. Campbell, A. Jaworowski, and D. C. Shaw. 1981. Nucleotide sequence coding for the respiratory NADH dehydrogenase of Escherichia coli UUG initiation codon. Eur. J. Biochem. 116:165-170. [DOI] [PubMed] [Google Scholar]