Abstract
It was found that the growth of Aeropyrum pernix was severely inhibited in a medium containing reducing sugars and tryptone due to the formation of Maillard reaction products. The rate of the Maillard browning reaction was markedly enhanced under aerobic conditions, and the addition of Maillard reaction products to the culture medium caused fatal growth inhibition.
Recently, hyperthermophiles have attracted much interest due to their potential industrial applications. Despite their biotechnological potential, however, it has been difficult to cultivate hyperthermophiles in the laboratory due to the sparsity of knowledge on their physiological characteristics at high temperatures. Since the growth of hyperthermophiles is generally very poor, low biomass yield has been one of the major challenges encountered in the cultivation of hyperthermophiles (4, 9, 22). To meet this challenge, previous studies have focused on the factors that affect the growth of Sulfolobus solfataricus (12, 15-20), a thermoacidophilic archaeon that normally grows at 75 to 85°C and pH 2.0 to 4.0 (7, 24). One recent study showed that prolonged incubation of l-glutamate under culture conditions with S. solfataricus resulted in the conversion of l-glutamate to l-pyroglutamate and that the l-pyroglutamate acted as a potent growth inhibitor of S. solfataricus (20). This indicated that a chemical modification of the medium at elevated temperatures might be one of the major constraints that limit the efficient growth of hyperthermophiles.
In this report, we present another example of a chemical modification of the culture medium at high temperatures and its toxic effect on cell growth. During cultivation of Aeropyrum pernix, a marine hyperthermophilic archaeon which grows under strictly aerobic conditions at temperatures up to 100°C (21), we observed the Maillard browning reaction between sugars and amino acids in the medium and significant growth inhibition by these Maillard reaction products.
A. pernix strain K1 (JCM 9820), which was isolated from a coastal solfataric vent in Japan (21), was obtained from the Japan Collection of Microorganisms. The base medium was composed of tryptone (4.0 g/liter), Na2S2O3 (0.61 g/liter), and ASN-III salts (artificial seawater salts containing NaCl [29.8 g/liter], MgCl2 [1.1 g/liter], MgSO4 [2.0 g/liter], CaCl2 [0.45 g/liter], KCl [0.6 g/liter], and Na2CO3 [0.024 g/liter]). Cell culture experiments were carried out using base medium (100 ml) supplemented with specific carbohydrates (3.0 g/liter) in screw-cap 500-ml flasks unless stated otherwise. The flasks were inoculated with 10% seed culture cells grown in YT medium (yeast extract [2.0 g/liter], tryptone [4.0 g/liter], Na2S2O3 [0.61 g/liter], and ASN-III salts) for 48 h. The initial pH of the culture medium was set at 7.0, and the incubation was carried out at 90°C in a rotary shaker at 150 rpm (Jeiotech Co., Seoul, Korea). Cell growth was observed by monitoring optical density at 660 nm (OD660).
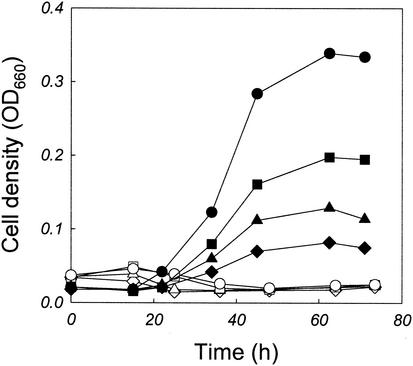
The formation of Maillard reaction products and their inhibitory effects were first observed when the cells were cultivated in a mixture of tryptone and d-glucose. A. pernix was able to grow on various proteinaceous complex media, such as tryptone, peptone, Casitone, and yeast extract. Among the proteinaceous complex media tested, the highest cell density was observed with tryptone, and cell densities increased with increasing concentrations of tryptone, up to 4.0 g/liter, in the culture medium (Fig. 1). As the content of carbohydrates in tryptone was low, we examined whether the supplementation of the base medium with d-glucose could increase the maximum cell density. Contrary to our expectation, however, cell growth was completely abolished by the addition of d-glucose (3.0 g/liter) to the base medium (Fig. 1). In addition, the color of the culture broth gradually turned to a dark brown, which was not observed when the cells were cultivated in the medium containing tryptone alone.
FIG. 1.
Growth of A. pernix in the base medium without (closed symbols) or with (open symbols) the addition of 3.0 g of d-glucose/liter. The concentrations of tryptone in the base medium were 4.0 (circles), 2.0 (squares), 1.0 (triangles), and 0.5 (diamonds) g/liter.
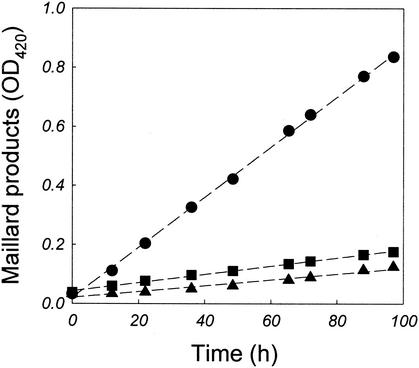
Browning of culture broth in the presence of d-glucose reminded us of the Maillard reaction, which concerns a series of complex reactions between reducing sugars and amino acids (1, 8, 10, 14). The extent of this Maillard browning reaction of the culture medium was examined using separate experiments. A thermochemical reaction was carried out at 90°C in a screw-cap flask (100 ml) containing 4.0 g of tryptone/liter with or without 3.0 g of d-glucose/liter. The initial pH of the reaction mixture was 7.0. After the samples were cooled to room temperature and insoluble materials were removed by centrifugation, the formation of advanced Maillard reaction products was monitored by measuring the OD420s of the solutions (2, 3). In the absence of d-glucose, the browning of tryptone solution was largely insignificant (Fig. 2). However, upon the addition of d-glucose to the tryptone medium, the rate of the browning reaction was increased some sixfold compared to that observed without d-glucose. This indicated that the Maillard reaction had occurred between the glucose added to the medium and the amino acids contained in tryptone in the culture (at 90°C and pH 7.0 and under aerobic conditions).
FIG. 2.
Effects of oxygen on formation of Maillard reaction products. Reaction conditions were as follows: tryptone (4.0 g/liter) plus d-glucose (3.0 g/liter), aerobic conditions (•); tryptone (4.0 g/liter), aerobic conditions (▪); and tryptone (4.0 g/liter) plus d-glucose (3.0 g/liter), anaerobic conditions (▴). In the case of anaerobic operation, a redox indicator (resazurin, 1.0 mg/liter) and reducing agents (240 mg of Na2S · 9H2O/liter and 30 mg of Na2S2O4/liter) were added to the reaction mixture and the flask was sparged with N2 until the solution became colorless. Dashed lines were obtained from the linear regression of experimental data. The slopes of the regression lines (from the top) were 8.5 × 10−3, 1.4 × 10−3, and 1.0 × 10−3 (h−1).
A. pernix is a strictly aerobic hyperthermophile, while the majority of the hyperthermophilic archaea isolated so far are anaerobes. Interestingly, a number of anaerobic hyperthermophiles grow well on carbohydrates. To examine the role of oxygen in the Maillard reaction, we repeated the experiment under anaerobic conditions. Resazurin (1.0 mg/liter) was added to the medium as a redox indicator, and the culture flask was sparged with inert gas (N2) until the solution became colorless. As shown in Fig. 2, the formation of Maillard reaction products was remarkably reduced by lowering the oxygen content in the medium. In view of this result, it appears that the extent of the Maillard browning reaction becomes most rigorous when aerobic hyperthermophiles such as A. pernix are cultivated. There is some evidence which supports the role of oxygen as a catalyst of the Maillard reaction (10). It is interesting that S. solfataricus, an aerobic, extremely thermophilic archaeon, can grow in the media containing sugars and proteinaceous complex media. From the separate experiments, we found that the formation of Maillard reaction products could be greatly reduced at lower pHs. This may be the reason why S. solfataricus grows well on carbohydrates under culture conditions (pH 3.0, 78°C).
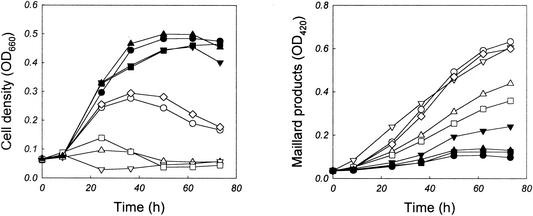
We examined the formation of Maillard reaction products and their effects on cell growth with various carbohydrates, namely, sugars (d-glucose, d-fructose, d-galactose, d-xylose, methyl α-d-glucopyranoside, methyl-β-d-glucopyranoside, maltose, lactose, sucrose, trehalose, raffinose, starch, and dextrin), sugar alcohols (d-sorbitol, d-mannitol, and myoinositol), and organic acids (pyruvate, malate, citrate, α-ketoglutarate, fumarate, and transaconitate). In Fig. 3, the time course profiles of cell growth and the Maillard reaction are shown for eight sugars. Maximum cell densities were much reduced in the media containing reducing sugars (i.e., d-glucose, d-fructose, d-galactose, lactose, and maltose) compared to those in the media containing nonreducing sugars (i.e., methyl-α-d-glucopyranoside, methyl-β-d-glucopyranoside, trehalose, sucrose, and dextrin). In the case of reducing sugars, cell death was accompanied by an increase in the level of Maillard reaction products. The results summarized in Table 1 indicate that there is an approximate inverse relationship between increased cell density (change in OD660 [ΔOD660]) and Maillard product formation (ΔOD420). The rate of the Maillard reaction was lowest when sugar alcohols or organic acids were added to the base medium, particularly when myoinositol, malate, or citrate was used as a supplement. The addition of these species enhanced cellular growth and reduced the Maillard reaction product levels.
FIG. 3.
Effects of carbohydrates on the growth of A. pernix. Cells were cultivated at 90°C in a medium composed of tryptone (4.0 g/liter), carbohydrate (3.0 g/liter), and ASN-III salts. Symbols: •, trehalose; ▪, sucrose; ▾, dextrin; ⋄, lactose; ○, maltose; ▵, d-galactose; ▿, d-fructose; □, d-glucose. Control data obtained without addition of carbohydrate are also shown (▴). Reducing sugars are depicted as open symbols.
TABLE 1.
Effects of carbohydrates on cell growth and Maillard product formation during cultivation of A. pernixa
| Group | Carbohydrate | Cell growth (ΔOD660) | Maillard product formation (ΔOD420) |
|---|---|---|---|
| Control | None | 0.38 | 0.06 |
| Monosaccharides | d-Glucose | −0.02 | 0.32 |
| d-Fructose | −0.01 | 0.57 | |
| d-Galactose | −0.01 | 0.41 | |
| d-Xylose | 0.10 | 0.93 | |
| Methyl-α-d-glucopyranoside | 0.35 | 0.07 | |
| Methyl-β-d-glucopyranoside | 0.31 | 0.07 | |
| Oligosaccharides | Maltose | 0.10 | 0.60 |
| Lactose | 0.11 | 0.57 | |
| Raffinose | 0.30 | 0.07 | |
| Sucrose | 0.40 | 0.09 | |
| Trehalose | 0.41 | 0.06 | |
| Polysaccharides | Starch | 0.27 | 0.16 |
| Dextrin | 0.34 | 0.20 | |
| Sugar alcohols | d-Sorbitol | 0.31 | 0.08 |
| d-Mannitol | 0.38 | 0.09 | |
| Myoinositol | 0.43 | 0.04 | |
| Organic acids | Transaconitate | 0.47 | 0.06 |
| Fumarate | 0.49 | 0.07 | |
| α-Ketoglutarate | 0.50 | 0.11 | |
| Malate | 0.53 | 0.04 | |
| Citrate | 0.53 | 0.05 | |
| Pyruvate | 0.53 | 0.23 |
Cells were cultivated at 90°C in a medium composed of tryptone (4.0 g/liter), carbohydrate (3.0 g/liter), and ASN-III salts. Changes in cell density (ΔOD660) and Maillard reaction product levels (ΔOD420) were determined after cultivation of the cells for 74 h.
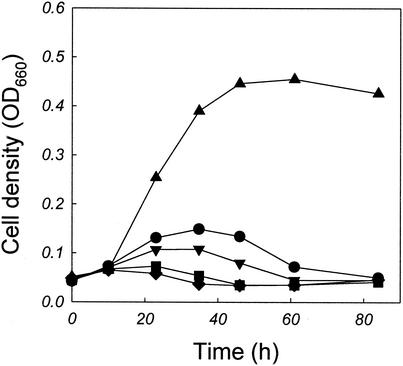
Although the results in Table 1 indirectly support the view that the growth of A. pernix is inhibited by the compounds formed between reducing sugars and tryptone, it is possible that the cell growth is inhibited by the loss of nutrient availability due to thermal decomposition of labile components. To examine whether the compounds, which had an inhibitory effect on the cell growth, were in fact Maillard reaction products, we synthesized these products from the well-defined substrates d-glucose and l-lysine. The reaction was carried out at 90°C with a mixture of d-glucose and l-lysine (each at a concentration of 100 mM, pH 7.0). Aliquots (10 ml) were withdrawn after 0, 4, 8, and 16 h of incubation and added to the culture medium (90 ml) before cell inoculation. The cell culture conditions used were identical to those employed for the previous experiments. As can be seen in Fig. 4, the growth of A. pernix was significantly inhibited by the Maillard reaction products obtained from d-glucose and l-lysine. The degree of growth inhibition was more severe with the Maillard reaction products formed after an extended reaction time.
FIG. 4.
Effects of Maillard reaction products on the growth of A. pernix. The Maillard reaction products (10 ml), which were obtained by incubating a mixture of d-glucose (100 mM) and l-lysine (100 mM) in a screw-cap flask at 90°C for 0 (•), 4 (▾), 8 (▪), or 16 (♦) h, were added to the base medium (90 ml) before cell inoculation. The cell growth observed without the addition of Maillard reaction products is also shown (▴).
Relatively little data are available on the effects of Maillard reaction products on microbial growth (6, 11, 13, 23), and most of the relevant studies concerned the formation of these products in autoclaved growth media. Recently, Driskill et al. (5) observed browning of culture medium during the cultivation of Pyrococcus furiosus, an anaerobic hyperthermophilic archaeon that grows optimally at about 100°C, in the medium containing d-glucose. They also observed low growth yields of P. furiosus on d-glucose compared to the yields obtained on larger carbohydrates such as maltose, cellobiose, and laminarin. Although these workers considered this a consequence of the loss of substrate availability due to thermal lability of the monosaccharide, it is possible that the Maillard reaction products formed from the medium components inhibited the growth of P. furiosus. Unfortunately, they did not pursue this possibility.
The results presented in this report indicate that the cultivation of hyperthermophilic microorganisms in media containing reducing sugars and amino compounds is undesirable due to the accelerated formation of Maillard reaction products at elevated temperatures. Thus, when hyperthermophiles are cultivated, the periodic addition of culture medium to fed-batch cultures is desirable to increase the cell densities (5, 17). Alternatively, the use of nonreducing sugars, sugar alcohols, or organic acids as carbon sources should result in a biomass increase due to the reduced production of Maillard reaction products, as is the case with A. pernix. However, it remains to be seen whether Maillard reaction products are toxic to other hyperthermophiles, and this will be the subject of our future study.
Acknowledgments
This work was supported by the Korean Ministry of Science and Technology (21C Frontier Microbial Genomics and Applications Program) and KoBioTech Co., Ltd.
REFERENCES
- 1.Ames, J. M. 1988. The Maillard browning reaction—an update. Chem. Ind. (London) 17:558-561. [Google Scholar]
- 2.Baisier, W. M., and T. P. Labuza. 1992. Maillard browning kinetics in a liquid model system. J. Agric. Food Chem. 40:707-713. [Google Scholar]
- 3.Carabasa-Giribet, M., and A. Ibarz-Ribas. 2000. Kinetics of colour development in aqueous glucose systems at high temperatures. J. Food Eng. 44:181-189. [Google Scholar]
- 4.Clark, D., and R. Kelly. 1990. Hot bacteria. Chemtech 20:554-562. [Google Scholar]
- 5.Driskill, L. E., K. Kusy, M. W. Bauer, and R. M. Kelly. 1999. Relationship between glycosyl hydrolase inventory and growth physiology of the hyperthermophile Pyrococcus furiosus on carbohydrate-based media. Appl. Environ. Microbiol. 65:893-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Einarsson, H., B. G. Snygg, and C. Eriksson. 1983. Inhibition of bacterial growth by Maillard reaction products. J. Agric. Food Chem. 31:1043-1046. [Google Scholar]
- 7.Grogan, D. W. 1989. Phenotypic characterization of the archaebacterial genus Sulfolobus: comparison of five wild-type strains. J. Bacteriol. 171:6710-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodge, J. E. 1953. Dehydrated foods: chemistry of browning reactions in model systems. J. Agric. Food Chem. 1:928-943. [Google Scholar]
- 9.Holst, O., A. Manelius, M. Krahe, H. Markl, N. Raven, and R. Sharp. 1997. Thermophiles and fermentation technology. Comp. Biochem. Physiol. 118A:415-422.
- 10.Ikan, R. (ed.). 1996. The Maillard reaction: consequences for the chemical and life sciences. John Wiley & Sons, Chichester, England.
- 11.Jemmali, M. 1969. Influence of the Maillard reaction products on some bacteria of the intestinal flora. J. Appl. Bacteriol. 32:151-160. [DOI] [PubMed] [Google Scholar]
- 12.Kan, E. S., C. B. Park, and S. B. Lee. 1997. Optimization of culture conditions for hyperthermophilic archaeon Sulfolobus solfataricus. Korean J. Biotechnol. Bioeng. 12:121-126. [Google Scholar]
- 13.Lanciotti, R., M. Anese, M. Sinigaglia, C. Severini, and R. Massini. 1999. Effects of heated glucose-fructose-glutamic acid solutions on the growth of Bacillus stearothermophilus. Lebensm.-Wiss. Technol. 32:223-230. [Google Scholar]
- 14.Namiki, M. 1988. Chemistry of Maillard reactions: recent studies on the browning reaction mechanism and the development of antioxidants and mutagens. Adv. Food Res. 32:115-184. [DOI] [PubMed] [Google Scholar]
- 15.Park, C. B., and S. B. Lee. 1997. Constant-volume fed-batch operation for high density cultivation of hyperthermophilic aerobes. Biotechnol. Tech. 11:277-281. [Google Scholar]
- 16.Park, C. B., and S. B. Lee. 1998. Ammonia production from yeast extract and its effect on growth of Sulfolobus solfataricus. Biotechnol. Bioprocess Eng. 3:115-118. [Google Scholar]
- 17.Park, C. B., and S. B. Lee. 1999. Inhibitory effect of mineral ion accumulation on high density growth of the hyperthermophilic archaeon Sulfolobus solfataricus. J. Biosci. Bioeng. 87:315-319. [DOI] [PubMed] [Google Scholar]
- 18.Park, C. B., and S. B. Lee. 1999. Cultivation of the hyperthermophilic archaeon Sulfolobus solfataricus in low-salt media. Biotechnol. Bioprocess Eng. 4:21-25. [Google Scholar]
- 19.Park, C. B., and S. B. Lee. 2000. Effect of exogenous compatible solutes on growth of the hyperthermophilic archaeon Sulfolobus solfataricus. J. Biosci. Bioeng. 89:318-322. [DOI] [PubMed] [Google Scholar]
- 20.Park, C. B., S. B. Lee, and D. D. Y. Ryu. 2001. l-Pyroglutamate spontaneously formed from l-glutamate inhibits growth of the hyperthermophilic archaeon Sulfolobus solfataricus. Appl. Environ. Microbiol. 67:3650-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sako, Y., N. Nomura, A. Uchida, Y. Ishida, H. Morii, Y. Koga, T. Hoaki, and T. Maruyama. 1996. Aeropyrum pernix gen. nov., sp. nov., a novel aerobic hyperthermophilic archaeon growing at temperatures up to 100°C. Int. J. Syst. Bacteriol. 46:1070-1077. [DOI] [PubMed] [Google Scholar]
- 22.Schiraldi, C., and M. De Rosa. 2002. The production of biocatalysts and biomolecules from extremophiles. Trends Biotechnol. 20:515-521. [DOI] [PubMed] [Google Scholar]
- 23.Stecchini, M. L., P. Giavedoni, I. Sarais, and C. R. Lerici. 1991. Effect of Maillard reaction products on the growth of selected food-poisoning microorganisms. Lett. Appl. Microbiol. 13:93-96. [Google Scholar]
- 24.Zillig, W., K. O. Stetter, S. Wunderl, W. Schulz, H. Priess, and J. Scholz. 1980. The Sulfolobus-“Caldariella” group: taxonomy on the basis of the structure of DNA-dependent RNA polymerase. Arch. Microbiol. 125:259-269. [Google Scholar]