Abstract
The thermotolerant, restrictive methylotroph Bacillus methanolicus MGA3 (ATCC 53907) can secrete 55 g of glutamate per liter (maximum yield, 0.36 g/g) at 50°C with methanol as a carbon source and a source of ammonia in fed-batch bioreactors. A homoserine dehydrogenase mutant, 13A52-8A66, secreting up to 35 g of l-lysine per liter in fed-batch fermentations had minimal 2-oxoglutarate dehydrogenase activity [7.3 nmol min−1 (mg of protein)−1], threefold-increased pyruvate carboxylase activity [535 nmol min−1 (mg of protein)−1], and elevated citrate synthase (CS) activity [292 nmol min−1 (mg of protein)−1] and simultaneously secreted glutamate (20 to 30 g per liter) and l-lysine. The flow of carbon from oxaloacetate is split between transamination to aspartate and formation of citrate. To investigate the regulation of this branch point, the B. methanolicus gene citY encoding a CSII protein with activity at 50°C was cloned from 13A52-8A66 into a CS-deficient Escherichia coli K2-1-4 strain. A citY-deficient B. methanolicus mutant, NCS-L-7, was also isolated from the parent strain of 13A52-8A66 by N-methyl-N′-nitro-N-nitrosoguanidine mutagenesis, followed by selection with monofluoroacetate disks on glutamate plates. Characterization of these strains confirmed that citY in strain 13A52-8A66 was not altered and that B. methanolicus possessed several forms of CS. Analysis of citY cloned from NCS-L-7 showed that the reduced CS activity resulted from a frameshift mutation. The level of glutamate secreted by NCS-L-7 was reduced sevenfold and the ratio of l-lysine to glutamate secreted was increased 4.5-fold compared to the wild type in fed-batch cultures with glutamate feeding. This indicates that glutamate secretion in l-lysine-overproducing mutants can be altered in favor of increased l-lysine secretion by regulating in vivo CS activity.
The unusual metabolic properties of aerobic, restrictive, thermotolerant methylotrophic bacteria make them useful for the production of recombinant proteins, vitamins, amino acids, coenzymes, and cytochromes (12). Bacillus methanolicus MGA3 (ATCC 53907) is a gram-positive bacterium with a growth optimum of 50°C to 53°C. Cell extracts of this bacterium grown on methanol contain an NAD-dependent methanol dehydrogenase (3, 13), have 3-hexulose-6-phosphate synthase, fructose bisphosphate aldolase, and transaldolase activity indicative of the fructose bisphosphate aldolase/transaldolase variant of the ribulose monophosphate pathway, RuMP (4, 14, 44). The precursors (acetyl-coenzyme A, pyruvate, oxaloacetate, and 2-oxoglutarate) for lipids, amino acids, nucleic acids, and assimilation of ammonia are all synthesized from the products of the RuMP pathway (2). The dissimilation of carbon from formaldehyde to CO2 in B. methanolicus, which may function to detoxify accumulating formaldehyde, has recently been confirmed by 13C nuclear magnetic resonance and isotope ratio mass spectrometry (39). The theoretical yield of secreted amino acids varies depending on the percentage of formaldehyde carbon dissimilated to CO2 (39), and therefore control of the feeding of methanol to fed-batch cultures is critical (29).
Homoserine dehydrogenase mutants (HSD−) of B. methanolicus, which are threonine plus methionine auxotrophs, have been shown to secrete substantial quantities of l-lysine when grown at 50°C in fed-batch cultures on methanol as a sole carbon source and supplied with a source of ammonia (18, 29, 44). B. methanolicus HSD− strain 13A52-8A66 can secrete over 60 g of l-lysine plus glutamate (glutamate) per liter, with a mass yield of 0.50 to 0.63 g/g. However, no mutants that secrete only l-lysine have been found with this approach; all simultaneously secrete significant quantities of glutamate and smaller amounts of alanine.
Limited knowledge exists for most aerobic methylotrophs of the genetic basis of the regulation of pyruvate metabolism, the activity of tricarboxylic acid (TCA) cycle enzymes during rapid growth on methanol (7, 13, 54), and particularly regulation of the flow of carbon to amino acid biosynthesis (20, 31-33, 46). To accomplish high-level l-lysine production, the percentage of carbon dissimilated to CO2 must be minimized to generate an abundant supply of aspartate (37). In Bacillus subtilis, aspartate is synthesized from oxaloacetate by aspartate aminotransferase (glutamate:oxaloacetate transaminase) encoded by aspB, which appears to be constitutively expressed (5).
The simultaneous production of glutamate and l-lysine in B. methanolicus mutants indicates a substantial flow of carbon both to aspartate and into the first three reactions of the TCA cycle catalyzed by citrate synthase (CS), aconitase, and isocitrate dehydrogenase (Fig. 1). Since many methylotrophs generate reducing power by the oxidation of formaldehyde to CO2, catalyzed by an NAD(P)-dependent formaldehyde dehydrogenase and formate dehydrogenase, the TCA cycle in B. methanolicus is mainly used for supply of intermediates such as glutamate, which is the source of many cellular compounds and assimilation of ammonia (5, 13, 14, 49). Only the first three enzymes in the TCA cycle are needed for 2-oxoglutarate biosynthesis, which is transaminated to glutamate, catalyzed by glutamate synthase. The level of 2-oxoglutarate is regulated by the activity of isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase. Glutamate synthase expression is high when B. subtilis is grown in the presence of ammonia, and intracellular levels of glutamate can reach 100 mM (49).
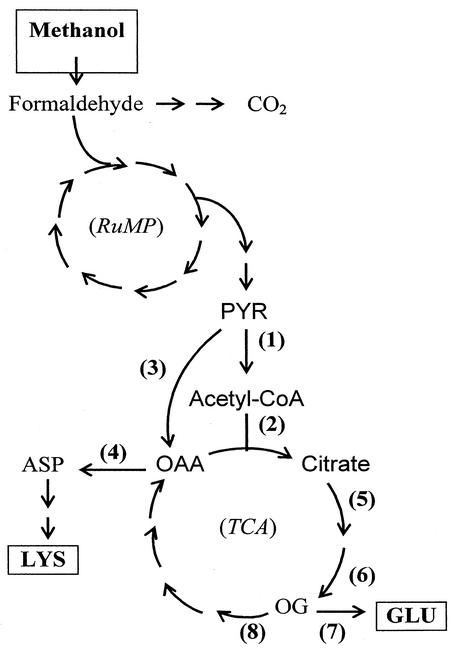
FIG. 1.
Methanol metabolism in B. methanolicus. Methanol is initially oxidized to formaldehyde, which may be dissimilated to CO2 or assimilated to pyruvate via the RuMP pathway. From pyruvate, the carbon flow is split between entering the TCA cycle or conversion to aspartate and its related amino acids. Abbreviations: TCA, tricarboxylic acid cycle; RuMP, ribulose monophosphate pathway; PYR, pyruvate; ASP, aspartate; OAA, oxaloacetate; OG, 2-oxoglutarate; GLU, glutamate; LYS, l-lysine; CoA, coenzyme A. Enzymes: 1, pyruvate dehydrogenase, PDH; 2, citrate synthase, CS; 3, pyruvate carboxylase, PC; 4, glutamate:oxaloacetate aminotransferase, GOT; 5, aconitase, ACN; 6, isocitrate dehydrogenase, IDH; 7, glutamate synthase, GOGAT; 8, 2-oxoglutarate dehydrogenase, OGDC.
The reactions that convert pyruvate to oxaloacetate and condensation of acetyl-coenzyme A to citrate may then be uniquely regulated in l-lysine-secreting HSD− strains of B. methanolicus because 2-oxoglutarate levels are critical for both carbon and nitrogen metabolism during growth on methanol. B. subtilis contains a pyruvate carboxylase (PC), encoded by pycA, to convert pyruvate to oxaloacetate, and lacks phosphoenolpyruvate carboxylase activity (50). By understanding the regulation of PC and CS activity in vivo, it should therefore be possible to increase the flow of carbon to oxaloacetate (and aspartate) by altering or deregulating either PC or CS activity or both activities.
The gene encoding PC activity has not been cloned from any thermotolerant low-G+C gram-positive bacterium. In Corynebacterium glutamicum, there is a single CS gene, and knocking out this gene results in glutamate auxotrophy (16). However, in Bacillus subtilis, two distinct CS genes have been found (citA and citZ, encoding CSI and CSII, respectively) that may catalyze the same reaction under different metabolic conditions (22, 23). CSII is the major form contributing to more than 90% of all CS activity in this organism (22, 24), and CS activity is regulated by the intracellular concentration of 2-oxoglutarate (50).
Here we describe the fed-batch reactor cultivation of B. methanolicus MGA3 (ATCC 53907) resulting in the secretion of 55 g of glutamate per liter from methanol at 50°C. Comparison of enzyme and metabolite levels involved in the flow of carbon leading to the secretion of glutamate or l-lysine of this strain and an l-lysine-overproducing mutant (13A52-8A66) indicated that CS activity may have a key role in determining the ratio of these two secreted amino acids. The citY gene, encoding a CSII protein, was cloned from the mutant strain, and a citY-deficient mutant, NCS-L-7, was isolated and characterized. These strains were used to investigate the effects of altering the in vivo CS activity on the ratio of l-lysine and glutamate produced in B. methanolicus from methanol in fed-batch cultures at 50°C.
(Portions of this work were performed by Christine Kaufmann to fulfill the requirements for a Diplomarbeit, Technische Fachhochschule Berlin, D-13347 Berlin, Germany.)
MATERIALS AND METHODS
Bacterial strains and plasmids and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli cells were grown at 37°C in Luria-Bertani and M9 media (43) supplemented with the appropriate antibiotics (ampicillin, 100 μg/ml; chloramphenicol, 150 μg/ml). B. methanolicus strains were grown at 50°C in a minimal vitamin medium (MVcM) containing l-methionine (1.5 mM), l-threonine (1.0 mM), MgSO4 · 7H2O (1 mM), methanol (150 mM), high-salt buffer, complete vitamins, and a concentrated solution of trace metals as described by Schendel et al. (44). MVcMY is MVcM medium supplemented with 0.025% yeast extract (Difco, Detroit, Mich.). Glutamate was added when appropriate to a final concentration of 1 g per liter. Solid media were made by adding 1.5% agar (Difco, Detroit, Mich.).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotypea | Reference or source |
|---|---|---|
| Strains | ||
| B. methanolicus | ||
| NOA2 | Wild-type strain | 44 |
| MGA3 | Wild-type strain | 44 |
| PB1 | Wild-type strain | American Type Culture Collection |
| 13A52-8A66 | Met and Thr auxotroph mutant of NOA2 | 18 |
| NCS-L-7 | Mutant of NOA2 with low CS activity | This study |
| E. coli | ||
| DH5α | General cloning host | Bethesda Research Laboratories |
| K2-1-4 | CS-deficient mutant | 45 |
| Plasmids | ||
| pUC19 | Apr | 56 |
| pUC19cm | Clmr | 45 |
| pTBCS-L7 | 1.2-kb DNA fragment from NCS-L-7 chromosome encoding citY structural gene cloned into PstI and EcoRI sites of pUC19 | This study |
| Clone 11 | pUC19cm clone from 13A52-8A66 gene library, encoding the citY gene | This study |
Ap, ampicillin; Clm, chloramphenicol; Met, methionine; Thr, threonine.
For the time course CS activity experiments, overnight cultures were diluted 5% in 400 ml of warmed MVcMY (shake flasks) medium. For all other enzyme activity experiments, the cells were grown in 20 ml of MVcMY medium overnight. Cell growth was monitored by measuring optical density at 500 nm (OD500) with a Lambda 40 UV/Vis spectrophotometer (Perkin Elmer, Norwalk, Conn.), and cell dry weight values were obtained by dividing OD500 values by the factor 3.2.
Sources of strains and DNA.
Sequencing of 16S rRNA genes from B. methanolicus strain PB1 (ATCC 51357) and two wild-type B. methanolicus strains isolated in our laboratory, MGA3 (ATCC 53907) and NOA2, was performed by MIDI Labs (Newark, Del.). The full-length sequences (data not shown) were compared with databases from MIDI Labs, GenBank, and the Ribosomal Database Project, and species identity was analyzed through a comparison of strain sequences. The calculated percent genetic distance between strains MGA3 and NOA2 was found to be 0.03%, and they were found to be closely related to PB1 (calculated values of 0.88% and 0.84%, respectively). Differences of less than 1% are indicative of a species-level match (51), supporting that they are all of the same species. The closest related Bacillus species was B. niacini, whose 16S rRNA sequence differed from that of PB1, MGA, and NOA2 by 4.10%, 3.87%, and 3.90%, respectively. Because of previous genetic manipulation work on strain NOA2 (9), its near identity with MGA3, as well as being the parent of the l-lysine- plus glutamate-secreting strain 13A52-8A66, DNA from NOA2 was isolated for subsequent cloning of CS-encoding genes.
DNA manipulations.
Plasmid preparations, endonuclease digestions, ligations, transformation, and PCR were performed according to Sambrook et al. (43). Electroporation of E. coli K2-1-4 was performed with a Gene Pulser apparatus (Bio-Rad Laboratories, Richmond, Calif.) as described previously (46). Total DNA from B. methanolicus was isolated with the Qiagen DNeasy Tissue kit (Qiagen Inc., Stanford, Calif.). Cells were lysed by adding lysozyme (15 mg/ml) and incubation at 37°C for 1 h and otherwise in accordance with the manufacturer's instructions. DNA sequencing was performed by the Advanced Genetic Analysis Center (University of Minnesota), and the data were analyzed with the GCG software (11).
Preparation of crude extracts and measurement of enzyme activities.
All operations were performed at 4°C. E. coli extracts were prepared as described previously (46). Crude extracts of B. methanolicus MGA3 and 13A52-8A66 for measurements of glutamate:oxaloacetate transaminase, PC, phosphoenolpyruvate carboxylase, pyruvate dehydrogenase, 2-oxoglutarate dehydrogenase, and CS activities were prepared as follows: 20-ml overnight cultures were harvested by centrifugation (9,000 × g, 10 min), and pellets were washed twice in 20 ml of potassium phosphate buffer (20 mM, pH 7.0) and resuspended in 20 ml of the same buffer. Lysozyme (0.1 mg/ml) was added to the suspension and incubated for 15 min at 37°C, and the cells were disrupted by two passes through a French pressure cell (Hiratio, catalog no. 4-3398; SLM Aminco Instruments, Urbana, Ill.) at 638 lb/in2. Cell debris was removed by centrifugation (27,000 × g, 20 min), and solid ammonium sulfate was added to the supernatant to 65% saturation. The precipitate was collected by centrifugation (27,000 × g, 20 min), resuspended in 20 ml of potassium phosphate buffer (20 mM, pH 7.0), and dialyzed three times against 200 ml of potassium phosphate buffer for 5 to 11 h.
The dialyzed material was collected and used as a crude extract for enzyme activity and total cell protein analysis. Crude extracts of B. methanolicus NOA2 and NCS-L-7 for time course CS measurements were prepared as follows: 10 ml of cells was harvested by centrifugation (3,200 × g, 15 min), pellets were washed twice in 10 ml of lysis buffer (10 mM Tris-HCl, 1 mM EDTA, 1 mM citrate, 100 mM KCl, adjusted to pH 7.5), resuspended in 1.5 ml of lysis buffer supplemented with lysozyme (1 mg/ml), RNase (0.2 mg/ml), and DNase (0.2 mg/ml), and incubated at 37°C for 30 min. Suspensions were sonicated for 3 min (output control 4, 50% pulses) with a sonicator (Bronwell Scientific, Rochester, N.Y.). After sonication, cell debris was removed by centrifugation (12,000 × g, 15 min), and the supernatants were collected as crude cell extracts for enzyme activity and total cell protein analysis.
All spectroscopic assays were performed at 50°C (unless otherwise stated) with a Perkin Elmer Lambda UV/Vis recording spectrophotometer (Perkin Elmer, Norwalk, Conn.), and all assays were run in replicate. CS activity was assayed in the direction oxaloacetate to citrate at 412 nm by monitoring the formation of mercaptide ions produced from cleavage of 5,5-dithiobios-(2-nitrobenzoic acid) by acetyl-coenzyme A as described previously (45). 2-Oxoglutarate dehydrogenase activity was assayed with 2-oxoglutarate as the substrate by a coupled assay as described by Reed and Mukherjee (40). PC activity was assayed by measurement of oxaloacetate production with excess malate dehydrogenase and NADH by monitoring the decrease in absorbance at 340 nm due to the formation of NAD+ (48). Pyruvate dehydrogenase activity was assayed with pyruvate as the substrate in a coupled assay as described by Mukherjee et al. (34). Phosphoenolpyruvate carboxylase activity was assayed similar to PC, following the method of Canovas and Kornberg (6). Finally, glutamate:oxaloacetate transaminase was assayed in a coupled assay by measurement of oxaloacetate production with excess malate dehydrogenase and NADH, by monitoring the decrease in absorbance at 340 nm due to the formation of NAD+ (55). Protein assays were calibrated with bovine serum albumin (Pierce Chemical, Rockford, Ill.; assay no. 23223).
Preparation of cell extracts and measurement of intracellular metabolite concentrations.
B. methanolicus MGA3 and mutant 13A52-8A66 were grown in 100 ml of MVcMY medium in 1-liter shake flasks at 50°C until stationary phase. Cells were harvested by centrifugation (9,000 × g, 10 min) at 4°C, washed in 100 ml of potassium phosphate buffer (20 mM, pH 7.0), and resuspended in the same buffer to an OD500 of 20. The suspensions were boiled (15 min) and centrifuged (12,000 × g, 10 min), and the supernatants were assayed for internal metabolite concentrations.
The amino acids l-lysine, glutamate, and aspartate were assayed by high-pressure liquid chromatography as described below. Pyruvate, oxaloacetate, and 2-oxoglutarate were assayed spectrophotometrically at room temperature with a Perkin Elmer Lambda 3B recording spectrophotometer (Perkin Elmer, Norwalk, Conn.). The buffer used in all cases was 20 mM Tris-EDTA (pH 8). Pyruvate was assayed by the lactate dehydrogenase enzyme assay, where the decrease in NADH concentration, measured by the change in absorbance at 340 nm, is proportional to the amount of pyruvate reduced. Oxaloacetate and 2-oxoglutarate were assayed as described for pyruvate except that malate dehydrogenase and glutamate dehydrogenase were added instead of lactate dehydrogenase for oxaloacetate and 2-oxoglutarate, respectively.
The concentrations of the metabolites were calculated for the supernatants obtained after boiling the resuspended cells, assuming that boiling the cells released all the intercellular metabolites. For calculation of the substrate concentrations inside the cells, the cell volume in the cell samples was estimated. Assuming that cells are cylinders with a volume (V) of πr2 × the length (l) and that r = 0.45 μm and l = 3.5 μm (44), the volume of one cell was calculated to be 2.2 × 10−12 ml. With an OD500 value of 1 representing 108 cells, the total cell volume in a sample equals the number of cells in the sample (OD500 of sample × 108 × sample size) times the volume of one cell. The intracellular substrate concentrations were calculated for each sample by multiplication of the concentration in the supernatant with the sample volume divided by the total cell volume in the sample.
Fed-batch bioreactor experiments.
B. methanolicus strains were grown in 14-liter fermentors (BioLafette & Moritz, Mauze, France) in fed-batch MVcMY medium with the following fermentation parameters: antifoam, Mazu DF204; temperature, 50°C; pH 6.8 (maintained through addition of 30% NH4OH); agitation, 600 rpm; dissolved oxygen control level, 30%; methanol control level, 100 mM; airflow, 1 vvm (volume of air per volume of medium per minute); and vessel pressure, 4 lb/in2. Glutamate was fed at 6 mM when appropriate. The methanol concentration was measured in uncondensed off gas with a model MC-168 methanol concentration monitor and controller (PTI Instruments, Kathleen, Ga.) and controlled at 100 mM. A neat methanol-trace metal solution was added under liquid level in order to maintain 100 mM methanol in the vessel. When appropriate, 150 mM dl-methionine was added at 25% the rate of methanol-trace metals on methanol demand.
Amino acid analysis.
Samples taken from the reactors at various time points were centrifuged (12,000 × g, 10 min), and supernatants were analyzed for amino acid contents by reverse column high-pressure liquid chromatography (Alltech Associates, Deerfield, Ill; Absorbosphere HS-OPA 5U column). Samples were derivatized with o-phthaldialdehyde reagent prior to injection with an auto sampler (WISP-710B; Millipore Waters Chromatography Division, Milford, Mass.). An automated gradient controller was used to deliver methanol and 50 mM sodium acetate (pH 5.7). Amino acids were detected with a fluorescent detector (model 420-AC; Millipore Waters Chromatography Division, Milford, Mass.), and peaks were integrated as described previously (44).
Cloning the citrate synthase gene, citY, from B. methanolicus 13A52-8A66.
A gene library of B. methanolicus 13A52-8A66 was constructed by partial Sau3A1 digestion of its chromosomal DNA. A sucrose density gradient was used to fractionate the digested material, and fragments in the size range from 3 to 5 kbp were isolated and ligated into pUC19cm previously digested with BamHI and treated with alkaline phosphatase. The ligation mixture was transformed into E. coli DH5α by electroporation, and recombinant cells were selected on Luria-Bertani plates supplemented with chloramphenicol. The DNA library in plasmid pUC19cm was collected and electroporated into E. coli K2-1-4 (CS deficient), and cells were plated on M9 minimal medium plates. Citrate synthase-positive colonies were identified by the ability to grow on M9 minimal medium lacking glutamate.
Mutagenesis of B. methanolicus and selection of mutants with low CS activity.
B. methanolicus NOA2 was grown to mid-log phase in 10 ml of MVcMY broth, 0.5 ml of N-methyl-N′-nitro-N-nitrosoguanidine (0.8 mg per liter) was added to the culture and incubated at 50°C with aeration (200 rpm) for 20 min. Cells were centrifuged at 4,000 × g for 5 min, the cell pellets were washed and resuspended in 10 ml of warm (50°C) MVcMY broth, and 0.1 ml of resuspended cells are mixed with 2.5 ml of MVcM plus 10 mM glutamate top agar (0.6%) and spread onto MVcM plus 10 mM glutamate plates. After hardening, a paper disk saturated with monofluoroacetate (a sterile 6-mm paper disk dipped into 20 ml of 5 M monofluoroacetate; Schleicher & Schuell, Keene, N.H.) was applied to the lawn, and the plates were incubated at 50°C for 3 to 4 days. Thirty colonies growing near the disk were subcultured onto MVcM agar with and without 10 mM glutamate, and three colonies demonstrating the greatest disparity of growth were selected for CS activity measurements.
PCR-assisted cloning and sequencing of NCS-L-7 citY gene.
PCR primers were designed for both the 5′-terminal (two alternative primers, CS-F1 [5′-TTTTGAATTCATGACAGTTACACGTGG-3′, sense] and CS-F2 [5′-TTTTCATATGACAGTTACACGTGG-3′, sense]), and the 3′-terminal (CS-R1, 5′-TTTTCTGCAGTTATCCTCTTTGCTCAATTGG-3′, antisense) ends of the citY structural gene. Underlined in CS-F1 and CS-R1 are EcoRI and PstI restriction sites, respectively, for cloning. With primers CS-F1 and CS-R1, a fragment of the expected size was amplified from NCS-L-7 total DNA by PCR. The fragment was digested with EcoRI and PstI and ligated into the corresponding sites of pUC18, resulting in plasmid pTBCS-L7. This plasmid was subjected to DNA sequencing. As a control, a fragment of similar size was amplified with the primer pair CS-F2 and CS-R1, and this PCR fragment was purified and used for DNA sequencing.
Nucleotide sequence accession number.
The sequence of the structural citY gene has been deposited in GenBank under accession number AF424980.
RESULTS
Amino acid secretion by B. methanolicus MGA3 in 14-liter fed-batch bioreactors with methanol feeding.
To fully explore the ability of B. methanolicus MGA3 for glutamate secretion, we performed fed-batch bioreactor experiments. MGA3 cells were grown in a 14-liter fermentor in MVcMY medium under controlled conditions with methanol feeding, and samples were collected at various time points for analysis of secreted amino acids. l-Lysine was not detected in the medium. Glutamate secretion was, and it increased throughout the growth phase, reaching a maximum of 55 g per liter in the stationary phase. The carbon conversion (glutamate/methanol) was 0.36 g/g, and the average productivity was 2.78 g of glutamate liter−1 h−1. The maximum productivity was estimated to be 4.03 g of glutamate liter−1 h−1.
Comparison of specific enzyme activities and intracellular metabolite levels in B. methanolicus MGA3 and mutant 13A52-8A66.
In order to understand the enzymes involved in the flow of carbon from pyruvate to aspartate, the specific activities of CS, phosphoenolpyruvate carboxylase, PC, glutamate:oxaloacetate transaminase, 2-oxoglutarate dehydrogenase, and pyruvate dehydrogenase were measured in crude extracts prepared from B. methanolicus wild-type MGA3 and mutant 13A52-8A66 (Table 2). No phosphoenolpyruvate carboxylase activity was detected. The very low 2-oxoglutarate dehydrogenase activities observed were in agreement with previous reports indicating that the TCA cycle is not important for energy generation in methylotrophic bacteria (13, 14, 49). Also, the higher level of PC activity in the l-lysine-secreting mutant strain 13A52-8A66, particularly compared to pyruvate dehydrogenase activity in both strains, indicates that the dominant route for pyruvate is conversion to oxaloacetate. Interestingly, glutamate:oxaloacetate transaminase activity was reduced in strain 13A52-8A66, and the CS activity levels of the two strains correlate with the high glutamate production observed in both strains.
TABLE 2.
Enzyme activities in wild-type (MGA3) and l-lysine-overproducing mutant (13A52-8A66) B. methanolicus strainsa
| Strain | Activity [nmol min−1 (mg of protein)−1]
|
|||||
|---|---|---|---|---|---|---|
| PC | Pyruvate dehydrogenase | Glutamate: oxaloacetate aminotransferase | CS | 2-Oxoglutarate dehydrogenase | Phosphoenolpyruvate carboxylase | |
| MGA3 | 161 ± 45 | 33 ± 4.4 | 142 ± 7.3 | 126 ± 5.6 | 0.96 ± 0.11 | ND |
| 13A52-8A66 | 535 ± 22 | 5.5 ± 0.67 | 55 ± 8.1 | 292 ± 28 | 7.3 ± 0.61 | ND |
Cells were grown in shake flasks in MVcMY medium (see text). All measurements were made at 50°C in crude extracts prepared from cells harvested in stationary phase. All experiments were performed in duplicate or triplicate, and values are reported as means ± 1 standard deviation. ND, not detected.
We then proceeded to compare the internal concentrations of pyruvate, oxaloacetate, aspartate, 2-oxoglutarate, glutamate, and l-lysine in these two strains (Table 3). The concentrations of pyruvate, 2-oxoglutarate, and glutamate in the wild-type strain were similar to the corresponding values obtained in B. subtilis (15, 21, 47), whereas the oxaloacetate level was more than 600-fold higher. Both the l-lysine and glutamate levels in the mutant strain were substantially higher (29- and 6-fold, respectively), whereas the pyruvate, 2-oxoglutarate, and aspartate levels were somewhat lower compared to the wild type. These results are in agreement with the enzyme activity data showing that both the PC and CS levels are higher in the former strain. Moreover, these results indicate that a substantial portion of RuMP-derived carbon enters the TCA cycle via CS in the mutant strain (see Fig. 1).
TABLE 3.
Intracellular metabolite concentrations in B. methanolicus MGA3 and mutant 13A52-8A66a
| Strain | Intracellular metabolite concn (mM)
|
|||||
|---|---|---|---|---|---|---|
| Pyruvate | Oxaloacetate | 2-Oxoglutarate | Aspartate | Glutamate | l-Lysine | |
| MGA3 | 3.2 | 449 | 2.7 | 8.1 | 87.7 | 2.3 |
| 13A52-8A66 | 0.6 | 448 | 0.6 | 5.0 | 428 | 66.8 |
Cells were grown in MVcMY medium in 1-liter baffled shake flasks at 50°C until stationary phase, and crude extracts were prepared and measured for metabolite concentrations as described in the text.
Cloning of a gene, citY, encoding citrate synthase II from B. methanolicus 13A52-8A66.
The l-lysine- plus glutamate-overproducing B. methanolicus mutant 13A52-8A66 was previously generated by multiple rounds of mutagenesis and selection (18, 29). This strain was chosen for making a B. methanolicus chromosomal gene library. The library was transformed to the CS-deficient E. coli K2-1-4 (see Table 1), and three clones that could complement CS deficiency in this strain were identified. Restriction analysis of the recombinant plasmids showed that they were similar (data not shown), and one of the plasmids, designated clone 11, was chosen for further characterization. CS assays in crude extracts of recombinant E. coli cells harboring clone 11 showed that a protein with CS activity was expressed from the recombinant plasmid. The activity level was higher (100 mU/mg of protein) when assayed at 50°C than at 37°C (76 mU/mg of protein). The 4.1-kbp insert of clone 11 was sequenced, and one large open reading frame of 1,119 bp was identified. A putative E. coli-like promoter sequence (−35 region) and a putative Shine-Dalgarno consensus sequence (AAGGAG) were located upstream of the start codon (ATG) of the open reading frame. The identified gene was designated citY, and computer analysis of the deduced 373-amino-acid sequence of the citY gene product showed that it had 77.8% identity with the B. subtilis CSII protein (24), indicating that citY encodes a CSII protein (see Fig. 4).
FIG. 4.
Amino acid alignment of citrate synthase genes from B. coagulans, B. subtilis, B. methanolicus, M. smegmatis, C. glutamicum, and Sus scrofa indicating conserved helical regions that contribute to thermostability. Sequences were aligned with Clustal W. Secondary structure was assigned based on the alpha-helices from pig heart (Sus scrofa) enzyme. ∗, residues identical in all sequences; colon, conserved substitution of residues; period, semiconserved residues. Boxed amino acids represent the five ion pair network identified in P. furiosus.
Generation of a CS-deficient B. methanolicus mutant, NCS-L-7.
Assuming that B. methanolicus is similar to B. subtilis in possessing more than one CS gene, it was of interest to investigate the effects of knocking out the citY gene on growth and l-lysine/glutamate production. Because no efficient methods for specific homologous recombination have been developed for B. methanolicus, we used mutagenesis and selection. NOA2, the parental strain of 13A52-8A66, was subjected to N-methyl-N′-nitro-N-nitrosoguanidine mutagenesis, and selection of mutants with low CS activity was based on the ability to grow on glutamate-enriched plates in the presence of monofluoroacetate. This compound complexes with citrate produced by CS to make fluorocitrate, which is toxic for the cells. Mutants with no or low CS activity do not produce much fluorocitrate and therefore survive. Mutants were selected with this procedure, and CS activity measurements indicated that one of them, NCS-L-7, had approximately 90% reduced CS activity (data not shown) compared to the wild type.
In an attempt to map the mutations that caused the observed phenotype of NCS-L-7, the citY gene was amplified from its chromosome by PCR with primers CS-F1 and CS-R1 (see Materials and Methods). The 1.2-kb fragment obtained was cloned into pUC19, resulting in plasmid pTBCS-L7. Both strands of the insert were sequenced, and the results showed that the cloned fragment was identical to the previously obtained citY sequence with the exception of a frameshift mutation at bp 240 (T), which generated multiple translational stop codons. As a control to check whether the identified mutation could be caused as a result of an incorrect PCR amplification, the same procedure was used to amplify and sequence the citY gene from the parental (13A52-8A66) chromosome. In this case, no mutations were found. Finally, the citY gene was amplified from the NCS-L-7 chromosome with an alternative PCR primer pair (CS-F2 and CS-R1), and sequence analysis of this fragment identified the same frameshift mutation as described above. In conclusion, these experiments prove that NCS-L-7 is a citY-deficient mutant, supporting the assumption that citY encodes a CSII protein contributing to the major portion of CS activity in B. methanolicus, similar to what has been described in other Bacillus species (22, 52). Also, these results indicate that the citY sequence of mutant 13A52-8A66 is identical to the wild-type citY sequence of B. methanolicus NOA2.
Physiological characterization of mutant NCS-L-7.
In Bacillus spp., it is known that the CS genes are regulated at the transcriptional level according to the metabolic and physiological state of the cells (23, 26, 53). The citZ gene is repressed during growth in rich medium containing a rapidly metabolized carbon source (e.g., glucose), glutamate, or glutamine. In the absence of such compounds, however, its expression is increased (23). Upon growth of NCS-L-7 and NOA2 in MVcM medium without glutamate, the specific growth rate of the mutant strain was much lower (0.23 h−1) than that of the wild-type strain (0.53 h−1). These results demonstrate that B. methanolicus possesses several CS proteins, but the low residual CS activity in NCS-L-7 is insufficient to generate the glutamate levels necessary to maintain maximum cell growth.
In order to compare the CS activity levels of the two strains under conditions relevant for high-level l-lysine secretion, we grew the cells in MVcMY medium supplemented with glutamate (1 g per liter). The CS activity of the mutant strain was between 10-fold (exponential phase) and 80-fold (stationary phase) reduced compared to that of the wild type (Fig. 2). These results demonstrate that under presumably CS-repressive conditions, the wild type retained high CS activity relative to the mutant strain. Both strains displayed the highest CS activities during early exponential growth; however, the uncertainties in these particular measurements are high due to low protein levels. Interestingly, the growth rates of NCS-L-7 and the wild-type strain were comparable (0.48 h−1 and 0.58 h−1, respectively) under these conditions, indicating that the two strains may be similar in other respects. This suggests that it should be possible to grow any citY-deficient B. methanolicus mutant in the presence of glutamate, which should increase the amount of methanol-derived carbon from the RuMP directed towards aspartate.
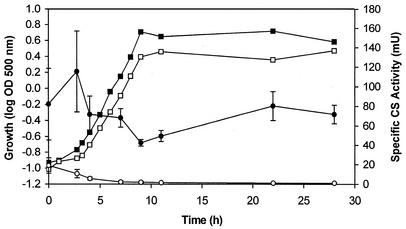
FIG. 2.
Time course of CS activities of B. methanolicus mutant NCS-L-7 and B. methanolicus wild-type NOA2. Cells were grown in shake flasks at 50°C in MVcMY medium supplemented with glutamate (1 g per liter), and samples were taken throughout the growth phase for OD500 and CS activity measurements. Mean values of duplicate experiments ± 1 standard deviation are shown. Growth: NCS-L-7, □; NOA2, ▪. CS specific activity: NOA2, •; NCS-L-7, ○.
Amino acid production by B. methanolicus NCS-L-7 in 14-liter fed-batch bioreactors with glutamate feeding.
Based on the genetic and physiological characteristics of mutant NCS-L-7 (above), we decided to study the effect of the low CS activity in this strain on the secreted l-lysine/glutamate ratio in 14-liter fed-batch bioreactors. To obtain results relevant for high-level l-lysine secretion, cells were grown in MVcMY medium with an exogenous supply of glutamate for maintaining maximum growth rates (see above). Overnight inoculum cultures in MVcMY plus glutamate medium were diluted to 5% in the same medium in the bioreactors, and the fermentors were run with continuous methanol (100 mM), glutamate (1 g per liter), and methionine (150 mM) feeding. Samples were collected and centrifuged at 4,000 × g for 15 min, and the supernatants were analyzed for amino acids. The results of these experiments are summarized in Fig. 3.
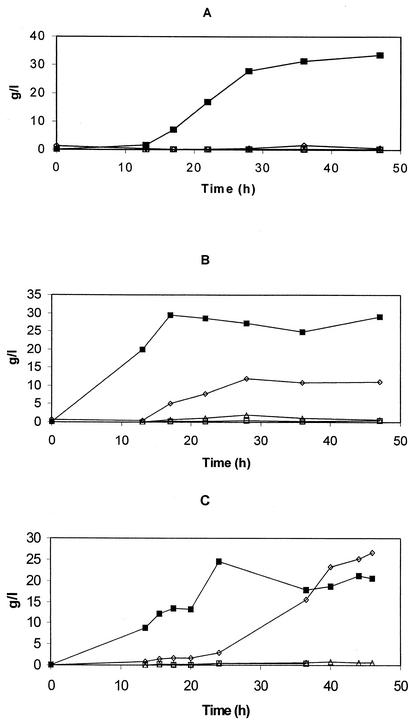
FIG. 3.
Fed-batch growth and amino acid secretion by B. methanolicus strains with glutamate feeding. Cells were grown in 14-liter bioreactors at 50°C in MVcMY medium and with methanol, glutamate, and methionine feeding. Samples were removed throughout the growth phase for OD500 measurements and analyzed for amino acid content. (A) NCS-L-7 (with glutamate and methionine feeding), (B) NOA2 (with glutamate and methionine feeding), (C) NOA2 (without glutamate or methionine feeding). Glutamate, ⋄; l-lysine, □; aspartate, ▵; cell dry weight, ▪.
Interestingly, both aspartate and l-lysine production in NCS-L-7 (Fig. 3A) and its wild-type NOA2 (Fig. 3B) were similar (between 0.30 and 0.79 g per liter). However, the secretion of glutamate was reduced approximately sevenfold in the former strain (1.7 versus 12 g per liter), leading to a 4.5-fold increase in the ratio of secreted l-lysine to glutamate in the mutant compared to its wild-type strain. As a control, NOA2 was analyzed in a similar experiment (Fig. 3C) but without glutamate and methionine feeding. This experiment demonstrates that the feeding of these two amino acids did not affect l-lysine and aspartate production, whereas glutamate production was reduced by approximately 50%. It has been shown in B. subtilis that glutamate may depress CS activity, and this may be why that less glutamate is produced under these conditions in B. methanolicus. These results demonstrate that deregulating the CS activity may be an efficient way to diminish undesired production of glutamate in l-lysine-secreting B. methanolicus strains. However, in order to achieve higher l-lysine production, deregulating the pathway of l-lysine biosynthesis and l-lysine transport out of the cell will presumably also be required.
DISCUSSION
The yield of 55 g of secreted glutamate per liter by Bacillus methanolicus MGA3 is the first report of high-level secretion of glutamate by a thermotolerant gram-positive methylotroph with the fructose bisphosphate aldolase/transaldolase variant of the RuMP pathway of carbon assimilation. The yield of 0.36 g/g is 86% of the theoretical maximum yield proposed by Ackerman and Babel (1), assuming that 1 mol of ATP is required to export this amino acid. Glutamate secretion to a level of 33 g per liter has previously been reported for the methylotroph Pseudomonas insueta, with a maximum yield of 0.17 g/g (35). The influence of magnesium limitation on glutamate secretion by B. methanolicus as a function of dilution rate in chemostats at 50°C has been reported by Pluschkell (38). Efficient glutamate secretion by B. methanolicus at elevated temperature is important because aerobic conversion of methanol, a reduced carbon source, to amino acids generates substantial heat, and only at temperatures at or above 50°C can the cooling requirements of large-volume bioreactors (>450 m3) be satisfied with reasonable cooling water temperatures and flow rates (38).
The B. methanolicus citY gene encodes a CSII protein which is active at 50°C and is 77.8% identical to the B. subtilis CSII. CS exists as a dimer in all gram-positive bacteria, eukaryotes, and archaea. In gram-negative bacteria, CS is a hexamer, with the exception of the minor dimeric form of 2-methylcitrate synthase induced in E. coli when grown on propionate (17). The structural basis for the thermostability of CS activity is known better than for any other protein. The crystal structures of CS have been determined and compared for organisms capable of growth over a very wide range of temperatures, from the Antarctic bacterium Arthrobacter sp. strain DS2-3R (maximum growth at 30°C) (42), mesophilic organisms such as chicken and porcine heart (37°C), thermotolerant organisms such as Thermoplasma acidophilum (55°C) (28), and the extreme thermophiles Sulfolobus solfataricus (88°C) (8), and Pyrococcus furiosus (100°C) (28).
The greater complementarity of the helical sandwich of four antiparallel pairs of helices at the subunit interface contributes to CS thermostability, as do shorter surface loops, reduction in internal cavities, and reduction in cysteine, methionine, asparagine, and glutamine residues (10). Protein analysis with Clustal W matched the deduced citY gene product to the highly conserved CS motif pattern G-[FYA]-[GA]-H-x-[IV]-x-(12)-[RKT]-x (2)-D-[PS]-R previously identified in the ctsA gene cloned from another thermotolerant gram-positive bacterium, Bacillus coagulans (45). This motif is also present in both isozymes of CS from B. subtilis, CitZ and CitW, and the CS sequences from Mycobacterium smegmatis, Corynebacterium glutamicum, and porcine heart (Fig. 4). The regions of secondary structure found to be important in CS stability in the porcine heart protein can be used to identify similar alpha-helical regions in citY and citZ that may contribute to thermostability. The boxed regions in Fig. 4 depict identical amino acid residues within the helix. The residues within the G and M helices of citY and citZ were all conserved with the exception of the following: in helix G, Thr 96 and Ile 98 in citZ were replaced with Ser 96 and Val 98 in citY. In helix M, only one residue is not conserved; Ile 206 in citZ is replaced with Val 206 in citY. The substitution of these amino acids may play a role in the thermostability of the B. methanolicus CSII activity in vivo compared to in vivo B. subtilis CSII activity.
In vivo CSII activity is important in regulating pyruvate and oxaloacetate levels, which are important branch point intermediates in the synthesis of aspartate (see Fig. 1). In B. subtilis (5, 36), the major route for aspartate synthesis involves the carboxylation of pyruvate to oxaloacetate by PC and subsequent transamination to aspartate catalyzed by glutamate:oxaloacetate transaminase. Wild-type strains of B. methanolicus lack phosphoenolpyruvate carboxylase activity, and the function of PC under normal growth conditions is anaplerotic, providing oxaloacetate, the substrate for CS. The affinities of PC and pyruvate dehydrogenase for pyruvate (Km values of 0.23 mM and 0.40 mM, respectively) are in the same range (19, 41). Both enzymes catalyze metabolically irreversible reactions, and the Km values for their reactants are much lower (70- and 150-fold, respectively) than for their products (19, 30).
Glutamate:oxaloacetate transaminase and CS both consume oxaloacetate and thus represent another pair of enzymes in central metabolism that catalyze opposing reactions. Glutamate:oxaloacetate transaminase catalyzes a near-equilibrium reaction (27), and the reported Km values of the B. subtilis glutamate:oxaloacetate transaminase for aspartate (3.0 mM) and oxaloacetate (2.0 mM) are similar (5, 36). Flux through a near-equilibrium reaction is controlled by changes in substrate and product concentration. CS catalyzes a metabolically irreversible reaction and is thus nearly unaffected by changes in substrate and product concentrations. CS has a much lower Km for oxaloacetate (0.03 mM, 25 mM) than glutamate:oxaloacetate transaminase, indicating that cellular carbon is preferentially converted to citrate by CS rather than to aspartate by glutamate:oxaloacetate transaminase.
In order to increase the level of secreted l-lysine, it will be important to prevent a majority of carbon from the RuMP cycle from entering the TCA cycle, leading to a high intracellular concentration of glutamate, which leaks out of the cells. Interestingly, despite a threefold-elevated PC activity, the oxaloacetate level in the l-lysine- plus glutamate-overproducing B. methanolicus mutant 13A52-8A66 was similar to that of the wild-type strain (Tables 2 and 3). However, the ability of the mutant strain to overproduce l-lysine indicates that certain l-lysine biosynthetic genes are deregulated, causing a drain of both aspartate and oxaloacetate in this direction. Besides, the secretion of glutamate in the l-lysine-secreting mutant strain indicates that CS converts a considerable portion of oxaloacetate to citrate. Moreover, the much higher oxaloacetate concentration compared to the aspartate concentration in 13A52-8A66 correlates with the observed low glutamate:oxaloacetate transaminase activity (threefold lower than the wild type), suggesting that this enzyme may represent a bottleneck for l-lysine overproduction in B. methanolicus.
In C. glutamicum, inactivation of PC led to 60% reduced lysine production, whereas overexpression of this gene in a C. glutamicum l-lysine-overproducing mutant resulted in 50% higher production of this amino acid (37). These experiments demonstrate the importance of efficient generation of aspartate for obtaining high-level l-lysine synthesis. An alternative approach to raising aspartate levels would be to introduce a glyoxylate shunt into B. methanolicus. This organism does not grow or grows very poorly on acetate (4), which suggests that it may be similar to B. subtilis in lacking the enzymes for conversion of isocitrate to malate and succinate (50). A functional glyoxylate shunt would regulate isocitrate levels and may reduce intracellular 2-oxoglutarate accumulation by drawing off oxaloacetate via transamination to aspartate.
Two important observations were evident as a result of measuring the intracellular enzyme specific activity of B. methanolicus enzymes involved in this pathway. First, B. methanolicus has low 2-oxoglutarate dehydrogenase activity, confirming previous reports describing that the TCA cycle in methylotrophic bacteria may have a minor role in energy metabolism. Second, the CS activity level in the l-lysine- plus glutamate-overproducing mutant 13A52-8A66 was greater than twofold higher than in the wild-type strain. Based on these observations, further investigations may focus on altering the regulation of CS, together with increasing the activity of glutamate:oxaloacetate transaminase and PC, in order to increase the flux of carbon from pyruvate into l-lysine biosynthesis in B. methanolicus.
Acknowledgments
We thank Nobuharo Tsujimoto (Ajinomoto Co., Kawasaki-shi, Japan) for construction of the B. methanolicus gene library, Fred Schendel, Grace Okamoto, Hong Lam, and Heather A. Hrodey for enzyme assays, and Matt Laudon for selection of mutants.
This work was supported by grants from the University of Minnesota Biotechnology Institute and SINTEF, Trondheim, Norway.
REFERENCES
- 1.Ackermann, J.-U., and W. Babel. 1994. Glutamic acid synthesis form methanol: theoretical considerations. J. Basic Microbiol. 4:211-216.
- 2.Anthony, C. 1991. Assimilation of carbon by methylotrophs, p. 79-109. In I. Goldberg and J. S. Rokem (ed.), Biology of methylotrophs. Butterworth-Heinemann, Boston, Mass. [DOI] [PubMed]
- 3.Arfman, N., E. M. Watling, W., Clement, R. J. van Oosterwijk, G. E. de Vries, W. Harder, M. M. Attwood, and L. Dijkhuizen. 1989. Methanol metabolism in thermotolerant methylotrophic Bacillus strains involving a novel catabolic NAD-dependent methanol dehydrogenase as a key enzyme. Arch. Microbiol. 152:280-288. [DOI] [PubMed] [Google Scholar]
- 4.Arfman, N., L. Dijkhuizen, G. Kirchhof, W. Ludwig, K.-H. Schleifer, E. S. Bulygina, K. M. Chumakov, N. I. Govorukhina, Y. A. Totsenko, D. White, and R. J. Sharp. 1992. Bacillus methanolicus sp. nov., a new species of thermotolerant, methanol-utilizing, endospore-forming bacteria. Int. J. Syst. Bacteriol. 42:439-445. [DOI] [PubMed] [Google Scholar]
- 5.Belitsky, B. R. 2002. Biosynthesis of amino acids of the glutamate and aspartate families, alanine, and polyamines, p. 203-231. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives. ASM Press, Washington, D.C.
- 6.C<novas, J. L., and H. L. Kornberg. 1969. Phosphoenol pyruvate carboxylase from Escherichia coli. Methods Enzymol. 13:288-292. [Google Scholar]
- 7.Chistoserdova, L., L. Gomelsky, J. A. Vorholt, M. Gemelsky, Y. D. Tsyganov, and M. Lidstrom. 2000. Analysis of the formaldehyde oxidation pathways in Methylobacillus flagellatus KT, a ribulose monophosphate cycle methylotroph. Microbiology 146:233-238. [DOI] [PubMed] [Google Scholar]
- 8.Connis, H., S. M. West, D. W. Hough, and M. J. Danson. 1998. Cloning and overexpression in Escherichia coli of the gene encoding citrate synthase from the hyperthermophilic archaeon Sulfolobus solfataricus. Extremophiles 2:61-66. [DOI] [PubMed] [Google Scholar]
- 9.Cue, D., H. Lam, R. L. Dillingham, R. S. Hanson, and M. C. Flickinger. 1997. Genetic manipulation of Bacillus methanolicus, a gram-positive thermotolerant methylotroph. Appl. Environ. Microbiol. 63:1406-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danson, M. J., and D. W. Hough. 1998. Structure, function and stability of enzymes from the Archaea. Trends. Microbiol. 6:307-315. [DOI] [PubMed] [Google Scholar]
- 11.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vries, G. E., U. Kües, and U. Stahl. 1990. Physiology and genetics of methylotrophic bacteria. FEMS Microbiol. Rep. 75:57-102. [DOI] [PubMed] [Google Scholar]
- 13.De Vries, G. E., N. Arfman, P. Terpstra, and L. Dijkhuizen. 1992. Cloning, expression, and sequence analysis of the Bacillus methanolicus C1 methanol dehydrogenase gene. J. Bacteriol. 174:5346-5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dijkhuizen, L., P. R. Levering, and G. E. de Vries. 1992. The physiology and biochemistry of aerobic methanol-utilizing gram negative and gram positive bacteria, p. 149-181. In J. C. Murrell and H. Dalton (ed.), Methane and methanol utilizers. Plenum Press, New York, N.Y.
- 15.Donohue, T. J., and D. A. Bernlohr. 1978. Carbon and nitrogen catabolite repression, metabolite pools, and the regulation of sporulation in Bacillus licheniformis, p. 293-298. In G. Chambliss and J. C. Vary (ed.), Spores VII. American Society for Microbiology, Washington, D.C.
- 16.Eikmanns, B. J., N. Thum-Schmitz, L. Eggeling, K. U. Ludtke, and H. Sahm. 1994. Nucleotide sequence, expression and transcriptional analysis of the Corynebacterium glutamicum gltA gene encoding citrate synthase. Microbiology 140:1817-1828. [DOI] [PubMed] [Google Scholar]
- 17.Gerike, U., D. W. Hough, N. J. Russell, M. L. Dyall-Smith, and M. J. Danson. 1998. Citrate synthase and 2-methylcitrate synthase: structural, functional and evolutionary relationships. Microbiology 144:929-935. [DOI] [PubMed] [Google Scholar]
- 18.Hanson, R. S., R. L. Dillingham, P. Olson, G. H. Lee, D. Cue, F. J. Schendel, C. Bremmon, and M. C. Flickinger. 1996. Production of l-lysine and some other amino acids by mutants of B. methanolicus, p. 227-234. In M. E. Lidstrom and F. R. Tabita (ed.), Microbial growth on C1 compounds. Kluywer Academic Publishers, Dordrecht, The Netherlands.
- 19.Hodgson, J. A., P. N. Lowe, and R. N. Perham. 1983. Wild type and mutant forms of pyruvate dehydrogenase multienzyme complex from Bacillus subtilis. Biochem. J. 211:463-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jagusztyn-Krynicki, E. K., and A. Malashewska-Keough. 1989. Cloning and expression of Thiobacillus versutus aspartate-semialdehyde dehydrogenase gene in E. coli. FEMS Microbiol. Lett. 59:21-26. [DOI] [PubMed] [Google Scholar]
- 21.Jeong, J. W., J. Snay, and M. M. Ataai. 1990. Mathematical model for examining growth and sporulation processes of Bacillus subtilis. Biotechnol. Bioeng. 25:160-184. [DOI] [PubMed] [Google Scholar]
- 22.Jin, S., and A. L. Sonenshein. 1994. Identification of two distinct Bacillus subtilis citrate synthase genes. J. Bacteriol. 176:4669-4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin, S., and A. L. Sonenshein. 1994. Transcriptional regulation of Bacillus subtilis citrate synthase genes. J. Bacteriol. 176:4680-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin, S., and A. L. Sonenshein. 1996. Characterization of the major citrate synthase of Bacillus subtilis. J. Bacteriol. 178:3658-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, D. E., and R. S. Hanson. 1974. Bacterial citrate synthase: purification, molecular weight and kinetic mechanism. Biochim. Biophys. Acta 350:336-353. [DOI] [PubMed] [Google Scholar]
- 26.Jourlin-Castelli, C., N. Mani, M. M. Nakano, and A. L. Sonensheim. 2000. CcpC, a novel regulator of the LysR family required for glucose repression of the citB gene in Bacillus subtilis. J. Mol. Biol. 295:865-878. [DOI] [PubMed] [Google Scholar]
- 27.Kuramitsu, S., K. Hiromo, H. Hayashi, Y. Morino, and H. Kagamiyama. 1990. Pre-steady-state kinetics of Escherichia coli aspartate aminotransferase catalyzed reactions and thermodynamic aspects of its substrate specificity. Biochemistry 29:5469-5476. [DOI] [PubMed] [Google Scholar]
- 28.Kurz, L. C., G. Drysdale, M. Riley, M. A. Tomar, J. Chen, R. J. Russell, and M. J. Danson. 2000. Kinetics and mechanism of the citrate synthase from the thermophilic archaeon Thermoplasma acidophilum. Biochemistry 39:2283-2296. [DOI] [PubMed] [Google Scholar]
- 29.Lee, G. H., W. Hur, C. E. Bremmon, and M. C. Flickinger. 1996. l-lysine production from methanol at 50°C with Bacillus methanolicus: modeling volume control, l-lysine concentration, and productivity with a three-phase continuous simulation. Biotechnol. Bioeng. 49:639-653. [DOI] [PubMed] [Google Scholar]
- 30.Libor, S. M., T. K. Sundaram, and M. C. Scrutton. 1978. Pyruvate carboxylase from a thermophilic Bacillus. Biochem. J. 169:543-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchenko, G. N., N. D. Marchenko, Y. D. Tsyganov, and A. Y. Chistoserdov. 1999. Organization of threonine biosynthesis genes from the obligate methylotroph Methylobacillus flagellatus. Microbiology 145:3273-3282. [DOI] [PubMed] [Google Scholar]
- 32.Mills, D. A., and M. C. Flickinger. 1992. Cloning and sequencing of the meso-diaminopimelate decarboxylase gene from Bacillus methanolicus MGA3 and comparison to other decarboxylase genes. Appl. Environ. Microbiol. 59:2927-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motoyama, H., K. Maki, H. Anazawa, S. Ishino, and S. Teshiba. 1994. Cloning and nucleotide sequences of the homoserine dehydrogenase genes (hom) and the threonine synthase genes (thrC) of the gram-negative obligate methylotroph Methylobacillus flagellatus. Appl. Environ. Microbiol. 60:111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukherjee, B. B., J. Matthews, D. L. Horney, and L. J. Reed. 1965. Resolution and reconstitution of Escherichia coli α-ketoglutarate dehydrogenase complex. J. Biol. Chem. 240:2268-2269. [PubMed] [Google Scholar]
- 35.Nakayama, K., M. Kobata, Y. Tanaka, T. Nomura, and R. Katsumata. 1975. Verfahren zur biotechnischen Herstellung von l-Glutaminsäure und Mikroorgansimen zur Durchführung des Verfahrens. DE 2438206.
- 36.Paulus, H. 1993. Biosynthesis of the aspartate family of amino acids, p. 237-267. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria. American Society for Microbiology, Washington, D.C.
- 37.Peters-Wendisch, P. G., B. Schiel, V. F. Wendisch, E. Katsoulidis, B. Mockel, H. Sahm, and B. J. Eikmanns. 2001. Pyruvate carboxylase is a major bottleneck for glutamate and l-lysine production by Corynebacterium glutamicum. J. Microbiol. Biotechnol. 3:295-300. [PubMed] [Google Scholar]
- 38.Pluschkell, S. B. 1999. Characterization and mathematical modeling of growth and glutamic acid production by Bacillus methanolicus MGA3. Ph.D. thesis, University of Minnesota, Minneapolis, Minn.
- 39.Pluschkell, S. B., and M. C. Flickinger. 2002. Dissimilation of 13C-methanol by continuous cultures of Bacillus methanolicus MGA3 at 50°C studied by 13C NMR and isotope ratio mass spectrometry. Microbiology 148:3223-3233. [DOI] [PubMed] [Google Scholar]
- 40.Reed, L. J., and B. B. Mukherjee. 1969. α-Ketoglutarate dehydrogenase complex from Escherichia coli. Methods Enzymol. 13:55-61. [Google Scholar]
- 41.Renner, E. D., and R. W. Bernlohr. 1972. Characterization and regulation of pyruvate carboxylase of Bacillus licheniformis. J. Bacteriol. 213:53-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell, R. J. M., U. Gerike, M. J. Danson, D. W. Hough, and G. L. Taylor. 1998. Structural adaptations of the cold-active citrate synthase from an Antarctic bacterium. Structure 6:351-361. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Schendel, F. J., C. E. Bremmon, M. C. Flickinger, M. Guettler, and R. S. Hanson. 1990. l-Lysine production at 50°C by mutants of a newly isolated and characterized Bacillus sp. Appl. Environ. Microbiol. 56:963-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schendel, F. J., P. R. August, C. R., Andersen, R. S. Hanson, and M. C. Flickinger. 1992. Cloning and nucleotide sequence of the gene coding for citrate synthase from a thermotolerant Bacillus sp. Appl. Environ. Microbiol. 58:335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schendel, F. J., and M. C. Flickinger. 1992. Cloning and nucleotide sequence of the gene encoding aspartokinase II from a thermophilic methylotrophic Bacillus sp. Appl. Environ. Microbiol. 58:2806-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schreier, H. J., T. M. Smith, T. J. Donohue, and R. W. Bernlohr. 1981. Regulation of nitrogen metabolism and sporulation in Bacillus licheniformis, p. 138-141. In H. S. Levinson, A. L. Sonenshein, and D. J. Tipper (ed.), Sporulation and germination. American Society for Microbiology, Washington, D.C.
- 48.Seubert, W., and H. Weicker. 1969. Pyruvate carboxylase from Pseudomonas. Methods Enzymol. 13:258-262. [Google Scholar]
- 49.Shrishinka, V. N., and Y. A. Trotsenko. 1982. Multiple enzymic lesions in obligate methanotrophic bacteria. FEMS Microbiol. Lett. 13:237-242. [Google Scholar]
- 50.Sonenshein, A. L. 2002. The Krebs citric acid cycle, p. 151-162. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives. ASM Press, Washington, D.C.
- 51.Stackebrandt, E., and B. M. Gobel. 1994. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 52.Takami, H., K. Nakasone, Y. Takaki, G. Maeno, R. Sasaki, N. Masui, F. Fuji, C. Hirama, Y. Nakamura, N. Ogasawara, S. Kuhara, and K. Horikoshi. 2000. Complete genome sequence of the alkaliphilic bacterium Bacillus halodurans and genomic sequence comparison with Bacillus subtilis. Nucleic Acids Res. 28:4317-4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tobisch, S., D. Zulcke, J. Bernhardt, J. Stulke, and M. Hecker. 1999. Role of CcpA in regulation of central pathways of carbon catabolism in Bacillus subtilis. J. Bacteriol. 181:6996-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vorholt, J. A., L. Chistoserdova, S. M. Stolyar, R. K. Thauer, and M. Lidstrom. 1999. Distribution of tetrahydrometanopterin-dependent enzymes in methylotrophic bacteria and phylogeny of methenyl tetrahydrometanopterin cyclohydrolases. J. Bacteriol. 181:5750-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yagi, T., H. Kagamiyama, M. Nozaki, and K. Soda. 1985. Glutamate-aspartate transaminase from microorganism. Methods Enzymol. 113:83-89. [DOI] [PubMed] [Google Scholar]
- 56.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]