Abstract
Infections with the group B coxsackieviruses either can be asymptomatic or can lead to debilitating chronic diseases. To elucidate the mechanism by which these viruses cause chronic disease, we developed a mouse model of chronic pancreatitis by using a virulent variant of coxsackievirus B4, CVB4-V. Infection with CVB4-V results in an early, severe pancreatitis, which can lead to mortality or progress to chronic pancreatitis. Chronic pancreatitis, in this model, is due to immunopathological mechanisms. We investigated whether interleukin-12 (IL-12) could modulate the outcome of CVB4-V infection. Eighty-five percent of the infected mice treated with 500 ng of IL-12 survived, whereas all untreated mice succumbed. To understand the mechanism underlying the beneficial effect of IL-12, we investigated the role of gamma interferon (IFN-γ). Three lines of evidence suggest that the protective effect of IL-12 is due to IFN-γ. First, administration of IL-12 increased the production of endogenous IFN-γ in CVB4-V-infected mice. Both NK and NKT cells were identified as the source of IFN-γ. Second, IFN-γ knockout mice treated with IL-12 succumbed to infection with CVB4-V. Third, wild-type mice treated with IFN-γ survived infection with CVB4-V. Due to the antiviral effects of IFN-γ, we examined whether IL-12 treatment affected viral replication. Administration of IL-12 did not decrease viral replication in the pancreas, but it did prevent extensive tissue damage and the subsequent development of chronic pancreatitis. The data suggest that IL-12 treatment during CVB4-V infection is able to suppress the immunopathological mechanisms that lead to chronic disease.
The group B coxsackieviruses are enteroviruses belonging to the Picornaviridae family. In humans, most infections with the group B viruses are asymptomatic (25, 30). Generally, symptomatic infections result in mild illnesses of the upper respiratory tract, the gastrointestinal tract, and the skin. Occasionally, the group B coxsackieviruses cause chronic inflammatory disease of the pancreas (insulin-dependent type I diabetes mellitus, idiopathic chronic pancreatitis), heart (dilated cardiomyopathy), and central nervous system (7). The mechanism by which the group B viruses cause chronic disease is a topic of ongoing debate. One view is that coxsackieviruses cause chronic disease via viral persistence (18, 19, 38, 40). Viral persistence may be due to the prolonged presence of low levels of infectious virus that continue to cause tissue injury. Alternatively, viral persistence may be due to the prolonged presence of viral RNA in the absence of infectious virus. The implication is that a low level of translation results in the synthesis of viral proteins that continue to stimulate immune responses, which cause prolonged tissue destruction. Another view is that coxsackieviruses cause chronic disease via immune-mediated mechanisms directed against self-antigens. In mouse models, extensive evidence indicates that immunopathological mechanisms contribute to coxsackievirus-induced chronic disease (11, 15, 16, 32, 35, 36).
We have developed a mouse model of coxsackievirus B4-induced disease by using two serologically indistinguishable variants of the B4 serotype, CVB4-P and CVB4-V (5). The avirulent CVB4-P variant induces a mild, transient inflammation of the pancreas (pancreatitis) with full recovery of the tissues by 10 days of infection. The virulent CVB4-V variant induces a severe pancreatitis, which can lead to mortality or progress to chronic disease (chronic pancreatitis). We have shown that the severity of CVB4-V-induced chronic disease is influenced by the major histocompatibility complex (31), cytokines (33), and T cells (33). Chronic pancreatitis, in this model, is due to immunopathological mechanisms.
Given that CVB4-V-induced disease is due to immunopathological mechanisms, we addressed whether the outcome of CVB4-V infection could be altered by treatment with cytokines. The cytokine chosen for the present study was interleukin-12 (IL-12), because several studies have shown that administration of IL-12 can be beneficial during infection with a variety of viruses (21). In addition, IL-12 is made early in infection (34) and has a broad range of effects on both innate and adaptive immunity (34, 39). Our reasoning was that administration of IL-12 early in the course of CVB4-V infection could alter the development of immunopathology.
In the present study, we show that (i) mice treated with IL-12 survive infection with the lethal CVB4-V variant, (ii) the beneficial effect of IL-12 is mediated via gamma interferon (IFN-γ), (iii) IFN-γ is produced by NK and NKT cells after IL-12 treatment, and (iv) the beneficial effect of IL-12 is not due to a decreased viral load in the target organ.
MATERIALS AND METHODS
Cells and viruses.
The present study used a strain of coxsackievirus B4 that had been previously designated CB4-V (31). In keeping with the nomenclature for the group B coxsackieviruses, the CB4-V strain is renamed CVB4-V. The passage history of CVB4-V has been described (31). A large-scale stock of plaque-purified CVB4-V was prepared in LLC-MK2(D) cells. Viral infectivity was assessed by plaque assay with LLC-MK2(D) cells.
Mouse strains.
BALB/cByJ mice and IFN-γ KO (GKO) mice (10) were bred and maintained in the Animal Core Facility of the Wadsworth Center (Albany, N.Y.). The GKO strain is on the BALB/cByJ genetic background.
Infection of mice.
Four-week-old male mice (18 to 20 g) were used in the present study. Mice were injected intraperitoneally (i.p.) with 100 PFU of virus diluted in phosphate-buffered saline (PBS). Control mice were injected with PBS. All injected mice were monitored daily. Mice that became moribund (displayed dramatic weight loss, shivering, huddling, and inactivity) were euthanized immediately. In a previous study (33), we have shown that exocrine pancreatic insufficiency contributes to morbidity and mortality in CVB4-V-infected mice and that supplements of pancreatic enzymes and sucrose minimize morbidity in this model. For studies (morphological and infectivity) that extended over a 2-week period, CVB4-V-infected mice were given supplements of pancreatic enzymes (1 mg of pancreatin/ml) and sucrose (10%) in their drinking water to minimize morbidity. Mice were sacrificed at various times postinfection, and their organs were harvested. Organ homogenates were prepared as previously described (31) and assayed for infectivity by plaque assay. Pancreatic tissues, fixed with Bouin's solution (Sigma-Aldrich, St. Louis, Mo.), were processed for routine histology, followed by staining with hematoxylin and eosin. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center.
Administration of cytokines.
Recombinant, murine IL-12 (Genetics Institute, Cambridge, Mass.) was administered i.p. as a single dose immediately after viral infection. Recombinant, murine IFN-γ was purchased from Roche Diagnostics Corporation (Indianapolis, Ind.) and was administered i.p. as multiple doses at various times after infection. Control mice were injected with vehicle alone (1% normal mouse sera in PBS). All mice were monitored daily. If mice became moribund, they were euthanized immediately.
ELISA.
The amount of IFN-γ present in sera and organ homogenates was determined by enzyme-linked immunosorbent assay (ELISA). Serum samples were obtained from blood collected from the tail vein of infected mice. Organs (pancreas, heart, and spleen), harvested at various times postinfection, were homogenized in lysis buffer (50 mM Tris, 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, 30 μM phenylmethylsulfonyl fluoride, 420 μM leupeptin, 0.02% aprotinin, 3 mM NaN3) (17) and clarified by low-speed centrifugation (1,800 × g for 10 min). Immulon-4 microtiter plates (Dynex Technologies, Inc., Chantilly, Va.) were coated with anti-IFN-γ capture antibody (R4-6A2; BD Pharmingen, San Diego, Calif.) (0.2 μg/well) in 0.1 M sodium phosphate buffer (pH 9.0) at 4°C overnight. Plates were washed three times in PBS-0.05% Tween 20 and blocked with 10% fetal calf serum (FCS) in PBS for 1 h at room temperature. Undiluted samples (serum, organ homogenate) were added to the plates, followed by incubation at 4°C overnight. Plates were washed four times and incubated with biotinylated anti-IFN-γ antibody (XMG1.2; BD Pharmingen, San Diego, Calif.) (50 ng/well) for 1 h at room temperature. After four washes, plates were incubated with horseradish peroxidase-conjugated streptavidin (Southern Biotechnology Associates, Inc., Birmingham, Ala.) diluted 1:2,000 in PBS-10% FCS-0.05% Tween 20 for 30 min at room temperature. After five washes, the enzymatic reaction was developed with a substrate solution, tetramethylbenzidine (Pierce Chemical Co., Rockford, Ill.), for 10 min at room temperature. The optical density was measured at 450 nm with a Thermomax microplate reader (Molecular Devices, Sunnyvale, Calif.). Samples were tested in duplicate. Recombinant, murine IFN-γ (Genzyme Corp., Cambridge, Mass.) diluted in PBS-10% FCS-0.01% Tween 20 was used to generate a standard curve. The limit of detection of the assay was 70 pg of IFN-γ per ml of serum or tissue homogenate.
Intracellular staining and flow cytometry.
A modified procedure for intracellular staining of IFN-γ was performed on freshly isolated splenocytes (2). Since IFN-γ production peaked in the spleen of infected mice 1 day after administration of IL-12, splenocytes were harvested and assessed at this time point. Splenocytes were isolated after mechanical dissociation and hypotonic lysis of red blood cells with 1.5 mM ammonium chloride (22). To increase IFN-γ production, splenocytes (resuspended at 4 × 106/ml) were stimulated for 4 h at 37°C and 5% CO2 with 20 ng of phorbol 12-myristate 13-acetate/ml, 1 μM ionomycin, and 2 μM monensin (Sigma-Aldrich) in complete RPMI 1640 (10% FCS, 2 mM l-glutamine, 50 μM β-mercaptoethanol, 100 U of penicillin/ml, 100 μg of streptomycin sulfate/ml). Cells were washed with Hanks balanced salt solution-2% FCS-4 μM monensin and resuspended in Hanks balanced salt solution-2% FCS-2 μM monensin-0.1% sodium azide (NaN3). Nonspecific staining associated with binding of antibodies to Fc receptors was blocked with anti-Fc (2.4G2) for 10 min at 4°C. Cells (106) were labeled with a phycoerythrin-conjugated pan-NK cell antibody, DX5 (eBioscience, San Diego, Calif.), and a fluorescein isothiocyanate-conjugated anti-T-cell receptor alpha/beta (TCRα/β) antibody, H57-597, for 30 min at 4°C. Control samples were stained with isotype-matched antibodies. After two washes with PBS-0.1% NaN3, cells were fixed with 4% paraformaldehyde for 5 min at room temperature, washed twice with PBS-1% bovine serum albumin (BSA), and stored overnight at 4°C in PBS-1% BSA. Cells were permeabilized with PBS-1% BSA-0.1% saponin-5% nonfat dry milk-1 mM CaCl2-1 mM MgSO4-0.05% NaN3-10 mM HEPES (pH 7.4) for 30 min at room temperature, incubated with 2.5 μg of the nonspecific antibody anti-staphylococcal enterotoxin B (B344.1.13) for 30 min at room temperature, and then treated with 0.25 μg of allophycocyanin-conjugated anti-IFN-γ (XMG1.2; BD Pharmingen, San Diego, Calif.) for 30 min at room temperature. Control samples were incubated with 2.5 μg of unlabeled XMG1.2 and treated with allophycocyanin-XMG1.2. Finally, samples were washed twice with PBS-1% BSA-0.1% saponin-1 mM CaCl2-1 mM MgSO4-0.05% NaN3-10 mM HEPES (pH 7.4) and analyzed with a FACSCalibur system (BD Bioscience, San Jose, Calif.) by using the CellQuest software package.
Statistical analysis.
Cox proportional hazards regression (9) was used to evaluate survival data obtained after the administration of cytokines to infected mice. The body weight of each mouse prior to infection was used as a covariant in the Cox proportional hazards regression. Statistical evaluation of the ELISA data and the intracellular IFN-γ staining data was performed by using the Mann-Whitney test (8, 24).
RESULTS
Exogenous IL-12 protects BALB/c mice from lethal infection with CVB4-V.
We have previously shown that there is a gender difference in survival rates of CVB4-V-infected BALB/c mice (33). The mortality rates for male and female mice are 100 and 67%, respectively. For these studies, the more sensitive male BALB/c mice were used. To determine the lowest dose of CVB4-V that induced 100% morbidity and mortality, male BALB/c mice were infected with 50 to 500 PFU of CVB4-V. Mice infected with 50 and 100 PFU of CVB4-V had mortality rates of 25 and 100%, respectively (data not shown). Thus, for these studies, a dose of CVB4-V (100 PFU) capable of causing 100% mortality was used.
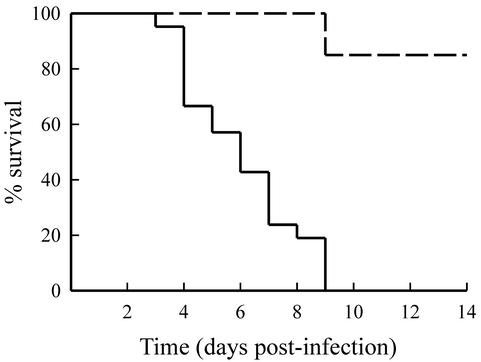
To determine whether exogenous administration of IL-12 could modulate the outcome of infection with CVB4-V, infected mice were given various amounts (100 to 1,000 ng) of murine, recombinant IL-12. Due to the limited supply of IL-12, initial studies were done with small groups (n = 4) of mice. During the 2-week, follow-up period, mice that became moribund (weight loss, shivering, huddling, inactive) were euthanized. Control mice infected with CVB4-V all succumbed to infection. The administration of exogenous IL-12 significantly (P = 0.0032) increased survival of CVB4-V-infected mice (Table 1). CVB4-V-infected mice treated with 500 ng of IL-12 had the highest survival rate. To obtain a more accurate assessment of survival, we increased the number of infected mice treated with 500 ng of IL-12. The survival rate of CVB4-V-infected mice treated with 500 ng of IL-12 was 85% (Fig. 1). Infected mice treated with a higher dose (750 ng) of IL-12 exhibited a decrease in survival rate (25%). All mice given 1,000 ng of IL-12 succumbed to infection, similar to infected, control mice. The results suggest that, in this model system, the beneficial effect of IL-12 is optimal at a dose of 500 ng. Higher doses of IL-12 in this model were less effective and possibly associated with toxicity. Toxicity may result because of synergy between high doses of IL-12, a proinflammatory cytokine, and viral infection, an inflammatory state.
TABLE 1.
Administration of exogenous IL-12 results in survival of CVB4-V-infected mice
| Amt of IL-12 (ng) | No. of mice infected | % Survival |
|---|---|---|
| 0 | 21 | 0 |
| 100 | 4 | 25 |
| 250 | 8 | 25 |
| 500 | 20 | 85 |
| 750 | 4 | 25 |
| 1,000 | 4 | 0 |
FIG. 1.
Survival of CVB4-V-infected, IL-12-treated mice. Infected mice treated with 500 ng of IL-12 (dashed line) or vehicle alone (solid line) were monitored daily for 2 weeks. Mice that became moribund were euthanized immediately. Each group contained 20 to 21 mice. Treatment with 500 ng of IL-12 significantly (P < 0.001) increased the survival of CVB4-V-infected mice.
The beneficial effect of IL-12 treatment is not due to decreased viral replication.
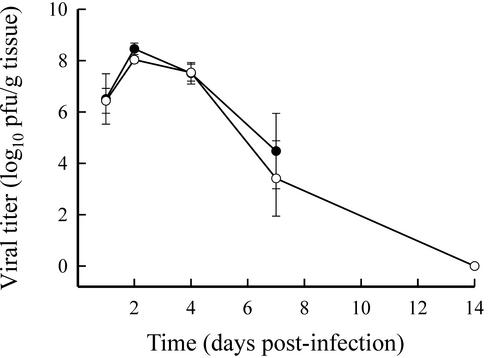
Since IL-12 was able to alter the outcome of infection with CVB4-V, we investigated whether survival was the result of decreased viral replication in the target organ (pancreas). The kinetics of viral replication in the pancreatic tissues of infected control mice and of infected mice treated with 500 ng of IL-12 were similar (Fig. 2). Viral titers in both groups of mice peaked 2 days after infection. In CVB4-V-infected, IL-12-treated mice, infectious virus was cleared by 14 days after infection. Infectivity assays were not done beyond 7 days of infection with CVB4-V due to the high mortality rate.
FIG. 2.
Viral replication in the pancreatic tissues of untreated and IL-12-treated mice. Pancreata harvested from CVB4-V-infected, IL-12-treated mice (○) and from infected, untreated mice (•) were assayed for viral infectivity by plaque assay. Organs from four mice were analyzed at each time point. Mean values and standard deviations are shown.
Treatment with IL-12 protects the exocrine pancreas of CVB4-V-infected mice.
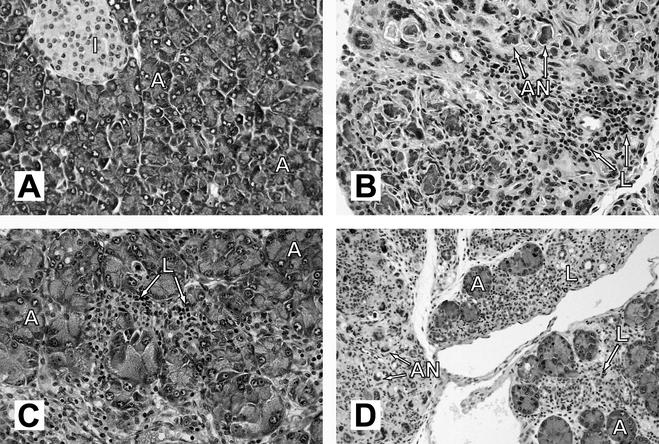
Our previous studies have shown that CVB4-V infection causes chronic pancreatitis in a variety of mouse strains (31). Disease severity correlates with destruction of the exocrine pancreas. In the most severe case wherein mice succumb to infection, there is total destruction of the exocrine pancreas. In mouse strains that survive infection, residual acini remain. We investigated whether IL-12 treatment of CVB4-V-infected mice was able to protect the exocrine pancreas from extensive destruction. At 14 days after infection with CVB4-V, pancreatic tissue from untreated mice displayed complete acinar cell necrosis with fibrosis (Fig. 3B). In addition, a mild inflammatory infiltrate consisting of lymphocytes was evident. Unlike tissues from untreated, infected mice, pancreatic tissues from infected mice treated with 500 ng of IL-12 appeared relatively undamaged (Fig. 3C). Intact acini were abundant. There was some focal destruction of acini with minimal necrosis and no fibrosis. Tissue damage was accompanied by a moderate inflammatory infiltrate consisting of lymphocytes.
FIG. 3.
Histopathology of pancreatic tissues from CVB4-V-infected mice treated with either IL-12 or IFN-γ. Pancreata were harvested at 14 days postinfection, processed for routine histology, and stained with hematoxylin and eosin. (A) Uninfected; (B) CVB4-V-infected; (C) CVB4-V-infected and treated with 500 ng of IL-12; (D) CVB4-V-infected and treated with 100 ng of IFN-γ on multiple days (0.5, 1, and 2) after infection. Labeling: A, acini; I, islet of Langerhans; L, lymphocytic infiltrate; AN, acinar cell necrosis. Magnifications: ×234 for panels A, B, and C; ×117 for panel D.
Administration of IL-12 increases IFN-γ production during CVB4-V infection.
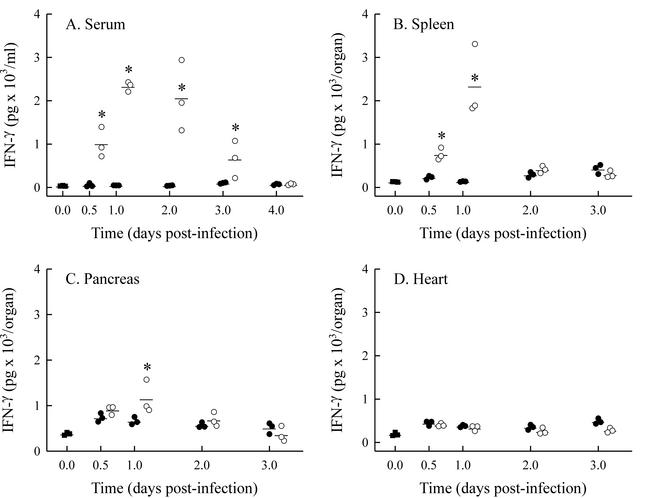
We investigated whether treatment with exogenous IL-12 altered IFN-γ production in organs that support virus replication, i.e., pancreas, heart, and spleen. Since IL-12 was administered immediately after infection and has a half-life of 5 to 10 h (1), the prediction was that IL-12 would alter IFN-γ production early in infection. As a result, tissue samples were collected 0.5 to 4 days after infection and assayed by ELISA. The level of sensitivity of the ELISA was 70 pg of IFN-γ per ml of sample. If organ weight remains relatively unchanged, data are usually reported as the amount of IFN-γ present per gram of tissue. However, during CVB4-V infection, the weights of the pancreas and spleen decrease by 20% within 4 days. As a result, the data are presented as total IFN-γ detected per organ. In addition to measuring IFN-γ in various organs, we assayed serum samples. During CVB4-V infection, the amount of IFN-γ present in the pancreas, heart, spleen, and serum was negligible (Fig. 4). When infected mice were treated with 500 ng of IL-12, there was a significant increase in serum IFN-γ (Fig. 4A). Increased amounts of IFN-γ in the sera of IL-12-treated, CVB4-V-infected mice were detected as early as 12 h after infection. IFN-γ, in sera of IL-12-treated, infected mice, peaked 1 day after infection and represented a 49-fold increase over the amount of IFN-γ observed in infected, control mice. At 4 days after infection, the amount of IFN-γ in the sera of IL-12-treated mice decreased to background levels. A significant, 17-fold increase in IFN-γ was also observed in the spleens of IL-12-treated mice by 1 day after infection (Fig. 4B). By 2 days after infection, the amount of IFN-γ in the spleens of IL-12-treated mice decreased to the level seen in untreated mice. Administration of IL-12 resulted in a significant, small increase in the amount of IFN-γ present in pancreata by 1 day after infection (Fig. 4C). The administration of IL-12 had no significant effect on the amount of IFN-γ present in the hearts of CVB4-V-infected mice (Fig. 4D).
FIG. 4.
IFN-γ production in CVB4-V-infected mice treated with IL-12. Organs and sera were collected from groups (n = 3) of IL-12-treated (○) or untreated (•) mice at various times after infection and assayed for IFN-γ by ELISA. Uninfected mice (▪) were included as controls. (A) Serum; (B) spleen; (C) pancreas; (D) heart. Each datum point represents the result for one mouse. The mean value for each group is shown. Statistically significant differences (P < 0.05) between IL-12-treated and untreated mice are denoted by asterisks.
Administration of IL-12 increases IFN-γ production by NK and NKT cells.
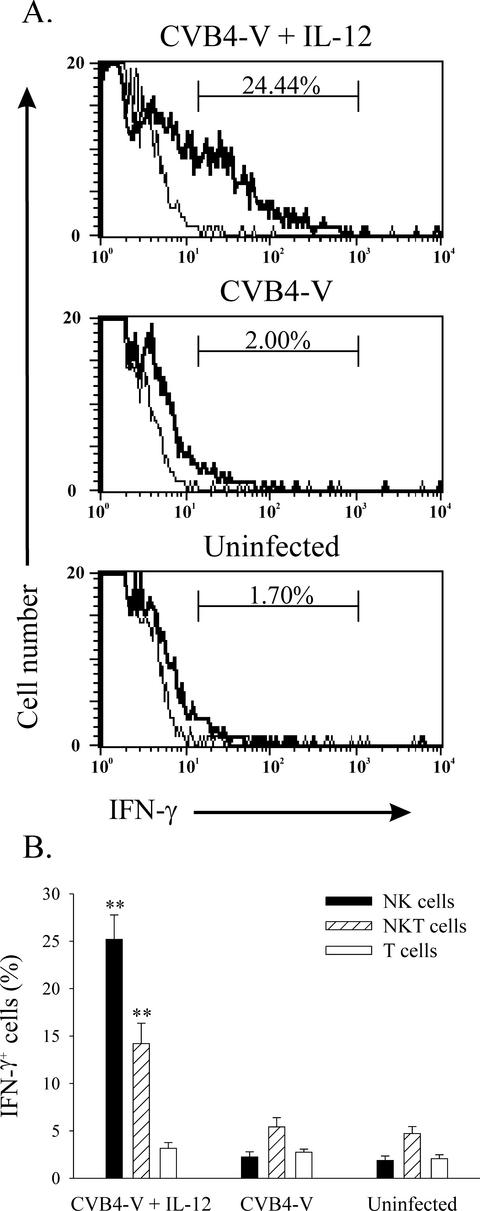
To determine the cellular source of IFN-γ after administration of IL-12, intracellular cytokine staining and flow cytometry was done. Splenocytes were labeled with the appropriate antibodies to distinguish NK, NKT, CD8, and CD4 T cells and subsequently labeled with an anti-IFN-γ antibody. Splenocytes from three groups of mice (CVB4-V, CVB4-V + IL-12, and uninfected), with each group containing six mice, were analyzed. Representative histograms for individual mice are shown in Fig. 5A, and a summary of the data is presented in Fig. 5B. After treatment of CVB4-V-infected mice with IL-12, there was a significant (P = 0.0026) increase in the percentage of NK cells (DX5+ TCRα/β−) staining for IFN-γ (Fig. 5A). In addition, a significant (P = 0.0026) increase in the percentage of NKT cells (DX5+ TCRα/β+) staining for IFN-γ (Fig. 5B) was observed after IL-12 treatment. A small percentage of T cells (TCRα/β+ DX5−) stained for IFN-γ. These cells were subsequently identified as CD8 T cells (data not shown). However, there was no significant difference in the percentage of T cells staining for IFN-γ among CVB4-V-infected, IL-12-treated mice, CVB4-V-infected mice, and uninfected mice (Fig. 5B).
FIG. 5.
Administration of IL-12 increases IFN-γ production by NK and NKT cells. Intracellular IFN-γ staining of splenocytes from IL-12-treated and CVB4-V-infected, CVB4-V-infected, and uninfected mice. (A) Representative histograms of IFN-γ staining (thick lines) NK cells and background staining (thin lines). Each panel represents data from one mouse. (B) Summary of IFN-γ staining NK cells, NKT cells, and T cells from CVB4-V-infected, IL-12-treated mice, CVB4-V-infected mice, and uninfected mice (n = 6 for each group). Theresults are expressed as the percentage of each cell population staining for IFN-γ minus the background staining. Mean values and standard deviations are shown. Statistically significant differences (P < 0.01) between IL-12-treated and untreated mice are denoted by asterisks.
IFN-γ is necessary for IL-12-mediated protection from lethal virus infection.
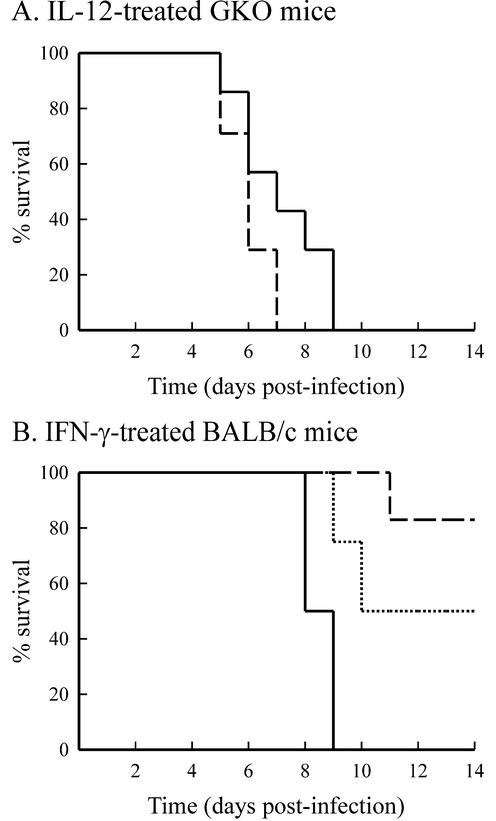
IL-12 treatment resulted in increased IFN-γ production early in infection. We next investigated whether IFN-γ was necessary for IL-12-mediated protection from lethal infection. To do so, BALB/c GKO mice were used. As was observed for CVB4-V-infected BALB/c mice, GKO mice succumbed to infection within 5 to 9 days (Fig. 6A). Unlike BALB/c mice, GKO mice treated with 500 ng of IL-12 also succumbed (100% mortality) to CVB4-V infection within 5 to 9 days (Fig. 6A).
FIG. 6.
Cytokine treatment of CVB4-V-infected mice. (A) Administration of IL-12 to CVB4-V-infected GKO mice was not protective. Each group contained seven mice. Curves: dashed line, 500 ng of IL-12; solid line, vehicle alone. (B) Administration of IFN-γ to CVB4-V-infected, BALB/c mice was protective. Each group contained four to six mice. IFN-γ treatment significantly (P < 0.05) increased survival of CVB4-V-infected mice. Curves: dotted line, 50 ng of IFN-γ; dashed line, 100 ng of IFN-γ; solid line, vehicle alone.
Exogenous IFN-γ protects BALB/c mice from lethal infection with CVB4-V.
During CVB4-V infection, little or no IFN-γ was detected in organs that support viral replication. However, administration of IL-12 resulted in increased production of serum and splenic IFN-γ early in infection. We next examined whether administration of IFN-γ would be sufficient to protect against lethal infection. Due to the short half-life of IFN-γ (25 to 35 min) (23) and the early burst of IFN-γ after exogenous administration of IL-12, CVB4-V-infected BALB/c mice were given multiple doses of IFN-γ during the first 2 days of infection. IFN-γ can be associated with toxicity (13). However, our IFN-γ treatment regimen was not associated with toxicity. Uninfected mice treated with multiple doses of IFN-γ gained weight similarly to untreated, control mice (data not shown). CVB4-V-infected BALB/c mice treated with 50 or 100 ng of IFN-γ on 0.5, 1, and 2 days after infection exhibited survival rates of 50 and 83%, respectively (Fig. 6B). The administration of exogenous IFN-γ significantly (P = 0.023) increased survival of CVB4-V-infected mice. We investigated whether IFN-γ treatment of CVB4-V-infected mice was able to protect the exocrine pancreas from extensive destruction. As was observed in IL-12-treated, CVB4-V-infected mice, intact acini were present in infected, IFN-γ-treated mice (Fig. 3D). In addition, ca. 50% of the acinar cells showed necrosis. Tissue damage was accompanied by a moderate inflammatory infiltrate consisting of lymphocytes.
DISCUSSION
We have shown that mice infected with a virulent strain of CVB4 and treated with IL-12 survive infection and are protected from developing chronic pancreatitis. The beneficial effect of IL-12 administered as a single dose immediately after viral infection was optimal at 500 ng. Higher doses of IL-12 were less effective and possibly associated with toxicity. Administration of IL-12 has been shown to be beneficial during infection with a variety of viruses (21). Treatment schedules (dosage and duration) that are protective vary for different model systems, suggesting that the underlying mechanisms by which IL-12 exerts its effect differ. For both lymphocytic choriomeningitis virus (LCMV) and murine cytomegalovirus (MCMV), daily low doses of IL-12 (1 or 10 ng) during the first week of infection were found to be beneficial, while higher doses led to wasting and death (3, 28). For encephalomyocarditis virus, a single dose of 20 ng of IL-12 administered 18 h prior to infection resulted in survival (29).
IL-12 is a potent inducer of IFN-γ both in vitro (6, 20) and in vivo (12, 14). To elucidate the mechanism by which IL-12 protects mice from developing chronic pancreatitis, we examined whether IL-12 exerts its effect via IFN-γ. Three lines of evidence suggest that the protective effect of IL-12 is due to IFN-γ. First, IL-12 treatment induced rapid production of IFN-γ. Both NK and NKT cells were identified as the source of IFN-γ. Second, IL-12 treatment of CVB4-V-infected mice lacking a functional IFN-γ gene was not protective. Third, the administration of IFN-γ to CVB4-V-infected mice had a beneficial effect similar to that observed with IL-12 treatment. Thus, as has been shown for encephalomyocarditis virus (29), LCMV (28), and MCMV (27), the beneficial effect of IL-12 during CVB4 infection is due to the induction of IFN-γ.
Studies by Biron and coworkers have helped to unravel the underlying mechanisms by which IL-12-induced IFN-γ is protective during infection with LCMV or MCMV (27, 28). In both model systems, IL-12-induced IFN-γ exerts an antiviral effect resulting in decreased viral replication in target organs. During LCMV infection, CD8 cytotoxic T cells play a major role in viral clearance (26). When LCMV-infected mice were treated with IL-12, there was an increase in the number of CD8 T cells in the spleen, as well as decreased viral replication, suggesting that IL-12-induced IFN-γ increases CTL activity, which results in a diminished viral load (28). During MCMV infection, NK cells play a major role in host defense (4, 37, 41). Treatment of MCMV-infected mice with IL-12 results in an accelerated NK cytotoxic response, decreased hepatitis and liver necrosis, and decreased viral replication (27). In this instance, IL-12-induced IFN-γ is thought to accelerate the NK cell response, which limits viral replication and liver damage. In these two model systems, IL-12-induced IFN-γ appears to act on different effector cells (CD8 T cells in LCMV infection; NK cells in MCMV infection) to exert an antiviral effect.
Since the beneficial effect of IL-12 in our model is mediated by the early induction of IFN-γ, we investigated whether IL-12 treatment altered CVB4-V replication in the target organ (pancreas). Viral loads in the pancreatic tissues of IL-12-treated and untreated mice were similar, indicating that the beneficial effect of IL-12 was not due to decreased viral replication in the target organ. Although treatment with IL-12 did not alter viral replication in the pancreas, IL-12 was able to protect the pancreas from extensive damage. In untreated mice, widespread damage eventually leads to exocrine pancreatic insufficiency, which is responsible for morbidity and mortality (31, 33). In mouse strains (B10, SJL) that survive infection, extensive pancreatic damage leads to chronic pancreatitis. BALB/c mice infected with CVB4-V and treated with IL-12 did not progress to chronic disease. Since IL-12 treatment protected the pancreas from damage and yet did not diminish viral replication, the data suggest that injury leading to exocrine pancreatic sufficiency in untreated mice is due to immunopathological mechanisms and not to virus-mediated destruction. Furthermore, the data suggest that IL-12 treatment is able to suppress the immunopathological mechanisms responsible for exocrine pancreatic damage. Suppression of immune-mediated damage prevents progression to chronic pancreatitis.
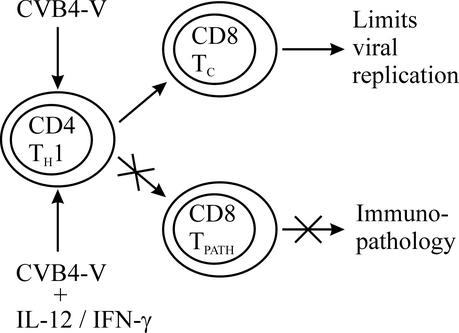
In our model of CVB4-V-induced chronic pancreatitis, we have shown that T cells are required for the survival of infection with CVB4-V, and yet T cells also exacerbate disease severity (33). Nude mice, which lack T-cell function, succumb to infection within 3 to 4 days. However, mice lacking CD4 T cells and mice depleted of CD8 T cells have survival rates of 100 and 50%, respectively. The dual nature of the T-cell response during CVB4-V infection suggests that T cells function both in limiting viral replication and in the immune-mediated destruction of the exocrine pancreas. Our working model is that CVB4-V infection results in the development of CD4 T cells of the Th1 subset, which activates both CD8 T cells (TC) that participate in controlling viral replication and CD8 T cells (TPATH) that cause immunopathology (Fig. 7). Two CD8 T-cell subsets are postulated, because administration of IL-12 did not alter viral replication in the target organ but did suppress the development of immunopathology associated with chronic pancreatitis. We infer that during treatment with IL-12 (or IFN-γ) the CD8 TC subset is functionally active and able to participate in limiting viral replication, while the effector function of the CD8 TPATH subset is suppressed. This model is supported by a recent study showing that during CVB3 infection T cells expressing the Vγ4 T-cell receptor initiate myocarditis through IFN-γ-mediated induction of Th1 cells (16). The CD4 Th1 cells then activate autoimmune CD8 effector T cells that are pathogenic. In the CVB3 model, IFN-γ is monitored by a highly sensitive method of intracellular staining and flow cytometric analysis. In our model, IFN-γ is measured by ELISA, a less sensitive assay with a detection limit of 70 pg per ml of sample. During CVB4-V infection, the amount of IFN-γ in the pancreas, heart, spleen, and serum is close to the detection limit of the ELISA. Our model predicts that low levels of IFN-γ, as measured by ELISA, drive the development of CD8 TPATH cells and also that a relatively large amount of IL-12 (or IFN-γ) administered immediately after infection is able to suppress the development or functioning of CD8 TPATH cells. Ongoing studies are focused on elucidating the mechanism by which IL-12-IFN-γ is able to suppress immunopathology during CVB4-V infection.
FIG. 7.
Model of IL-12-induced suppression of immunopathology during CVB4-V infection. Administration of IL-12 (or IFN-γ) early in infection prevents the development (or functioning) of effector CD8 T cells involved in immunopathology.
Acknowledgments
This work was supported by Public Health Service grants AI52705 (A.I.R.), HL62120 (D.W.M.), and AI41715 (D.W.M.) from the National Institutes of Health and by the American Heart Association (A.I.R.).
We thank the staff of the Department of Pathology for processing tissue samples for histology. We thank Andrew Reilly of the Computational Molecular Biology and Statistics Core Facility for doing the Cox proportional hazards regression. We thank Kenneth Class of the Immunology Core Facility for assistance with flow cytometry. The secretarial assistance of Maryellen Carl is greatly appreciated.
REFERENCES
- 1.Atkins, M. B., M. J. Robertson, M. Gordon, M. T. Lotze, M. DeCoste, J. S. DuBois, J. Ritz, A. B. Sandler, H. D. Edington, P. D. Garzone, J. W. Mier, C. M. Canning, L. Battiato, H. Tahara, and M. L. Sherman. 1997. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res. 3:409-417. [PubMed] [Google Scholar]
- 2.Babcock, G. F., and S. E. Frede. 1998. Intracellular cytokines, p. 9.9.1-9.9.10. In J. P. Robinson, Z. Darzynkiewicz, P. N. Dean, A. R. Hibbs, A. Orfao, P. S. Rabinovitch, and L. L. Wheeless (ed.), Current protocols in cytometry, vol. 2. John Wiley & Sons, Inc., New York, N.Y.
- 3.Biron, C. A., and J. S. Orange. 1995. IL-12 in acute viral infectious disease. Res. Immunol. 146:590-600. [DOI] [PubMed] [Google Scholar]
- 4.Bukowski, J. F., B. A. Woda, and R. M. Welsh. 1984. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J. Virol. 52:119-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caggana, M., P. Chan, and A. Ramsingh. 1993. Identification of a single amino acid residue in the capsid protein VP1 of coxsackievirus B4 that determines the virulent phenotype. J. Virol. 67:4797-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, S. H., B. Perussia, J. W. Gupta, M. Kobayashi, M. Pospisil, H. A. Young, S. F. Wolf, D. Young, S. C. Clark, and G. Trinchieri. 1991. Induction of interferon γ production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J. Exp. Med. 173:869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman, N. M., A. I. Ramsingh, and S. Tracy. 1997. Genetics of coxsackievirus virulence. Curr. Top. Microbiol. Immunol. 223:227-258. [DOI] [PubMed] [Google Scholar]
- 8.Conover, W. J. 1971. Practical nonparametric statistics. John Wiley & Sons, Inc., New York, N.Y.
- 9.Cox, D. R. 1972. Regression models and life-tables. J. R. Stat. Soc. Series B 34:187-220. [Google Scholar]
- 10.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 11.Gauntt, C. J. 1997. Roles of the humoral response in coxsackievirus B-induced disease. Curr. Top. Microbiol. Immunol. 223:259-282. [DOI] [PubMed] [Google Scholar]
- 12.Gazzinelli, R. T., S. Hieny, T. A. Wynn, S. Wolf, and A. Sher. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. USA 90:6115-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzel, F. P. 1990. The role of IFN-γ in the pathology of experimental endotoxemia. J. Immunol. 145:2920-2924. [PubMed] [Google Scholar]
- 14.Heinzel, F. P., D. S. Schoenhaut, R. M. Rerko, L. E. Rosser, and M. K. Gately. 1993. Recombinant interleukin 12 cures mice infected with Leishmania major. J. Exp. Med. 177:1505-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horwitz, M. S., L. M. Bradley, J. Harbertson, T. Krahl, J. Lee, and N. Sarvetnick. 1998. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat. Med. 4:781-785. [DOI] [PubMed] [Google Scholar]
- 16.Huber, S. A. S. 2002. Vγ4+ T cells promote autoimmune CD8+ cytolytic T-lymphocyte activation in coxsackievirus B3-induced myocarditis in mice: role for CD4+ Th1 cells. J. Virol. 76:10785-10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishikawa, H., and D. A. Lawrence. 1998. Differential production of interleukin-6 in the brain and spleen of mice treated with lipopolysaccharide in the presence or absence of lead. J. Toxicol. Environ. Health Pt. A 53:357-373. [DOI] [PubMed] [Google Scholar]
- 18.Klingel, K., C. Hohenadl, A. Canu, M. Albrecht, M. Seemann, G. Mall, and R. Kandolf. 1992. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc. Natl. Acad. Sci. USA 89:314-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klingel, K., S. Stephan, M. Sauter, R. Zell, B. M. McManus, B. Bultmann, and R. Kandolf. 1996. Pathogenesis of murine enterovirus myocarditis: virus dissemination and immune cell targets. J. Virol. 70:8888-8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi, M., L. Fitz, M. Ryan, R. M. Hewick, S. C. Clark, S. Chan, R. Loudon, F. Sherman, B. Perussia, and G. Trinchieri. 1989. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170:827-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komastu, T., D. D. Ireland, and C. S. Reiss. 1998. IL-12 and viral infections. Cytokine Growth Factor Rev. 9:277-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruisbeek, A. B. 2000. Isolation of mouse mononuclear cells, p. 3.1.1-3.1.5. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Stober (ed.), Current protocols in immunology, vol. 1. John Wiley & Sons, Inc., New York, N.Y. [DOI] [PubMed]
- 23.Kurzrock, R., M. G. Rosenblum, S. A. Sherwin, A. Rios, M. Talpaz, J. R. Quesada, and J. U. Gutterman. 1985. Pharmacokinetics, single-dose tolerance, and biological activity of recombinant γ-interferon in cancer patients. Cancer Res. 45:2866-2872. [PubMed] [Google Scholar]
- 24.Lowry, R. 1998-2003. Concepts and applications of inferential statistics. [Online.] http://faculty.vassar.edu/lowry/webtext.html
- 25.Melnick, J. L. 1996. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses, p. 655-712. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Fields virology, 3rd ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa.
- 26.Moskophidis, D., S. P. Cobbold, H. Waldmann, and F. Lehmann-Grube. 1987. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J. Virol. 61:1867-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orange, J. S., B. Wang, C. Terhorst, and C. A. Biron. 1995. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 182:1045-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orange, J. S., S. F. Wolf, and C. A. Biron. 1994. Effects of IL-12 on the response and susceptibility to experimental viral infections. J. Immunol. 152:1253-1264. [PubMed] [Google Scholar]
- 29.Ozmen, L., M. Aguet, G. Trinchieri, and G. Garotta. 1995. The in vivo antiviral activity of interleukin-12 is mediated by gamma interferon. J. Virol. 69:8147-8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pallansch, M. A. 1997. Coxsackievirus B epidemiology and public health concerns. Curr. Top. Microbiol. Immunol. 223:13-30. [DOI] [PubMed] [Google Scholar]
- 31.Ramsingh, A., J. Slack, J. Silkworth, and A. Hixson. 1989. Severity of disease induced by a pancreatropic Coxsackie B4 virus correlates with the H-2Kq locus of the major histocompatibility complex. Virus Res. 14:347-358. [DOI] [PubMed] [Google Scholar]
- 32.Ramsingh, A. I., N. Chapman, and S. Tracy. 1997. Coxsackieviruses and diabetes. Bioessays 19:793-800. [DOI] [PubMed] [Google Scholar]
- 33.Ramsingh, A. I., W. T. Lee, D. N. Collins, and L. E. Armstrong. 1999. T cells contribute to disease severity during coxsackievirus B4 infection. J. Virol. 73:3080-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romani, L., P. Puccetti, and F. Bistoni. 1997. Interleukin-12 in infectious diseases. Clin. Microbiol. Rev. 10:611-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose, N. R., and S. L. Hill. 1996. The pathogenesis of postinfectious myocarditis. Clin. Immunol. Immunopathol. 80:S92-S99. [DOI] [PubMed] [Google Scholar]
- 36.Schwimmbeck, P. L., S. A. Huber, and H. P. Schultheiss. 1997. Roles of T cells in coxsackievirus B-induced disease. Curr. Top. Microbiol. Immunol. 223:283-303. [DOI] [PubMed] [Google Scholar]
- 37.Shellam, G. R., J. E. Allan, J. M. Papadimitriou, and G. J. Bancroft. 1981. Increased susceptibility to cytomegalovirus infection in beige mutant mice. Proc. Natl. Acad. Sci. USA 78:5104-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tam, P. E., and R. P. Messner. 1999. Molecular mechanisms of coxsackievirus persistence in chronic inflammatory myopathy: viral RNA persists through formation of a double-stranded complex without associated genomic mutations or evolution. J. Virol. 73:10113-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251-276. [DOI] [PubMed] [Google Scholar]
- 40.Vella, C., C. L. Brown, and D. A. McCarthy. 1992. Coxsackievirus B4 infection of the mouse pancreas: acute and persistent infection. J. Gen. Virol. 73:1387-1394. [DOI] [PubMed] [Google Scholar]
- 41.Welsh, R. M., P. L. Dundon, E. E. Eynon, J. O. Brubaker, G. C. Koo, and C. L. O'Donnell. 1990. Demonstration of the antiviral role of natural killer cells in vivo with a natural killer cell-specific monoclonal antibody (NK 1.1). Nat. Immun. Cell Growth Regul. 9:112-120. [PubMed] [Google Scholar]