Abstract
A series of sites were established on Hawaiian volcanic deposits ranging from about 18 to 300 years old. Three sites occurred in areas that supported tropical rain forests; the remaining sites were in areas that supported little or no plant growth. Sites >26 years old consumed atmospheric CO and hydrogen at rates ranging from about 0.2 to 5 mg of CO m−2 day−1 and 0.1 to 4 mg of H2 m−2 day−1, respectively. Respiration, measured as CO2 production, for a subset of the sites ranged from about 40 to >1,400 mg of CO2 m−2 day−1. CO and H2 accounted for about 13 to 25% of reducing equivalent flow for all but a forested site, where neither substrate appeared significant. Based on responses to chloroform fumigation, hydrogen utilization appeared largely due to microbial uptake. In contrast to results for CO and hydrogen, methane uptake occurred consistently only at the forest site. Increasing deposit age was generally accompanied by increasing concentrations of organic matter and microbial biomass, measured as phospholipid phosphate. Exoenzymatic activities (acid and alkaline phosphatases and α- and β-glucosidases) were positively correlated with deposit age in spite of considerable variability within sites. The diversity of substrates utilized in Biolog Ecoplate assays also increased with deposit age, possibly reflecting changes in microbial community complexity.
Lava, tephra, and volcanic ash contain negligible amounts of organic matter and fixed nitrogen when first deposited (35). The absence or scarcity of vegetation at many active volcanic sites exacerbates the situation by limiting postdepositional inputs. The lack of organic matter substantially constrains the nature and rates of microbial colonization and succession. In such systems, atmospheric inputs or reduced substrates released by weathering (e.g., Mn2+) may support metabolism and growth. Nonexclusive carbon and energy sources for microbial community development under these conditions include (i) phototroph colonization, (ii) aeolian or aqueous organic matter inputs, and (iii) dry and wet deposition of NH4+ and trace gases (e.g., CO, H2, and CH4).
Relatively few studies have examined the significance of these sources or temporal patterns of microbial succession under extreme conditions (2). Data from cryptoendolithic communities suggest that bacterial colonization and succession depend on autochthonous photosynthesis (27, 47). Other studies indicate that desert varnishes form in part from substrates released by weathering (48). Microbiological analyses of recent volcanic materials have led to isolations of thermophiles, psychrotrophs, and other heterotrophs, but the activities and functions of these organisms remain essentially unknown (7, 22-24, 39, 45, 46, 49). In addition, it is unclear if the observed isolates represent dominant populations or fast-growing opportunistic strains.
Data from Hawaii and other systems indicate that various bacterial functional groups colonize lava and tephra relatively quickly. Burleigh and Dawson (6) cultured the actinomycete Frankia from unvegetated 25-year-old Hawaiian tephra. Crews et al. (13) and Kurina and Vitousek (33, 34) have documented nitrogen fixation on 10-year-old lava. Analyses of material collected from Mt. Erebus have revealed several taxa, including Bacillus schlegeli (22-24), a CO and H2 oxidizer reported from temperate soils and aquatic samples (36). Using molecular approaches, Nüsslein and Tiedje (40) have observed substantial diversity in 300-year-old lava-derived forest soils. Sequence information revealed the presence of genera that use trace gases and fix N2 (e.g., Pseudomonas, Bacillus, and Rhizobium), as well as numerous novel, uncultivated forms.
The research described here was undertaken to determine the potential significance of trace gases as carbon and energy sources for microbes colonizing recently deposited (18 to 300 years old) material near Kilauea volcano, Hawaii, and to assess some of the functional attributes of the microbial communities on these deposits. Atmospheric CO and hydrogen were consumed by all but the youngest deposits. In situ uptake rates were comparable to values observed for mature continental systems (10, 30). Atmospheric CO and hydrogen utilization were estimated to contribute 2 to 4% and 15 to 20% of total respiratory reducing equivalent flow, respectively, at all but a forested site.
MATERIALS AND METHODS
Site description.
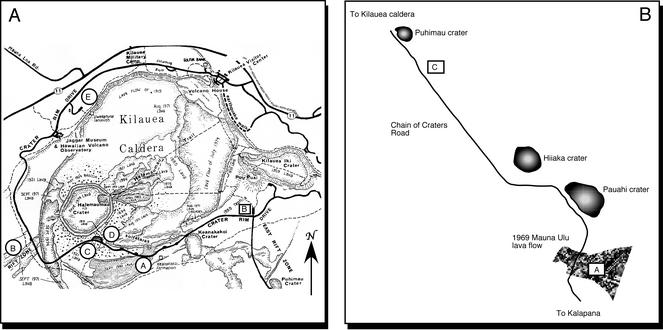
Field sites have been established within the caldera of Kilauea volcano and on its flank (Fig. 1). Vegetation and measured water contents (Table 1) provided a basis for organizing the sites into two groups. Group I sites occur west of or within the caldera (I-A to I-D) and on the north caldera rim (I-E). Group II sites occur southeast of the caldera (IIA-C) in a region supporting rain forest growth. Group I sites were generally drier and supported limited or no vegetation.
FIG. 1.
(A) Map of Kilauea caldera and approximate locations of sampling sites. Exact positions represent GPS data. I-A, 19o 25′ 29.2" N × 155o 16′ 55.6" W; I-B, 19o 23′ 52.7" N × 155o 17′ 44.6" W; I-C, 19o 23′ 56.7" N × 155o 16′ 59.5" W; I-D, 19o 24′ 06.0" N × 155o 16′ 40.1" W; I-E, 19o 23′ 50.6" N × 155o 16′ 26.3" W; II-B, 19o 24′ 22.5" N × 155o 15′ 18.2" W; II-C, 19o 23′ 13.3" N × 155o 14′ 37.7" W. (Redrawn from reference 21a with permission of the Hawaii Natural History Association.) (B) Map of Mauna Ulu region, about 8 km south of site II-C; II-A, 19o 21′ 38.1" N × 155o 13′ 12.9" W. (Redrawn from U.S. Geological Survey map 19155-D3-PF-100.)
TABLE 1.
Selected properties of Hawaiian volcanic depositsa
| Site | Date (age [yr])b | Water contents (%)c | Bulk density (gdw cm−3) | pH | % of:
|
Concn (nmol gdw of material−1) of:
|
||
|---|---|---|---|---|---|---|---|---|
| C | N | NO3− | PO43− | |||||
| I-A | 1982 (18) | 0.1-2 | NDetd | NDet | 0.02 ± 0.01 | NDe | 15 ± 10 | ND |
| I-B | 1974 (26) | 4-6 | NDet | NDet | 0.05 ± 0.01 | ND | 7 ± 1 | 3 ± 2 |
| I-C | 1921 (79) | 11-21 | 0.87 | 3.9 ± 0.1 | 0.05 ± 0.01 | ND | 7 ± 0.4 | ND |
| I-D | 1894 (106) | 12-16 | 1.71 | 4.4 ± 0.03 | 0.15 ± 0.03 | ND | 10 ± 2 | 13 ± 0.2 |
| I-E | 1790 (210) | 11-17 | 1.43 | 4.6 ± 0.03 | 0.7 ± 0.1 | 0.02 ± 0.01 | 7 ± 0.4 | 2 ± 1 |
| II-A | 1969 (31) | 5-7 | 0.65 | 5.4 ± 0.2 | 0.2 ± 0.1 | ND | 3 ± 1 | 10 ± 5 |
| II-B | 1959 (41) | 17-24 | 0.57 | 5.3 ± 0.1 | 0.5 ± 0.1 | ND | 4 ± 1 | 34 ± 2 |
| II-C | 1700 (300) | 51-114 | 0.79 | 5.7 ± 0.03 | 20.4 ± 1.1 | 1.1 ± 0.1 | 779 ± 213 | 166 ± 75 |
Data for all parameters but bulk density represent means of triplicate determinations ± 1 standard error.
Dates represent known or estimated primary deposition dates of material at each site, and ages are those for sample collection during the year 2000.
Shown is the percentage of water content (grams of water per gram [dry weight] of material).
NDet, not determined.
ND, not detected.
Sites I-A to I-D (Fig. 1) were established within the caldera, on lava flows from 1982 (I-A), 1971 (I-B), 1921 (I-C), and 1894 (I-D). Deposit ages were obtained from U.S. Geological Survey maps (Hawaii datum), with field locations established by using a Brunton “Multi-navigator” global positioning satellite receiver (GPS). Sites I-A and I-B contain loose millimeter- to centimeter-size material similar to that of site II-A; the deposits vary in thickness across the lava surface from about 1 to 10 cm. Sites I-C and I-D are characterized by lava-derived soil similar to that of I-E. A limited vascular plant community occurs on material at I-E, while the remaining group I sites are essentially unvegetated. The age of site I-E was taken as 210 years based on the date of a massive eruption in 1790 (D. Swanson, personal communication). It is characterized by relatively shallow (<20-cm depth) coarse- to fine-grained ash along with accretionary lapilli overlain by a thin (about 1 mm) mineral crust.
Sites II-A and II-B were established on a 1969 Mauna Ulu lava flow and a 1959 tephra deposit (Pu'u Puai), respectively. Site II-A consists of scoria (cinders and Pele's tears) in layers of varying thickness overlying an extensive “pahoehoe” lava flow; it supports very limited growth of ferns and Meterosideros polymorpha. Site II-B consists of cinders several meters thick and is colonized by M. polymorpha and Myrica faya (12), which form small stands interspersed with open patches from which samples were obtained. A forested site (II-C) dominated by M. polymorpha and M. faya is about 300 years old by analogy to similar sites nearby (12). Site II-C was characterized by a patchy litter layer <1 cm thick overlying an organic-rich, fine-grained deposit intermixed with millimeter- to centimeter-size tephra.
Bulk physical and chemical analyses.
Aluminum core tubes (7.2-cm inner diameter) were used to collect material to depths of 5 to 10 cm. Bulk densities were estimated from the dry mass of the upper 5 cm. Water contents were estimated by drying material at 110°C for 24 h. Slurries (1:2 mass ratio of samples and deionized water) were used to determine pH. Total organic carbon and nitrogen were analyzed with a Perkin-Elmer model 2400 elemental analyzer for ground material from the upper 2 cm. Extractable nitrate and phosphate were measured with supernatants from 2 g (fresh weight [gfw]) of material from a depth of 0 to 2 cm extracted with 8 ml of 2 N KCl. Nitrate assays were based on a commercial kit (Hach Chemical Co.), and phosphate assays were based on a standard method (15).
Phospholipid phosphate analysis.
Phospholipid phosphate contents were analyzed as a surrogate for microbial biomass (5). Triplicate 0.5- to 1-gfw samples from 0- to 2-cm depths were transferred to screw-cap glass tubes containing 5 ml of a 1:2 mixture of dichloromethane (DCM) and methanol. The tubes were sealed and stored at −20°C or on dry ice during transport to Maine. DCM (1.67 ml) and deionized water (5 ml) were added to each tube prior to centrifugation (1,000 × g). DCM (1 to 2 ml) phases were transferred to glass ampoules and evaporated to dryness at 30°C with nitrogen. Phospholipid phosphate was liberated by persulfate digestion and measured colorimetrically (5).
Gas flux analyses.
CO and hydrogen fluxes were measured in situ by using aluminum collars (about 67 cm2) fitted with a 1-liter quartz chamber forming a static headspace (30). At site I-B, collars were placed along ridges or cracks that had accumulated ≥10 cm of broken, loose material. At all other sites, surface deposits were more uniformly distributed. Approximately 6 to 24 h after deploying the collars, gas exchange assays were initiated by sealing a chamber to a collar, covering the chamber with aluminum foil, and removing 3-cm3 headspace samples with a needle and syringe at 3- to 4-min intervals for up to 15 to 20 min. Samples were assayed in the field with a Trace Analytical RGD or RGA gas chromatograph (29). Starting ambient CO and hydrogen values were typically about 100 and 550 ppb, respectively.
Additional assays were conducted with triplicate intact cores (7.5-cm inner diameter by 20-cm length). Cores were returned to a field laboratory within a few hours after collection. Core headspaces were subsampled for CO and hydrogen analysis as described above. Analyses of CO2 production were performed with headspace samples that were injected into an SRI flame ionization gas chromatograph equipped with a stainless steel column (3.2-mm outer diameter by 2 m) containing silica gel and a methanizer to convert CO2 to methane. Samples for CO2 production were collected at intervals over a 48-h period during incubations at room temperature. Methane uptake was measured similarly, with the exception that a methanizer was not used and headspace samples were assayed by using either the SRI or a Sente model 19A gas chromatograph equipped with a semiconductor detector sensitive to hydrogen, methane, and CO. Instrument responses to CO2 and methane were determined with various standards prepared from pure gases.
Chloroform inhibition of hydrogen uptake.
Surface material (0 to 2 cm) was collected from sites I-D, II-A, and II-B. Triplicate 10-gfw subsamples from each site were transferred to 110-cm3 glass jars sealed with neoprene stoppers. Hydrogen and CO uptake rates were measured as described above. The jars were opened, placed into a glass desiccator, and fumigated with chloroform for 24 h. Afterwards, the jars were equilibrated with ambient air and sealed, and then hydrogen and CO uptake rates were determined again.
Exoenzyme assays.
Triplicate 1-gfw samples from the 0- to 2-cm interval of selected sites were transferred to 50-ml disposable centrifuge tubes containing 5 ml of one of the following buffers: (i) modified universal buffer (1) at pH 6.0 with either p-nitrophenyl-α-d-glucoside or p-nitrophenyl-β-d-glucoside (5 mM final concentration), (ii) modified universal buffer at pH 6.5 or 11 with 3 mM p-nitrophenyl phosphate, or (iii) 0.5 M acetate buffer at pH 5.8 with 5 mM p-nitrophenyl sulfate. The tubes were shaken, incubated, and subsampled at intervals. Subsamples were supplemented with 0.5 M calcium chloride and 0.5 M NaOH. After centrifugation, p-nitrophenol absorbance was measured spectrophotometrically at 400 nm with an LKB model 1050 spectrophotometer.
Substrate utilization patterns.
Triplicate 1-gfw samples from the 0- to 2-cm interval were transferred to 15-ml disposable centrifuge tubes containing 10 ml of deionized water and vortexed for 60 s three times. The suspensions were centrifuged at 500 × g in an Eppendorf model 5810R to remove large particles while leaving cells suspended. One hundred-fifty-microliter subsamples were used to inoculate Biolog Ecoplates; each of the three replicated sets of substrates on a given plate was inoculated with one of the replicate extracts. Plates were incubated at 30°C. Well development was assessed visually at intervals of 1, 2, 4, and 8 days. Wells were scored as positive or negative relative to control wells.
Chlorophyll a analysis.
Triplicate 1-gfw samples from the 0- to 2-cm interval of various sites were transferred to 30-ml glass screw-cap tubes containing 10 ml of 90% acetone-10% dimethyl sulfoxide (DMSO). Tubes were sealed with Teflon-lined caps and held overnight at −20°C. Five milliliters of the acetone-DMSO extracts (or suitable dilutions in acetone-DMSO) was transferred to 13- by 100-mm culture tubes, and chlorophyll a fluorescence was determined with a Turner model TD-700 according to the manufacturer's instructions for EPA method 445.0. Extracts were acidified with 0.15 N HCl to correct for phaeophytin content.
RESULTS
Bulk physical and chemical analyses.
Water contents were low at all but the forested site (II-C), with values ranging from approximately 0.1 to 16% (g of H2O g [dry weight; gdw]−1) for group I. Water contents at group II sites were somewhat higher, ranging from approximately 5 to 114% (Table 1). Bulk densities were least for two recent tephra deposits (0.57 and 0.64 g cm−3 for sites II-A and -B, respectively). Bulk densities for the remaining sites varied between 0.79 and 1.71 g cm−3 (Table 1). The pH of material from the upper 2 cm of all sites varied between 3.9 and 5.7, with no consistent trends as a function of site age (Table 1). However, group II sites were significantly less acidic than group I sites. Carbon contents varied from a low of about 0.02% for material from site I-A to >20% for II-C; values increased with deposit age for both the wetter and drier sites, although the rate of increase was much greater for the former (Table 1). Nitrogen contents and C/N ratios were measurable only for the oldest sites, I-E and II-C, with values of 0.02 and 1.1% and 38.3 and 19.2, respectively.
Extractable nitrate concentrations were typically low, with no consistent variation among sites (values from 3 to 10 nmol of nitrate gdw−1; Table 1). Trends for extractable phosphate were similar; values for II-C were more than an order of magnitude greater than for all other sites (Table 1).
Phospholipid phosphate concentrations.
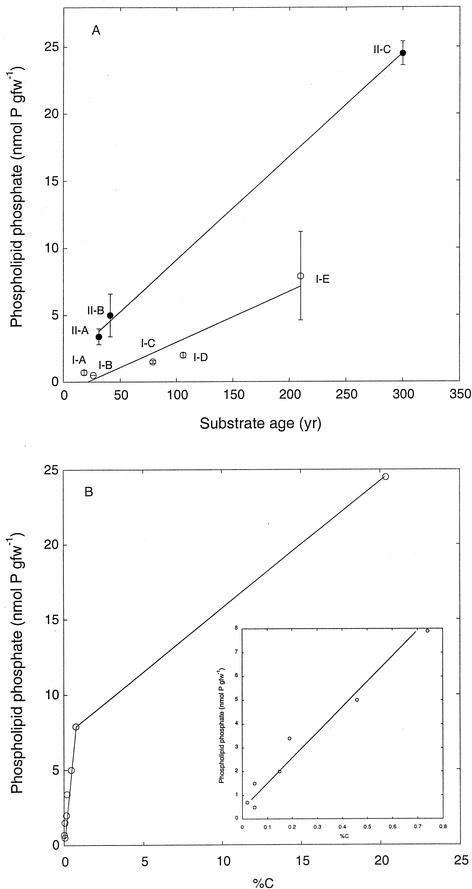
Phospholipid phosphate concentrations ranged from 0.5 ± 0.01 nmol of P gfw−1 to 24.5 ± 0.9 nmol of P gfw−1 (Fig. 2A). Use of a conversion factor of 190 nmol of phospholipid phosphate mg of microbial biomass−1 (5) yielded biomass estimates of about 3 μg gfw−1 to 130 μg gdw−1 (Table 2), which were a negligible fraction of organic carbon. For sites I-B to I-E, phospholipid phosphate contents increased with deposit age (r = 0.95; Fig. 2A). Phospholipid phosphate also increased with deposit age for group II sites (r = 0.99; Fig. 2A), but at a rate about threefold faster than for the other sites. Phospholipid phosphate contents correlated well with the percentage of C (r = 0.98) for sites with values of <1% C (Fig. 2B), irrespective of site location. Phospholipid phosphate correlated poorly (r = 0.35) with water content for sites I-B to I-E and strongly (r = 0.99) for the group II sites (data not shown).
FIG. 2.
(A) Phospholipid phosphate contents of volcanic deposits. Data are means of triplicate determinations ± 1 standard error. Open symbols represent group I sites, and closed symbols represent group II sites. (B) Relationship between phospholipid phosphate and organic contents (from Table 1) for all sites. The extreme point represents site II-C data.
TABLE 2.
Estimated microbial and photosynthetic biomass in volcanic deposits (means ± 1 standard error) and maximum potential areal gross carbon fixationa
| Site | Biomass (μg of biomass gdw−1)
|
Potential carbon fixation (μg of C m−2 day−1) | |
|---|---|---|---|
| Microbial | Photosynthetic | ||
| I-A | 4 ± 1 | 0.1 | NDb |
| I-B | 8 ± 0.5 | 0 | 0 |
| I-C | 3 ± 0.2 | 0 | 0 |
| I-D | 11 ± 0.4 | 0.4 ± 0.1 | 4-22 |
| I-E | 42 ± 18 | 14 ± 2 | 135-676 |
| II-A | 26 ± 8 | 10 ± 1 | 44-222 |
| II-B | 18 ± 3 | 31 ± 9 | 124-618 |
| II-C | 129 ± 5 | 23 ± 7 | 123-614 |
Biomass values are based on phospholipid phosphate (Fig. 2) and chlorophyll a contents (Fig. 5), with respective conversion factors of 5.3 μg (dry weight) nmol of phospholipid phosphorus−1 (5) and 36 mg of chlorophyll a g of cell C−1 (30), with relative carbon contents for cells of 40%. Carbon fixation is based on measured bulk densities (Table 1) and chlorophyll a fixation factors of 2 to 10 mg fixed mg of chlorophyll a−1 h−1 (17).
Not detected.
Gas flux analyses.
All sites except I-A consumed atmospheric hydrogen and CO during in situ or core-based assays (Table 3 and 4). In situ CO uptake varied between about 1 and 5 mg of CO m−2 day−1 (36 to 180 μmol of CO m−2 day−1). Among group I sites, CO uptake tended to increase with deposit age, with the highest values for site I-E. Hydrogen uptake was more variable, but the greatest rates were also observed for I-E (Table 3). Similar rates were observed for assays conducted by using intact cores from five of the sites, but hydrogen rather than CO uptake tended to increase with group I deposit age (Table 4).
TABLE 3.
In situ CO and hydrogen uptake rates for recent Hawaiian volcanic depositsa
| Site | Uptake of:
|
|
|---|---|---|
| CO (mg of CO m−2 day−1) | H2 (mg of H2 m−2 day−1) | |
| I-B | 1.6 ± 0.1 | 3.9 ± 0.3 |
| I-C | 1.2 ± 0.4-2.0 ± 0.8 | 0.1 ± 0.02-1.3 ± 0.2 |
| I-D | 2.7 ± 0.4 | 0.4 ± 0.1 |
| I-E | 3.2 ± 1.6-3.8 ± 1.8 | 0.8 ± 0.2-4.9 ± 2.5 |
| II-B | 4.8 ± 2.2 | 0.6 ± 0.1 |
| II-C | 0.1 ± 0.1 | 1.9 ± 1.1 |
Rates are means of triplicates (± 1 standard error). Ranges are presented for sites with multiple determinations of activity (n = 3 to 6).
TABLE 4.
Rates of atmospheric CO and hydrogen uptake and CO2 production (respiration) and percent contributions of CO and hydrogen to respiratory reducing equivalent flow for intact cores from selected group I and II sitesa
| Site | Uptake (mg m−2 day−1)
|
CO2 production (mg m−2 day−1) | % Contribu- tion ofb:
|
||
|---|---|---|---|---|---|
| CO | H2 | CO | H2 | ||
| I-C | 1.3 ± 0.7 | 0.7 ± 0.5 | 39.6 ± 13.2 | 1.6 | 19.9 |
| I-D | 1.0 ± 0.2 | 1.0 ± 0.1 | 57.2 ± 17.6 | 2.7 | 19.7 |
| I-E | 1.6 ± 0.3 | 3.3 ± 0.1 | 146.9 ± 39.6 | 1.6 | 24.0 |
| II-B | 2.3 ± 0.5 | 2.6 ± 0.30 | 246.4 ± 57.2 | 1.8 | 11.7 |
| II-C | 0.2 ± 0.8 | 1.4 ± 0.4 | 1412.4 ± 391.6 | 0.1 | 1.1 |
All rates are means ± 1 standard error for triplicate determinations.
Contributions of CO and H2 to respiratory reducing equivalent flow were calculated as described in the text.
In contrast, atmospheric methane uptake was typically undetectable, with consistently measurable activity occurring only for the forested site (II-C). Uptake at II-C was first order, with rates of 1.8 ± 0.2 mg of CH4 m−2 day−1 (115 ± 9 μmol of CH4 m−2 day−1) calculated for ambient methane concentrations of 1.8 ppm. When incubated anaerobically, methane was not produced by material from any of the sites.
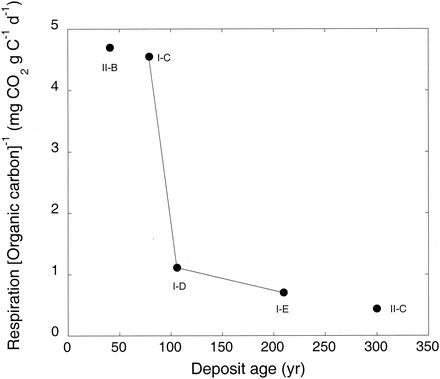
CO2 production (respiration) rates for intact cores ranged from 40 ± 13 mg m−2 day−1 (1 ± 0.3 mmol m−2 day−1) for site I-C to >1,400 ± 390 mg m−2 day−1 (32 ± 9 mmol m−2 day−1) for site II-C. Rates for group II sites were much greater than for group I sites, with activity for II-B almost double that of I-E, a much older site (Table 4). Respiration for group I sites increased approximately linearly with deposit age. CO2 production correlated well with carbon and phospholipid phosphate contents (r > 0.95) (data not shown). Although carbon content increased with deposit age, respiration per unit of carbon decreased sharply (Fig. 3).
FIG. 3.
Respiration per unit amount of organic carbon versus deposit age. Respiration data are from Table 2, and the amount of total organic carbon per square meter was estimated from data in Table 1. A solid line represents group I sites. Group II sites are also indicated on the figure.
Chloroform fumigation of surface material from selected sites indicated that most of the hydrogen uptake was due to intact microbes (Table 5). First-order uptake rate constants varied from about 6 to 33 min−1 prior to fumigation. After fumigation, uptake decreased by 60 to 80%, with no obvious relationship between the extent of inhibition and site location or age.
TABLE 5.
Effect of chloroform fumigation on hydrogen uptake by material from the 0- to 2-cm interval of selected sitesa
| Site | Rate of H uptake (min−1)a
|
% Cellular contributions | |
|---|---|---|---|
| Control | Fumigated | ||
| I-C | 32.9 ± 3.5 | 8.7 ± 0.8 | 73.6 |
| II-A | 6.2 ± 0.4 | 1.1 ± 0.1 | 82.4 |
| II-C | 29.7 ± 2.1 | 12.8 ± 2.8 | 56.8 |
First-order uptake rate constants (mean ± 1 standard error) were determined in triplicate before and after fumigation.
The contribution of cells reflects the activity lost upon fumigation: [1 − (control − fumigation)/control]·100%.
Estimates of the relative contributions of CO and hydrogen to respiratory reducing equivalent flow were calculated assuming a stoichiometry of 2 reducing equivalents per mol of CO or hydrogen oxidized (e.g., CO + 1/2 O2 → CO2) and 4 reducing equivalents per mol of “generic” organic matter oxidized (e.g., CH2O + O2 → CO2 + H2O). Organic matter oxidation was considered equivalent to CO2 production (Table 4). In addition, hydrogen uptake was assumed to result entirely from microbial activity. Relative contributions were calculated as follows: (i) mol of CO oxidized/[2 · (mol of CO2 produced − mol of CO oxidized)] and (ii) mol of hydrogen oxidized/[2 · (mol of CO2 produced)].
For group I sites, CO and hydrogen contributions ranged between about 1.6 and 2.7% and 20 and 24%, respectively, independent of deposit age (Table 4). For group II sites, CO and hydrogen contributions were lowest for the forested site (II-C, 0.1 and 1%), while values for II-B were similar to those of older group I sites (Table 4).
Exoenzymatic activity.
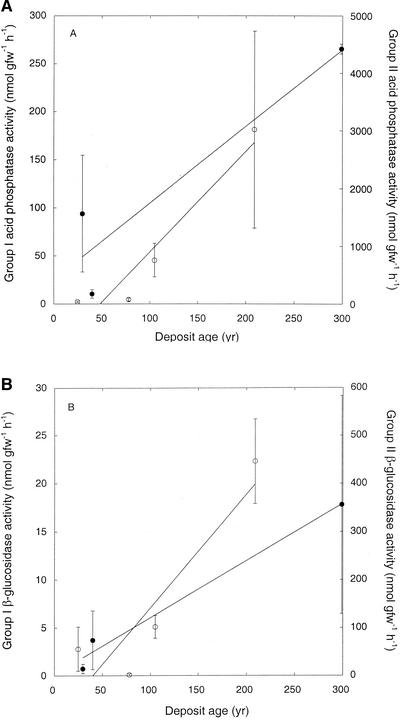
Acid and alkaline phosphatase activity generally increased with deposit age (Fig. 4A, acid phosphatase only; r = 0.67 for group I sites, r = 0.85 for group II sites), with greater rates for group II sites. Typically, acid phosphatase activity was more than an order of magnitude greater than alkaline phosphatase activity. A similar relationship was observed for α- and β-glucosidases (Fig. 4B), with activity of the latter consistently exceeding that of the former at group II sites. In contrast, α-glucosidase activity was greater for the youngest group I sites, increasing notably only for site I-E (data not shown). In spite of variability within sites, β-glucosidase activity was positively correlated with deposit age (r = 0.67 for group I sites, r = 0.85 for group II sites). Arylsulfohydrolase activity was not detected for sites II-A and II-B and was relativity low for the remaining sites; activity did not vary consistently with age (data not shown).
FIG. 4.
(A) Acid phosphatase activity versus deposit age for group I (open symbols, left axis) and group II (closed symbols, right axis) sites. (B) β-Glucosidase activity versus deposit age for group I (open symbols, left axis) and group II (closed symbols, right axis) sites. Data are means of triplicate determinations ± 1 standard error.
Ecoplate substrate utilization.
Substrate reactions developed within 72 h and remained stable for 1 week. For each of the sites, triplicate samples gave identical results. For group I sites, positive reactions were fewest for the youngest sites (I-A and I-B) and greatest for the oldest site (I-E), but the substrates utilized among sites varied substantially without an apparent relationship to deposit age (Table 6). Although the youngest group II site (II-A) used the fewest substrates, the fact that essentially all reactions were positive for the two older vegetated sites (Table 6) obviated development of a specific relationship between deposit age and substrate utilization.
TABLE 6.
Summary of Biolog Ecoplate reactions for bacterial extracts from Hawaiian volcanic deposits
| Substrate | Biolog Ecoplate reaction at sitea:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| I-A | I-B | I-C | I-D | I-E | II-A | II-B | II-C | |
| Arginine | − | − | − | w | + | − | + | + |
| Asparagine | + | − | + | w | + | − | + | + |
| Cellobiose | − | − | − | − | + | − | + | + |
| Cyclodextrin | − | − | − | − | − | − | + | + |
| Erythritol | − | − | − | − | − | − | + | + |
| Galactonic acid lactone | − | − | − | − | + | − | + | + |
| Galacturonic acid | − | − | − | − | + | − | + | + |
| Glucosaminic acid | − | − | − | − | + | − | + | + |
| Glucose-1-phosphate | − | − | − | − | − | − | + | + |
| d,l-α-Glycerol phosphate | − | − | − | − | − | − | + | + |
| Glycogen | − | − | − | − | + | − | + | + |
| Glycylglutamic acid | − | − | − | − | + | + | + | + |
| 2-Hydroxybenzoate | − | − | − | − | − | − | + | − |
| 4-Hydroxybenzoate | − | − | − | − | + | − | + | + |
| Hydroxybutyric acid | − | − | − | − | − | − | + | + |
| Itaconic acid | − | − | − | − | − | − | + | + |
| α-Ketobutyric acid | − | − | − | − | + | − | + | + |
| Lactose | − | − | − | − | + | − | + | + |
| Malic acid | − | − | − | − | + | − | + | + |
| Mannitol | − | − | − | − | + | − | + | + |
| β-Methyl-d-glucoside | − | − | − | − | − | − | + | + |
| N-Acetyl-d-glucosamine | + | − | + | − | + | − | + | + |
| Phenylalanine | − | − | + | − | + | − | + | + |
| Phenylethylamine | − | − | − | − | − | − | + | + |
| Putrescine | − | − | − | − | − | − | + | + |
| Pyruvic acid methyl ester | − | − | + | − | + | + | + | + |
| Serine | − | − | + | − | + | − | + | + |
| Threonine | − | − | − | − | + | − | + | + |
| Tween 40 | − | − | − | w | + | + | + | + |
| Tween 80 | − | − | − | − | + | + | + | + |
| Xylose | − | + | + | w | w | − | + | + |
Substrate reactions were scored positive (+), weakly positive (w), or negative (−) relative to control wells. All reactions for each site were assessed in triplicate.
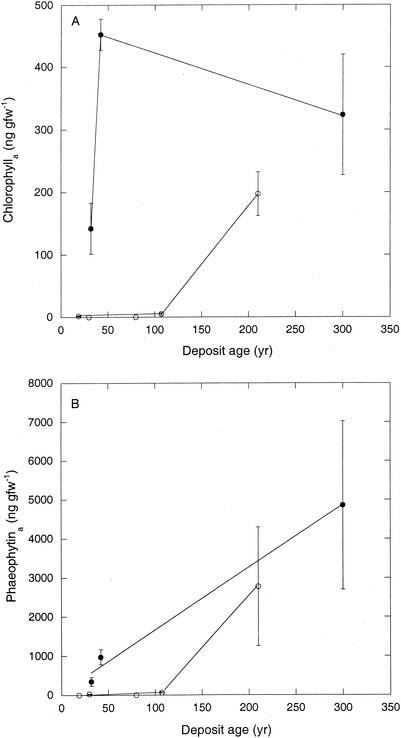
Chlorophyll a distribution.
Chlorophyll a concentrations were undetectable or very low for group I sites other than I-E (Fig. 5A). At site I-E, chlorophyll a concentrations (about 200 ng gfw−1) were comparable to values for group II sites. At all but the youngest group I site (I-A), phaeophytin a greatly exceeded chlorophyll a concentrations (Fig. 5B); for site I-A, chlorophyll a and phaeophytin concentrations were equivalent. Assuming a carbon content of 40% and a chlorophyll a/cell carbon conversion factor of 36 mg of chlorophyll a g of C−1 (32), phototroph biomass ranged from 0 to 31 μg gfw−1 (Table 2). With the exception of site II-B, phototroph biomass values were considerably lower than bacterial biomasses estimated from phospholipid phosphate (described above). For II-B, phototroph and bacterial biomasses were similar (31 versus 18 μg gfw−1). Assuming all measured chlorophyll a was photosynthetically active for 12 h day−1 and that a range of chlorophyll-specific fixation rates for phytoplankton (2 to 10 mg of C fixed mg of chlorophyll a−1 h−1) (17) can be applied to the various sites, microalgal production varied from 0 to 680 μg of C m−2 day−1 (Table 2).
FIG. 5.
(A) Chlorophyll a concentrations versus deposit age for group I (open symbols) and group II (closed symbols) sites. (B) Phaeophytin a concentrations versus deposit age for group I (open symbols) and group II (closed symbols) sites. Data are means of triplicate determinations ± 1 standard error.
DISCUSSION
Numerous studies have addressed the geology, biogeochemistry, and ecology of volcanic systems (3, 8, 12, 14, 25, 42, 44). Relatively little attention has been focused on microbial activities or the communities associated with them (6, 7, 13, 22-24, 33, 34, 39, 40). The results reported here provide new insights into the timing and patterns of lava and tephra colonization by microbes. Microbial colonization of and succession on volcanic deposits no doubt depend on the availability of suitable substrates for energy and biosynthetic metabolism. This is evident from the fact that trends in phospholipid phosphate concentrations, an index of microbial biomass (5), parallel those for organic carbon (Fig. 2), a pattern commonly observed for continental soils (41, 44), but less evident for much older cryptoendolithic communities in the Antarctic (16).
In this study, organic carbon accumulates approximately linearly with time, with different rates for group I and group II (Table 1 and Fig. 2). Differences in organic accumulation and vegetation between these groups likely reflect differences in precipitation or water availability as well as organic sources. Although site-specific precipitation data are not available, the presence of rain forests at or adjacent to group II sites indicates greater water input than for group I sites, which support sparse (I-A to I-D) or limited (I-E) vegetation.
While rooted plants affect organic concentrations at site II-C and perhaps I-E, vascular plant impacts appear negligible or small at other sites. The relative significance of alternate organic matter sources at these sites is uncertain, but includes material derived from aeolian dust inputs, wet deposition, microalgal production, and bacterial lithotrophic biosynthesis. Of these, dust inputs (about 250 mg m−2 year−1 for the Hawaiian Islands (8) can be discounted. Even with an unrealistically high organic content of 10%, dust would account for ≪10% of observed organic matter.
Wet deposition may be more important. Dissolved organic concentrations for Hawaiian rain have not been published, but data for coastal New Zealand suggest values of about 50 μM (as carbon) (28). Use of an approximate average annual rainfall of 200 cm (18) yields carbon inputs of 1.2 g year−1. If only 10% of this is retained, precipitation could contribute substantially to carbon pools at group I sites.
Microalgal inputs appear highly variable, but could be significant for sites other than IA to IC, which contain undetectable or very low chlorophyll a levels (Fig. 5A), implying little or no photosynthetic activity (Table 2). Based on optimal assumptions about relationships between chlorophyll a and net photosynthesis, microalgae could support a large fraction of the observed respiration at sites I-D, I-E, and II-B (Table 2). However, it must be noted that the estimated contributions are likely upper limits, since they do not account for microscale algal distribution and shading or for water and nutrient stresses. In addition, the conversion factors used to calculate photosynthetic activity are derived from phytoplankton and may not be applicable to terrestrial systems. For example, conversion factors derived from Antarctic cryptoendolithic communities under optimal conditions (20°C to 30°C) are orders of magnitude lower than those used here (16, 27, 50). In addition, the predominance of phaeophytin at all sites (Fig. 5B) suggests that algal communities may be severely stressed if not moribund. Nonetheless, the estimates presented here provide upper limits for microalgal contributions to carbon budgets and emphasize the significance of exogenous energy sources. Lichen development has previously been regarded as an important pioneering process for lava weathering and nitrogen cycling (9, 26, 33, 34), but additional effort is necessary to determine the roles of microalgae in bacterial dynamics.
Based on available data, lithotrophic biosynthesis adds relatively little to organic matter pools. Ammonia oxidation (data not shown) and methane oxidation are negligible at most sites. Using an optimal ratio of 10 CO oxidized to 1 CO2 fixed (37) and observed uptake rates (Tables 3 and 4) yields biosynthesis rates for CO-oxidizing bacteria of 0.04 to 0.1 mg of C m−2 day−1, which are considerably lower than inputs estimated for wet deposition and microalgal production. H2-linked biosynthesis may be more significant. Biosynthesis rates of 0.7 to 3.5 mg of C m−2 day−1 result from use of an optimal ratio of 6 H2 oxidized to 1 CO2 fixed (43) and observed uptake rates (Table 3). These values are >10-fold the potential rates for CO and rival inputs calculated for wet deposition. Nonetheless, respiration rates (Fig. 3 and Table 4) exceed even generous estimates of lithotrophic biosynthesis, suggesting minor net contributions at most.
Although apparently unimportant for biosynthesis and organic matter regimes, oxidation of CO and H2 likely contributes appreciably to cellular energy metabolism. Based on a simple organic matter respiration model, CO and H2 uptake account for important levels of reducing equivalent flow for four of five sites examined (Table 4). Each of these four sites contains very low levels of organic carbon (Table 1) and respires at correspondingly low rates (Table 4). CO and hydrogen uptake contribute negligibly to the fifth site (II-C) due to the presence of high organic inputs from the vascular plant community present there. The contrast between this site and the others emphasizes the importance of CO and hydrogen when organic carbon is limiting. In this context, relatively young terrestrial volcanic deposits may be somewhat similar to various hydrothermal systems in which microbial community metabolism depends in part on supplies of reduced gases.
The H2 uptake results are also novel because chloroform fumigation data indicate that activity is due to intact cells rather than “abiontic” hydrogenases (Table 5), which dominate activity in continental soils (11, 20). This may be due to the presence of very low organic concentrations at the onset of succession and selection for bacteria capable of efficiently utilizing available energy sources. If organic matter availability controls partitioning between cellular and exoenzymatic uptake, then other organic-poor systems (e.g., sands and desert soils) may also exhibit relatively high cellular activity.
The results presented here also suggest that organic matter regimes may play an important role in CO dynamics, as has been observed for continental systems (30). Mean CO uptake rates for sites I-B to I-E (from Tables 3 and 4) correlate positively with phospholipid phosphate and organic matter content (r2 = 0.97 and 0.95, respectively). For group I sites, which contain <1% organic carbon, increases in organic matter likely contribute to increases in CO oxidizer biomass along with increases in overall microbial biomass. This trend is consistent with results reported by Moxley and Smith (38) for nonvolcanic soils containing less than about 7% organic matter. A distinct decrease in CO uptake occurs for site II-C, however. This site, with a carbon content of about 20%, appears to behave similarly to continental forests, in which elevated abiological CO production linked to organic matter decreases net CO uptake from the atmosphere (19, 30, 31). Thus, recent Hawaiian volcanic deposits exhibit the full range of interactions between CO and organic matter dynamics.
Respiration data provide additional insights into organic matter dynamics. Decreasing CO2 production (per unit amount of organic matter) with increasing deposit age and organic concentrations (Fig. 3) suggests that a relative reduction in available substrate accompanies organic matter accumulation. This interpretation is supported by a parallel decrease in respiration per unit biomass (or phospholipid phosphate content) across the sites (data not shown). In spite of the fact that exoenzymatic activity and the capacity to use diverse organics increase with deposit age (Table 6 and Fig. 4), the supply of heterotrophic substrates appears to limit microbial populations throughout the initial stages of biotic development on young volcanic materials. In this context, utilization of CO and H2 likely remains a positively selected trait to the extent that CO and H2 contribute to maintenance of metabolism or growth.
Finally, colonization of volcanic substrates by CO- and hydrogen-oxidizing bacteria may contribute significantly to the development of complex microbial communities as documented by Nüsslein and Tiedje (40). Collectively, CO and hydrogen oxidizers express numerous physiological and functional traits that likely play significant roles in temporal and spatial patterns of nutrient cycling (e.g., nitrogen fixation and utilization of numerous organic substrates) (4, 36). In addition, the presence and activity of CO and hydrogen oxidizers have important implications for colonization of volcanic substrates by pioneer plant species, particularly legumes, since many legume symbionts (e.g., Bradyrhizobium, Mesorhizobium, and Sinorhizobium) have been shown to use either CO or hydrogen for lithotrophic growth (20; G. M. King and H. Crosby, Abstr. 102nd Gen. Meet. Am. Soc. Microbiol., abstr. I-4, p. 247, 2002). Thus, the direction and pace of ecosystem development on volcanic deposits may well hinge on microbial utilization of atmospheric carbon, nitrogen and energy sources.
Acknowledgments
This work was supported in part by funds from the NSF LExEn Program and the Clare S. Darling Endowment.
K. Hardy and K. Roache provided technical support. K. Nanba and C. Giardina assisted with fieldwork. K. Nüsslein and V. Gomez provided helpful feedback and insights. Special thanks go to Bill Gilmartin for his generosity in allowing Hale Mahana to be used as a field laboratory and staging center and to Hawaii Volcano National Park for providing access to sites.
Footnotes
Contribution 380 from the Darling Marine Center.
REFERENCES
- 1.Alef, K., P. Nannipieri, and C. Trazer-Cepedo. 1995. β-Glucosidases, p. 325-344. In K. Alef and P. Nannipieri (ed.), Methods in applied soil microbiology and biochemistry. Academic Press, London, United Kingdom.
- 2.Banfield, J. F., and R. J. Hamers. 1997. Processes at minerals and surfaces with relevance to microorganisms and prebiotic synthesis, p. 81-122. In J. F. Banfield and K. H. Nealson (ed.), Geomicrobiology: interactions between microbes and minerals. Mineralogical Society of America, Washington, D.C.
- 3.Banfield, J. F., B. F. Jones, and D. R. Veblen. 1991. An AEM-TEM study of weathering and diagenesis, Abert Lake, Oregon. I. Weathering reactions in the volcanics. Geochim. Cosmochim. Acta 55:2781-2793. [Google Scholar]
- 4.Bensen, D. R., and W. B. Silvester. 1993. Biology of Frankia strains, actinomycete symbionts of actinorhizal plants. Microbiol. Rev. 57:293-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinch-Iversen, J., and G. M. King. 1990. Effects of substrate concentration, growth state, and oxygen availability on relationships among bacterial carbon, nitrogen and phospholipid phosphorous content. FEMS Microbiol. Ecol. 74:345-355. [Google Scholar]
- 6.Burleigh, S. H., and J. O. Dawson. 1994. Occurrence of Myrica-nodulating Frankia in Hawaiian volcanic soils. Plant Soil 164:283-289. [Google Scholar]
- 7.Cameron, R. E., and R. E. Benoit. 1970. Microbial and ecological investigations of recent cinder cones, Deception Island, Antarctica—a preliminary report. Ecology 51:802-809. [Google Scholar]
- 8.Chadwick, O. A., L. A. Derry, P. M. Vitousek, B. J. Huebert, and L. O. Hedin. 1999. Changing sources of nutrients during four million years of ecosystem development. Nature (London) 397:491-497. [Google Scholar]
- 9.Cochran, M. F., and R. A. Berner. 1996. Promotion of chemical weathering by higher plants: field observations on Hawaiian basalts. Chem. Geol. 132:71-78. [Google Scholar]
- 10.Conrad, R., and W. G. Seiler. 1980. Role of microorganisms in the consumption and production of atmospheric carbon monoxide by soil. Appl. Environ. Microbiol. 40:437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conrad, R. 1996. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Rev. 60:609-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crews, T. E., K. Kitayama, J. H. Fownes, R. H. Riley, D. A. Herbert, D. Mueller-Dombois, and P. M. Vitousek. 1995. Changes in soil phosphorous fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76:1407-1424. [Google Scholar]
- 13.Crews, T. E., L. M. Kurina, and P. M. Vitousek. 2001. Organic matter and nitrogen accumulation and nitrogen fixation during early ecosystem development in Hawaii. Biogeochemistry 52:259-279. [Google Scholar]
- 14.del Moral, R., and C. A. Clampitt. 1985. Growth of native plant species on recent volcanic substrates from Mount St. Helens. Am. Midl. Nat. 114:374-383. [Google Scholar]
- 15.Forester, J. C. 1995. Soil phosphorus, p. 88-93. In K. Alef and P. Nannipieri (ed.), Methods in applied soil microbiology and biochemistry. Academic Press, London, United Kingdom.
- 16.Friedmann, E. I., P. A. LaRock, and J. O. Brunson. 1980. Adenosine triphosphate (ATP), chlorophyll, and organic nitrogen in endolithic microbial communities and adjacent soils in the dry valleys of southern Victoria Land. Antarct. J. U. S. 15:164-166. [Google Scholar]
- 17.Geider, R. J., H. L. MacIntyre, and T. M. Kana. 1997. Dynamic model of phytoplankton growth and acclimation: responses of the balanced growth rate and the chlorophyll a:carbon ratio to light, nutrient-limitation and temperature. Mar. Ecol. Prog. Ser. 148:187-200. [Google Scholar]
- 18.Giambelluca, T. W., M. A. Nullet, and T. A. Schroeder. 1986. Rainfall atlas of Hawaii. Department of Land and Natural Resources, Honolulu, Hawaii.
- 19.Gödde, M., K. Meuser, and R. Conrad. 2000. Hydrogen consumption and carbon monoxide production in soils with different properties. Biol. Fertil. Soils 32:129-134. [Google Scholar]
- 20.Hanus, F. J., R. J. Maier, and H. J. Evans. 1979. Autotrophic growth of H2-uptake positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc. Natl. Acad. Sci. USA 76:1788-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Häring, V., and R. Conrad. 1994. Demonstration of two different H2-oxidizing activities in soil using an H2 consumption and a tritium exchange assay. Biol. Fertil. Soils 17:125-128. [Google Scholar]
- 21a.Hazlett, R. 1993. Geological field guide: Kilauea volcano. Hawaii Natural History Association, Hawaii National Park, Hawaii.
- 22.Hudson, J. A., and R. M. Daniel. 1988. Enumeration of thermophilic heterotrophs in geothermally heated soils from Mount Erebus, Ross Island, Antarctica. Appl. Environ. Microbiol. 54:622-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson, J. A., R. M. Daniel, and H. W. Morgan. 1988. Isolation of a strain of Bacillus schlegelii from geothermally heated Antarctic soil. FEMS Microbiol. Lett. 51:57-60. [Google Scholar]
- 24.Hudson, J. A., R. M. Daniel, and H. W. Morgan. 1989. Acidophilic and thermophilic Bacillus strains from geothermally heated Antarctic soil. FEMS Microbiol. Lett. 60:279-282. [Google Scholar]
- 25.Huebert, B., P. Vitousek, J. Sutton, T. Elias, J. Heath, S. Coeppicus, S. Howell, and B. Blomquist. 1999. Volcano fixes nitrogen into plant-available forms. Biogeochemistry 47:111-118. [Google Scholar]
- 26.Jackson, T. A., and W. D. Keller. 1970. Comparative study of the role of lichens and inorganic processes in the chemical weathering of recent Hawaiian lava flows. Am. J. Sci. 269:446-466. [Google Scholar]
- 27.Johnston, C. G., and J. R. Vestal. 1991. Photosynthetic carbon incorporation and turnover in Antarctica cryptoendolithic microbial communities: are they the slowest-growing communities on Earth? Appl. Environ. Microbiol. 57:2308-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieber, R. J., B. Peake, J. D. Willey, and G. B. Avery. 2002. Dissolved organic carbon and organic acids in coastal New Zealand rainwater. Atmos. Environ. 36:3557-3563. [Google Scholar]
- 29.King, G. M. 1999. Attributes of atmospheric carbon monoxide oxidation in Maine forest soils. Appl. Environ. Microbiol. 65:5257-5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King, G. M. 2000. Impacts of land use on atmospheric carbon monoxide consumption by soils. Global Biogeochem. Cycles 14:1161-1172. [Google Scholar]
- 31.King, G. M., and M. Hungria. 2002. Soil-atmosphere CO exchanges and microbial biogeochemistry of CO transformations in a Brazilian agroecosystem. Appl. Environ. Microbiol. 68:4480-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirk, J. T. O. 1994. Light and photosynthesis in aquatic ecosystems. Cambridge University Press, Cambridge, United Kingdom.
- 33.Kurina, L. M., and P. M. Vitousek. 1999. Controls over the accumulation and decline of a nitrogen-fixed lichen, Stereocaulon vulcani, on young Hawaiian lava flows. J. Ecol. 87:784-794. [Google Scholar]
- 34.Kurina, L. M., and P. M. Vitousek. 2001. Nitrogen fixation rates of Stereocaulon vulcani on young Hawaiian lava flows. Biogeochemistry 55:179-194. [Google Scholar]
- 35.Macdonald, G. A. 1968. Composition and origin of Hawaiian lavas, p. 477-522. In R. R. Coates, R. L. Hay, and C. A. Anderson (ed.), Studies in volcanology: a memoir in honor of Howell Williams. Geological Society of America, Boulder, Colo.
- 36.Meyer, O., and H. G. Schlegel. 1983. Biology of aerobic carbon monoxide-oxidizing bacteria. Annu. Rev. Microbiol. 37:277-310. [DOI] [PubMed] [Google Scholar]
- 37.Morsdorf, G., K. Frunzke, D. Gadkari, and O. Meyer. 1992. Microbial growth on carbon monoxide. Biodegradation 3:61-82. [Google Scholar]
- 38.Moxley, J. M., and K. A. Smith. 1998. Factors affecting utilisation of atmospheric CO by soils. Soil Biol. Biochem. 30:65-79. [Google Scholar]
- 39.Nicholaus, B., F. Marsiglia, E. Esposito, A. Tricone, L. Lama, R. Sharp, G. DiPrisco, and A. Gambacarta. 1991. Isolation of five strains of thermophilic eubacteria in Antarctica. Polar Biol. 11:425-429. [Google Scholar]
- 40.Nüsslein, K., and J. M. Tiedje. 1998. Characterization of the dominant and rare members of a young Hawaiian soil bacterial community with small-subunit ribosomal DNA amplified from DNA fractionated on the basis of its guanine and cytosine composition. Appl. Environ. Microbiol. 64:1283-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40a.Ponderosa Publishing Co. Hawaii Volcanoes National Park, Hawaii. Ponderosa Publishing Co., Evergreen, Colo.
- 41.Powlson, D. S., P. C. Brookes, and B. T. Christensen. 1987. Measurement of soil microbial biomass provides an early indication of changes in the total soil organic matter due to straw incorporation. Soil Biol. Biochem. 19:159-164. [Google Scholar]
- 42.Raich, J. W., A. E. Russell, T. E. Crews, H. Farrington, and P. M. Vitousek. 1996. Both nitrogen and phosphorus limit plant production on young Hawaiian lava flows. Biogeochemistry 32:1-14. [Google Scholar]
- 43.Schlegel, H. G. 1986. General microbiology, 6th ed. Cambridge University Press, Cambridge, United Kingdom.
- 44.Schnürer, J., M. Clarholm, and T. Rosswall. 1985. Microbial biomass and activity in an agricultural soil with different organic matter contents. Soil Biol. Biochem. 17:611-618. [Google Scholar]
- 45.Siebert, J., and P. Hirsch. 1988. Characterization of 15 selected coccal bacteria isolated from Antarctic rock and soil samples from the McMurdo dry valleys (South-Victoria Land). Polar Biol. 9:37-44. [DOI] [PubMed] [Google Scholar]
- 46.Smith, H. G. 1985. Colonization of volcanic tephra on Deception Island by protozoa: long term trends. Br. Antarct. Surv. Bull. 66:19-33. [Google Scholar]
- 47.Sun, H. J., and E. I. Friedmann. 1999. Growth on geological time scales in the Antarctic cryptoendolithic microbial community. Geomicrobiol. J. 16:193-202. [Google Scholar]
- 48.Taylor-George, S., F. Palmer, J. T. Staley, D. J. Borns, B. Curtiss, and J. B. Adams. 1983. Fungi and bacteria involved in desert varnish formation. Microb. Ecol. 9:227-245. [DOI] [PubMed] [Google Scholar]
- 49.Tearle, P. V., and K. J. Richard. 1987. Ecophysiological grouping of Antarctic environmental bacteria by API 20 NE and fatty acid fingerprints. J. Appl. Bacteriol. 63:497-503. [Google Scholar]
- 50.Vestal, J. R. 1988. Biomass of the cryptoendolithic microbiota from the Antarctic desert. Appl. Environ. Microbiol. 54:957-959. [DOI] [PMC free article] [PubMed] [Google Scholar]