Abstract
A number of human immunodeficiency virus type 1 (HIV-1) non-B-subtype products have been developed for present or future vaccine trials; in Thailand, several studies using subtype B and/or CRF01_AE vaccines have been conducted. To better characterize the biologic properties of these subtypes, 70 HIV-1 subtype B and E isolates were phenotyped as syncytium-inducing (SI) or non-syncytium-inducing (NSI) isolates and assessed for sensitivity to neutralizing antibody (NAb). A significantly higher number of NSI subtype E viruses were neutralization sensitive than SI subtype E viruses (P = 0.009), while no association between viral phenotype and sensitivity to NAb was observed for subtype B (P = 0.856), suggesting a difference in the neutralization patterns of subtypes B and E. Strikingly, concurrent CD4 T-cell numbers were significantly lower for subtype E-infected patients whose isolates were more resistant to NAb, both for the overall study group (P < 0.001) as well as for the 22 patients with NSI isolates (P = 0.013). Characterization of the evolution of biologic properties of both B and non-B HIV-1 subtypes will provide a clearer understanding of the repertoire of antibodies that must be elicited for a vaccine to be effective against all phenotypes and subtypes.
The role of functional antibodies in human immunodeficiency virus type 1 (HIV-1) infection has been previously defined using subtype B reagents, and most vaccine studies to date have been conducted with subtype B products. However, the majority of the world's HIV-1 infections are caused by the following six subtypes or circulating recombinant forms: A, B C, D, CRF01_AE, and CRF02_AG (31, 35). Given the genetic diversity of HIV-1 and the geographic distribution of subtypes (19, 35), understanding the correlates of broadly reactive cellular and humoral immunity in patients infected with both B and non-B subtypes will be informative for the development of a global HIV-1 vaccine. Although the role of neutralizing antibody (NAb) in HIV protection and pathogenesis remains to be defined, eliciting broadly reactive NAb responses against primary isolates (PI) is a goal in most laboratories that are developing and testing HIV vaccine candidates.
Numerous studies have been done to characterize naturally induced antibody responses to the HIV-1 envelope (Env) in infected subjects. While conserved as well as type-specific Env epitopes clearly play a role in neutralization, interactions between the virus, antibodies, and host cells also influence in vitro neutralization sensitivity. A number of distinct differences between PI and T-cell line-adapted (TCLA) viruses have been identified. Many PIs show resistance to NAb, including monoclonal antibodies (MAbs) (24) and patient (27, 42) and vaccinee (18; J. Mascola, O. Weislow, S. Snyder, S. Belay, M. Yeager, F. McCutchan, J. McNeil, D. Burke, and M. C. Walker, abstract from the AIDS Vaccine Clinical Trials Network, AIDS Res. Hum. Retrovir. 10:S55, 1994) sera. It has been demonstrated that the sensitivity of PIs and TCLA HIV-1 isolates to neutralization by MAbs or CD4-based reagents (16, 34), as well as to polyclonal sera (6, 23), is independent of the coreceptor used by the virus. Most of these prior studies focused on subtype B isolates, while one report included five non-B subtypes; however, subtype E was not among those tested for the relationship between neutralization sensitivity and coreceptor usage or phenotype (6). While most TCLA viruses are syncytium inducing (SI) and utilize the CXCR4 (X4) coreceptor, a larger percentage of PIs are non-syncytium-inducing (NSI) isolates and utilize CCR5 (R5) as a coreceptor (44). In about 50% of subtype B-infected patients, a shift from early NSI monocyte-tropic isolates to a predominance of SI viruses later in disease occurs during the course of HIV-1 infection (1, 7, 15, 32, 45). In current vaccine trials, investigators have begun to shift from the use of Env proteins of SI isolates that are easy to propagate in T-cell lines to the use of Envs from NSI, R5-utilizing viruses. These isolates are thought to better represent early-stage transmitted or selected viruses (3, 4). It will be critical to dissect the immunogenicity of both R5 and X4 PI Envs and to characterize the NAb susceptibility of these HIV-1 biotypes within both B and non-B subtypes.
In this study of HIV-1 subtype E isolates from various stages of disease, 49 randomly assembled viruses (26 NSI and 23 SI) were tested for sensitivity to neutralization by pooled polyclonal antibodies. A total of 21 subtype B viruses were studied comparatively. This is the first report of a direct relationship between an indication of the host's immune status (CD4 cell count) and neutralization sensitivity of the concurrent replication-competent virus for subtype E-infected patients. The data also suggest a difference in neutralization sensitivity patterns among subtype E versus subtype B HIV-1 isolates of different phenotypes.
To establish the panel for studying differences in the neutralization of HIV-1 subtypes B and E, four NAb pools and 70 isolates were prepared. The NAb pools were from samples that had been serotyped as B or E with a V3 peptide enzyme-linked immunosorbent assay (36). Two pools of five subtype E plasmas (Ep5) or nine subtype E plasmas (Ep9) were prepared from specimens collected from 1994 to 1996 (Ep5) and 1998 to 1999 (Ep9) at the Army Institute of Pathology (AIP), Bangkok, Thailand. The two subtype B pools consisted of sera from 19 North American patients or plasma from 5 Thai patients infected with subtype B. The sera from the 19 North American patients and the plasma from the 5 Thai patients were collected from 1989 to 1992 (Walter Reed Army Medical Center, Washington, D.C.) and 1994 to 1996 (AIP), respectively. A total of 10 single plasma samples from subtype E-infected patients and Thai HIV-negative human plasma (NHP) samples were obtained from mucosal or natural history studies conducted at the Armed Forces Research Institute of Medical Sciences in Bangkok, Thailand, or from discarded blood bank samples from the AIP. The plasma or sera were heat inactivated, centrifuged, diluted (1:6.7), and filtered.
Patients from whom HIV-1-positive cultures were obtained were randomly selected for this study. A total of 33 isolates were obtained from natural history and mucosal studies conducted at the Armed Forces Research Institute of Medical Sciences. Seven maternal isolates were from a study conducted in Lampang, Thailand. Four isolates were from northern Thailand, and two isolates were from Siriraj Hospital in Bangkok, Thailand. One Thai isolate (92/TH023) was obtained from the UNAIDS/WHO working group, and one isolate (424896) was from a subject participating in a Royal Thai Army conscript screening. One additional subtype E isolate was from Indonesia (GS-025), and the subtype B viruses were from Brazil (BZ167), Haiti (92HT599), and the United States. Written informed consent was obtained from all subjects prior to venipuncture, and CD4 T-cell enumeration was conducted using standard two-color flow cytometry and a hematology analyzer. The Thai protocols were approved by the Ethical Review Boards of the Royal Thai Army and Thai Ministry of Public Health and by the office of the U.S. Surgeon General.
All clinical isolates were obtained by coculture of patient peripheral blood mononuclear cells (PBMC) (stimulated with phytohemagglutinin [PHA] for 3 to 4 days) obtained from HIV-negative donors, as previously described (29). Cellular DNA from cocultures was used for genotyping with subtype B- and E-specific gp41 PCR primers, as previously described (20). Viral isolates were passaged in PBMC to produce high-titer stocks and phenotyped as SI or NSI with an MT-2 cell assay (10).
Using fivefold virus dilutions and five replicate wells per dilution, the 50% tissue culture infectious dose (TCID50) for each virus stock was measured in PHA-stimulated PBMC. TCID50 values were calculated at day 8 by the Spearman-Karber method (10). A p24 antigen reduction neutralization assay was set up in quadruplicate, as previously described (29). The supernatant p24 was quantitated at days 4 and 8 postinfection. To normalize the amount of plasma or serum proteins and reduce the effects of nonspecific inhibition of HIV growth in NAb assays, we calculated the percentage of neutralization by using the level of p24 produced in the presence of HIV-NHP as a control. The percentage of reduction of p24 was calculated at day 4 or 8, when the control HIV-NHP supernatant p24 measured >2,000 pg/ml. The distribution of the data for all subtype E virus-E NAb pairs revealed the cutoff for the upper quartile (75th percentile) to be 84% neutralization. An isolate was considered to be sensitive when at least one NAb pool yielded neutralization values in the upper quartile (≥84%).
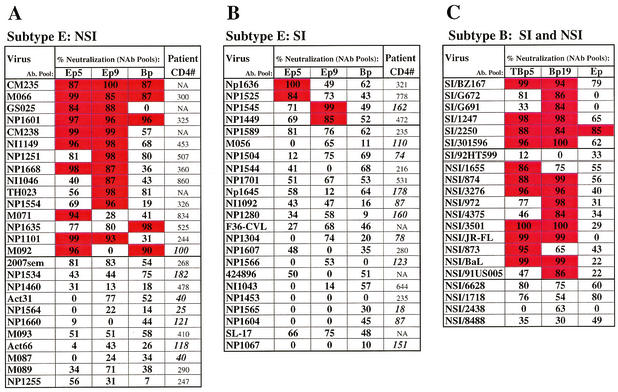
Neutralization sensitivity of subtype B and E isolates of different phenotypes.
The data for neutralization of all isolates are presented in Fig. 1. For subtype E viruses, percentages of neutralization for both E pools as well as for the stronger of the two B pools (column Bp) are presented (panels A and B); the data from both B pools and the most potent E pool (column Ep) are presented for each B virus (panel C). Compared with SI subtype E viruses, a significantly higher number of NSI subtype E viruses were neutralized (with neutralization values of at least 84%), while a larger proportion of the SI E isolates were not potently neutralized by any pool (panels A and B; P = 0.009). The number of subtype E virus-E NAb pairs with potent neutralization (≥84%) was significantly higher for the NSI (22/52 [42%]; panel A) than the SI (4/46 [9%]; panel B) subtype E viruses (P < 0.001). Furthermore, 35% (9/26) of the subtype E NSI viruses had neutralization values in the upper quartile (≥84%) for more than one pool, while 0 of 23 SI viruses showed this degree of sensitivity. Wilcoxon rank sum analysis revealed that when subtype E NAb pools were used, values for neutralization of the subtype E NSI isolates were significantly higher than the values for neutralization of SI E viruses (P < 0.001). Individually, neutralization values for NSI subtype E viruses were significantly higher than values for SI subtype E viruses for both Ep5 (P = 0.016) and Ep9 (P = 0.012). In contrast, no association between phenotype and susceptibility to neutralization was observed for the B viruses studied (P = 0.856; panel C); most of the SI or NSI B viruses were sensitive to one or more of the NAb pools (panel C). Comparing values for all B virus-B NAb pairs by Wilcoxon rank sum test showed no difference in values for NSI versus SI B isolates (P = 0.862).
FIG. 1.
The percentages of neutralization for each of three NAb pools are indicated. For subtype E viruses, the B pool with the highest percent neutralization is listed in the Bp column; for subtype B viruses, the data for the most potent E pool are shown under the Ep columns. Values of ≥84% (within the upper [4th] quartile of the distribution of data for all E NAb-E virus neutralization pairs) are indicated by red squares, while <84% neutralization is indicated by white squares. Viruses that were strongly neutralized (≥84%) by at least one pool were considered sensitive. These data represent the mean of two to six experiments (in quadruplicate) for each virus; the patient CD4 cell numbers coincident with virus isolation are indicated for subtype E isolates. CD4 counts of <200 are in bold and italicized (panels A and B). NA, data not available.
The NSI subtype E viruses were also more susceptible to cross-neutralization by subtype B NAb pools. As can be seen in Fig. 1A, 5 of 15 of the sensitive NSI subtype E viruses were strongly cross-neutralized by a B pool, while none of the SI E viruses showed ≥84% neutralization by a B pool (Fig. 1B). Only one sensitive SI subtype B virus (2250) was cross-neutralized by an E pool (Fig. 1C). Neutralization sensitivity for both subtype B and E viruses was not related to virus titer or level of virus growth at the endpoint of the NAb assays (data not shown).
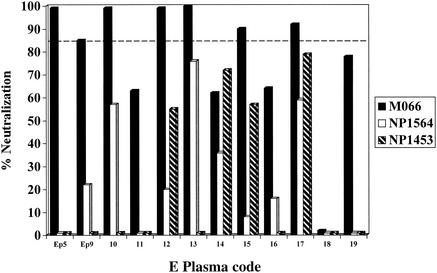
Neutralization of sensitive versus resistant viruses with individual plasmas.
The validity of typing the neutralization sensitivity of subtype E viruses with pooled polyclonal NAb was evaluated using single subtype E plasmas. The neutralization profiles of an NSI virus typed as sensitive (M066) and of the two most resistant (NSI/NP1564 and SI/NP1453) isolates were studied using 10 individual E plasmas (plasmas 10 to 19), as shown in Fig. 2. The M066 virus was potently neutralized (using the 84% cutoff) by 5 of 10 (50%) of the individual plasmas. In contrast, none of the single plasmas strongly neutralized either of the viruses typed as resistant. As previously observed by several laboratories, variation in the neutralization of PI is seen when using individual samples and the reactivities are often isolate or plasma specific. For example, plasma 18 is weak against all three viruses, while plasma 17 is broadly reactive. Additionally, 9 of 10 (90%) of these plasmas showed the strongest NAb activity against the sensitive M066 virus (Fig. 2). These data indicate that the results obtained using pooled polyclonal NAb to type viruses as sensitive or resistant are reflective of the proportion of samples from individual subtype E-infected patients that show neutralizing activity against these viruses.
FIG. 2.
Neutralization of sensitive versus resistant subtype E viruses using pooled and individual subtype E-infected patient plasmas. The percentages of neutralization by the two E NAb pools, as well as by 10 single E plasmas, are shown for three different viruses. M066 (black bars) is an NSI-sensitive isolate (strongly neutralized by three NAb pools), while NP1564 (white bars) and NP1453 (hatched bars) are the most resistant NSI and SI isolates. The dashed line indicates the 84% neutralization cutoff for sensitive viruses.
Coreceptor usage and neutralization sensitivity of subtype E viruses.
The coreceptor preferences of NSI and SI subtype E isolates were tested to assess whether neutralization sensitivity showed any relationship to sensitivity of the viruses to inhibition by a coreceptor ligand. A total of 10 viruses of each phenotype were tested for the use of R5, as determined in PBMC by inhibiting infection with the R5 ligand, RANTES (Regulated upon Activation, Normal T cell Expressed and Secreted). Briefly, using magnetic bead selection (Dynal, Inc.), PBMC were depleted of CD8+ cells and PHA stimulated. Duplicate aliquots of cells were incubated with 1 μg of RANTES/ml (or medium alone) for 2 h. Virus (at 200 to 500 TCID50) was then added, and cells were infected overnight, washed, and grown in cultures in complete RPMI-interleukin-2 alone (control) or in complete RPMI-interleukin-2 containing 250 ng of RANTES/ml. On day 4, using KC57-fluorescein isothiocyanate MAb (Beckman-Coulter) and surface CD4-phycoerythrin (Becton Dickinson) as previously described (9), cells were stained for intracellular p24. The number of p24 antigen-positive cells was enumerated by flow cytometry using a FACSCalibur apparatus, and the percentages of reduction of infected cells (in the presence of RANTES) were calculated in comparison to those of control media. When ≥50% inhibition of cell infection in the presence of RANTES was observed, the virus was considered to be an R5-utilizing virus. The formation of syncytia in MT-2 cells indicated the use of the X4 coreceptor. As shown in Table 1, all six of the sensitive NSI viruses (as well as all four of the more resistant NSI viruses) were >90% inhibited by RANTES, indicating a strong preference for the R5 coreceptor. Sensitivity to RANTES inhibition did not appear to be related to the neutralization sensitivity of NSI isolates. In contrast, 9 of 10 SI subtype E viruses showed little or no inhibition by RANTES, indicating a preference for X4 usage. Interestingly, one of the SI viruses (NP1589) that was moderately neutralized was a dualtropic R5/X4 isolate (Table 1), suggesting that this type of isolate might be an intermediate variant. The predominant replicating virus in vivo may transition from R5 using to dualtropic to X4 using and from neutralization sensitive to neutralization resistant.
TABLE 1.
Coreceptors used by HIV-1 subtype CRF01_AE isolates
| Virus isolate phenotype (isolate name/yr of isolation) | Subtype (MT-2 assay/ X4a) | % Inhibi- tion by RANTES/ R5b | Coreceptor preference (X4 and/or R5) |
|---|---|---|---|
| NSI | |||
| Neutralization sensitive | |||
| CM235/1991 | E | 94 | R5 |
| NP1601/1996 | E | 100 | R5 |
| NI1149/1996 | E | 100 | R5 |
| NP1251/1998 | E | 100 | R5 |
| NP1668/1997 | E | 100 | R5 |
| NP1635/1997 | E | 100 | R5 |
| Neutralization resistant | |||
| NP1460/1997 | E | 100 | R5 |
| 2007sem/1998 | E | 100 | R5 |
| NP1564/1996 | E | 100 | R5 |
| ACT-66/1998 | E | 96 | R5 |
| BaL/controlc | B | 83 | R5 |
| SI | |||
| Neutralization sensitive | |||
| NP1636/1997 | E | 0 | X4 |
| NP1525/1997 | E | 0 | X4 |
| NP1545/1996 | E | 0 | X4 |
| NP1449/1998 | E | 0 | X4 |
| Neutralization resistant | |||
| NP1589/1996 | E | 50 | R5/X4 |
| NI1043/1996 | E | 0 | X4 |
| NP1453/1997 | E | 0 | X4 |
| NP1565/1996 | E | 0 | X4 |
| NP1604/1996 | E | 0 | X4 |
| NP1067/1996 | E | 16 | X4 |
| NP03/controlc | E | 0 | X4 |
The use of X4 was deduced by viral induction of syncytium formation in MT-2 cells.
Usage of R5 was assessed by ≥50% reduction of cell infection by RANTES.
The NSI subtype B virus, HIV-1 BaL, was used as a control for R-5, and the cell line-adapted SI subtype E HIV-1 NP03 isolate was used as a control for X4.
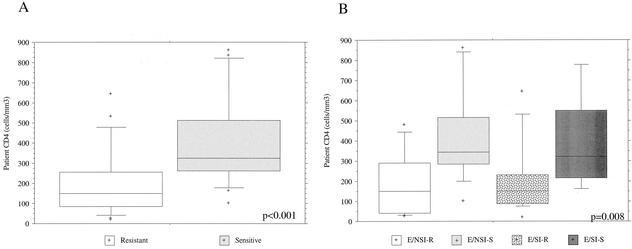
Relationship between patient CD4 cell count and neutralization sensitivity of concurrent isolates.
The concurrent patient CD4 cell counts for 42 of 49 of the subtype E isolates are indicated in Fig. 1A and B. The range of patient CD4 cell counts was 18 to 860 cells/μl. More than half (11/20; 55%) of the subtype E-infected patients with SI isolates had CD4 cells below 200, while 68% (15/22) of patients with NSI subtype E virus infections had CD4 cell counts of >200 cells/mm3 (Fig. 1A and B). The overall relationship between the neutralization sensitivity of subtype E virus isolates and concurrent patient CD4 cell count is shown in Fig. 3A. When all subtype E isolates were separated into the two groups of neutralization-sensitive versus less sensitive or neutralization-resistant viruses, the concurrent patient CD4 cell counts were found to be significantly lower in patients with resistant viruses (P < 0.001; Mann-Whitney U test). Given the observation that subtype E SI viruses are likelier to be neutralization resistant (Fig. 1) and that SI isolates are more frequently found later in disease, this association might be expected. Strikingly, even within the subset of 22 patients with NSI subtype E isolates, subjects with less-sensitive viruses had significantly lower CD4 cell numbers (Wilcoxon rank sum test; P = 0.013). The box plots shown in Fig. 3B provide a breakdown of neutralization sensitivity and CD4 counts within each phenotype. These data demonstrate that within the two groups of patients having either NSI or SI isolates, those patients with viruses less sensitive to NAb tended to have significantly lower CD4 cell counts independent of viral phenotype. Comparison of the four groups by a Kruskal-Wallis test demonstrated a significant difference between the groups (P = 0.008; Fig. 3B). For the subtype B viruses characterized, there were not sufficient CD4 data available to perform a similar analysis.
FIG. 3.
Differences in CD4 values of subtype E-infected patients with neutralization-sensitive and -resistant viruses. (A) All subtype E isolates were separated into two groups, based on neutralization sensitivity or resistance. The box plots show the distribution of concurrent patient CD4 values for each patient group, and the solid line represents the median. The boxed or shaded area displays the 25th to 75th percentiles, the top and bottom bars identify the 10th and 90th percentiles, and all plus signs represent CD4 counts falling outside the 10th and 90th percentiles. These data were analyzed by the Mann-Whitney U test (P < 0.001). (B) Box plots were prepared as described for panel A, except that CD4 counts for subtype E-infected patients having NAb-sensitive (S) or -resistant (R) isolates were further divided into four groups on the basis of SI or NSI viral phenotypes. There was a difference within the four groups as determined by a Kruskal-Wallis test (P = 0.008); the CD4 counts for patients with NSI-resistant viruses were significantly lower than those for patients with NSI-sensitive isolates (P = 0.013; Wilcoxon rank sums).
The identification of HIV-1 isolates of both SI and NSI phenotypes with diminished sensitivity to NAbs is critical in identifying factors involved in viral resistance to antibody-mediated control (27, 28). The neutralization resistance of PIs has remained an obstacle in HIV vaccine development (5, 8, 26, 42). This is one of the first studies to characterize a large number of viruses of a non-B subtype with respect to phenotype, coreceptor usage, and neutralization sensitivity and to relate these data to a contemporaneous clinical marker.
Early findings using TCLA isolates (especially cell line-passaged isolates like HIV-1MN) demonstrated the exquisite neutralization sensitivity of SI viruses (14, 49; Mascola et al., AIDS Res. Hum. Retrovir. 10:S55, 1994). Subsequent reports of vaccine-induced antibodies that neutralized SI PIs indicated that HIV vaccines could indeed induce PI neutralization, although these SI B viruses (such as BZ167) were atypically sensitive to NAb (25, 46, 47). It is now thought that data indicating that TCLA viruses are neutralization sensitive inaccurately resulted in the assumption that SI PIs are more sensitive to neutralization than NSI isolates. Among subtype B isolates, there appears to be a range of NAb sensitivities within each phenotype, indicating that for subtype B, phenotype is unrelated to neutralization sensitivity (6). Our data support this hypothesis. However, our study is the first to suggest that subtype E viruses might behave differently. Subtypes involved in temporally newer epidemics, such as subtype E (19-21), may retain an association between phenotype and overall sensitivity to antibody. As the subtype E epidemic matures and Env proteins increase in diversity (21), associations of this nature may be less pronounced. This may be affected by properties of the viruses, as well as the antibodies, which reflect host immune responses to different antigenic structures in the circulating quasispecies.
It has been shown that the genetic diversity within a particular isolate is not directly related to the general susceptibility of that virus population to NAb. Subtypes B, C, D, and E were studied, and higher levels of Env genetic diversity did not correlate with increased resistance to neutralization (11). Ongoing studies will determine whether there is a difference in genetic diversity within the sensitive NSI versus resistant SI subtype E isolates tested for this report. Although the quasispecies in donors transmitting HIV-1 may be heterogeneous, many recipients (early after infection) appear to acquire a relatively homogeneous viral population, indicating that specific variants are selectively transmitted or that the most prevalent variant has the highest probability of establishing infection (41, 43, 45). A recent report demonstrated that in the chimpanzee animal model, differential selection of HIV-1 species occurred during parenteral versus mucosal transmission (39). Clearly, virus- and host-specific factors play a role in defining the immune responses required to prevent transmission of different HIV-1 biotypes.
Early in infection, polymorphisms have been shown to exist in Env-coreceptor interactions. Changes in Env C1 and C4 were shown to account for phenotypic differences and differing sensitivities of isolates, from early infection to inhibition by beta-chemokines (17). Thus, not only V3 but also certain constant regions in Env affect virus-receptor interactions. It is interesting that subtype B SI viruses appear to be more promiscuous regarding coreceptor usage; one report showed that 4 of 6 SI subtype B isolates used both X4 and R5, while 9 of 9 SI isolates from clades A, C, D, E, and F used only X4. The majority of NSI viruses of all clades tested have shown preferential usage of R5 (33, 44). Progression from the R5 to the X4 phenotype has been suggested to occur due to the presence of a multi- or dualtropic intermediate Env (12); multiple domains, including V3 and V4/V5 (12) and C1 or C4 (17), may contribute to this transition.
In this study, 10 of 10 NSI viruses showed a strong preference for use of the R5 coreceptor, while 9 of 10 SI viruses preferentially used X4 and one dual X4/R5 SI isolate was identified. These data are in agreement with what has been previously reported for subtype B-infected patients (6, 17, 33). In a study of coreceptor usage and RANTES sensitivity of NSI isolates from subtype B-infected patients with AIDS, all NSI isolates, regardless of the clinical status of the patient, were dependent on R5 expression for entry. Broadening of coreceptor usage by NSI viruses from patients with AIDS was not observed. It was hypothesized that virus variants with decreased sensitivity to RANTES inhibition can evolve during disease progression not only in patients who undergo a switch from NSI to SI phenotype but also in patients who develop AIDS while maintaining R5 isolates (13). In our study, within the group of viruses that used R5, neutralization-sensitive and -resistant viruses did not appear to be differentially sensitive to RANTES inhibition (Table 1).
The biotype of HIV-1 has been associated with various aspects of infectivity; for example, other molecules on both the target cell membrane and in the Env can play a role in viral infectivity. It has been shown that viruses that use R5 have a higher ratio of major histocompatibility complex class II to lymphocyte function-related molecule 1 adhesion molecules in Env than viruses that use X4 (2). The proportions of host cell molecules in the Env of subtype E viruses of different phenotypes may contribute in some way to their neutralization sensitivities, and this may differ among subtypes. In addition, increased sensitivity to neutralization of simian immunodeficiency virus was recently shown to be associated with a decreased dependence on CD4 for virus entry (22). HIV-1 CD4 affinity may also contribute to the NAb sensitivity of different subtypes and phenotypes.
During the course of molecular evolution and disease progression, a series of changes that impact coreceptor usage or overall sensitivity to NAb may occur independently and contribute separately to pathogenesis in the subtype E-infected host. These changes are probably unrelated temporally, as some patients who retain NSI viruses develop neutralization-resistant isolates while a subset of patients who have undergone the switch to a predominant SI phenotype maintain isolates that are sensitive to heterologous NAb (Fig. 1). It remains to be determined whether any relationship exists between cytopathicity, replication fitness, coreceptor use, and neutralization sensitivity within different subtypes of HIV-1. Our study suggests that for subtype E-infected patients with disease progression and lowered CD4 cell numbers, the replication-competent viruses are less sensitive to heterologous NAb. This observation implies that sensitivity to antibody plays a role in viral selection during pathogenesis and that both SI and NSI viruses are subjected to this immune selective pressure. Recent reports have highlighted the rapid evolution of the autologous NAb response in vivo, which appears to be accompanied by complete replacement of neutralization-sensitive viruses by successive populations of resistant virus (30, 40). Wei et al. (40) have proposed an evolving glycan shield in which mutations confer the loss or acquisition of neutralization sensitivity in a context-dependent manner. They hypothesize that shifting the shield selectively accommodates receptor binding, but not NAb binding, thereby altering neutralization sensitivity (40). In light of these findings, it will be interesting to assess the glycosylation patterns in the Envs of sensitive versus resistant subtype E isolates.
Development of an effective global vaccine may require extensive characterization of the complete repertoire of antigenic structures and immunologic groupings of HIV-1. This would contribute to the rational choice of the minimum number of subtypes and biotypes that best represent the antigenic spectrum and induce the broadest cross-protective immune responses (37, 38, 48). The choice of using NSI/R5, SI/X4, or a combination of both types of HIV-1 PI Envs as vaccine components should therefore be carefully evaluated for all geographic subtypes.
Acknowledgments
We thank Pricha Singaraj, Puangmalee Buapunth, the staff of the Joint Clinical Research Center (JCRC) at AFRIMS in Bangkok, Thailand, and the clinical protocol volunteers for their invaluable contributions. For technical support, we acknowledge Chiraphan Khannapho, Rapee Trichavaroj, Nongluck Sannoi, Anna Sambor, and Shafaq Presswala. Viral isolates or clinical samples were contributed by Ruengpung Sutthent, Penprapa Chanbancherd, George Watt, Suda Louisirirotchanakul, and the NIH AIDS Research and Reference Reagent Program, Rockville, Md. We thank Phil Renzullo and Robin Garner for critical statistical analyses.
This work was supported by the U.S. Armed Forces Research Institute for Medical Sciences in Bangkok, Thailand, and the Henry M. Jackson Foundation Cooperative Agreement (no. DAMD17-98-2-7007).
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
REFERENCES
- 1.Asjo, B., L. Morfeldt-Manson, J. Albert, G. Biberfeld, A. Karlsson, K. Lidman, and E. M. Fenyo. 1986. Replicative properties of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet ii:660-662. [PubMed] [Google Scholar]
- 2.Bastiani-Lallos, L., D. Cecilia, E.-M. Fenyo, S. Laal, and S. Zolla-Pazner. 2000. HIV phenotype correlates with the relative amounts of lymphocyte function-related molecule 1 (LFA-1) and major histocompatibility complex (MHC) class II in the virion envelope. AIDS 14:1523-1531. [DOI] [PubMed] [Google Scholar]
- 3.Berman, P. W., W. Huang, L. Riddle, A. M. Gray, T. Wrin, J. Vennari, A. Johnson, M. Klaussen, H. Prashad, C. Kohne, C. deWit, and T. J. Gregory. 1999. Development of bivalent (B/E) vaccines able to neutralize CCR5-dependent viruses from the United States and Thailand. Virology 265:1-9. [DOI] [PubMed] [Google Scholar]
- 4.Brown, A. E., and J. G. McNeil. 1998. HIV vaccine development: a subtype E-specific strategy. Southeast Asian J. Trop. Med. Public Health 29:377-382. [PubMed] [Google Scholar]
- 5.Burton, D. R., and J. P Moore. 1998. Why do we not have an HIV vaccine and how can we make one? Nat. Med. 4:495-498. [DOI] [PubMed] [Google Scholar]
- 6.Cecilia, D., V. N. Kewalramani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 72:6988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng-Mayer, C., D. Seto, M. Tateno, and J. A. Levy. 1988. Biological features of HIV-1 that correlate with virulence in the host. Science 240:80-82. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, J. 2001. AIDS vaccines show promise after years of frustration. Science 291:1686-1689. [DOI] [PubMed] [Google Scholar]
- 9.Darden, J. M., V. R. Polonis, M. de Souza, S. Chantakulkij, A. Brown, D. L. Birx, and K. Pattanapanyasat. 2000. A flow cytometric method for measuring neutralization of HIV-1 subtype B and E primary isolates. Cytometry 40:141-150. [PubMed] [Google Scholar]
- 10.Division of AIDS, National Institute of Allergy and Infectious Diseases. 1997. DAIDS virology manual for HIV laboratories. Publication NIH-97-3828. U.S. Department of Health and Human Services, Washington, D.C.
- 11.Gordon, C. J., and E. L. Delwart. 2000. Genetic diversity of primary HIV-1 isolates and their sensitivity to antibody-mediated neutralization. Virology 272:326-330. [DOI] [PubMed] [Google Scholar]
- 12.Hu, Q.-X., A. P. Barry, Z.-X. Wang, S. M. Connolly, S. C. Peiper, and M. L. Greenberg. 2000. Evolution of the human immunodeficiency virus type 1 envelope during infection reveals molecular corollaries of specificity for coreceptor utilization and AIDS pathogenesis. J. Virol. 74:11858-11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansson, M., E. Backstrom, A. Bjorndal, V. Holmberg, P. Rossi, E-M. Fenyo, M. Popovic, J. Albert, and H. Wigzell. 1999. Coreceptor usage and RANTES sensitivity of non-syncytium-inducing HIV-1 isolates obtained from patients with AIDS. J. Hum. Virol. 2:325-338. [PubMed] [Google Scholar]
- 14.Katzenstein, D. A., L. K. Vujcic, A. Latif, R. Boulos, N. A. Halsey, T. C. Quinn, S. C. Rastogi, and G. V. Quinnan, Jr. 1990. Human immunodeficiency virus neutralizing antibodies in sera from North Americans and Africans. J. Acquir. Immune Defic. Syndr. 3:810-816. [PubMed] [Google Scholar]
- 15.Koot, M., I. P. M. Keet, A. H. V. Vos, R. E. Y. Degoede, M. T. L. Roos, R. A. Coutinho, F. Miedema, P. T. A. Schellekens, and M. Tersmette. 1993. Prognostic value of HIV-1 synctium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann. Intern. Med. 118:681-688. [DOI] [PubMed] [Google Scholar]
- 16.LaCasse, R. A., K. E. Follis, T. Moudgil, M. Trahey, J. M. Binley, V. Planelles, S. Zolla-Pazner, and J. H. Nunberg. 1998. Coreceptor utilization by human immunodeficiency virus type 1 is not a primary determinant of neutralization sensitivity. J. Virol. 72:2491-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis, J., P. Balfe, C. Arnold, S. Kaye, R. S. Tedder, and J. A. McKeating. 1998. Development of neutralizing antibody response during acute primary human immunodeficiency virus type 1 infection and the emergence of antigenic variants. J. Virol. 72:8943-8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews, T. J. 1994. Dilemma of neutralization resistance of HIV-1 field isolates and vaccine development. AIDS Res. Hum. Retrovir. 10:631-632. [DOI] [PubMed] [Google Scholar]
- 19.McCutchan, F. E. 2000. Understanding the genetic diversity of HIV-1. AIDS 14:S31-S44. [PubMed] [Google Scholar]
- 20.McCutchan, F. E., P. A. Hegerich, T. P. Brennan, P. Phanuphak, P. Singaraj, A. Jugsudee, P. W. Berman, A. M. Gray, A. K. Fowler, and D. S. Burke. 1992. Genetic variants of HIV-1 in Thailand. AIDS Res. Hum. Retrovir. 8:1887-1895. [DOI] [PubMed] [Google Scholar]
- 21.McCutchan, F. E., K. Viputtigul, M. S. de Souza, J. K. Carr, L. E. Markowitz, P. Buapunth, J. G. McNeil, M. L. Robb, S. Nitayaphan, D. L. Birx, and A. E. Brown. 2000. Diversity of envelope glycoprotein from human immunodeficiency virus type 1 of recent seroconverters in Thailand. AIDS Res. Hum. Retrovir. 16:801-805. [DOI] [PubMed] [Google Scholar]
- 22.Means, R. E., T. Matthews, J. A. Hoxie, M. H. Malim, T. Kodama, and R. C. Desrosiers. 2001. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J. Virol. 75:3903-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montefiori, D. C., R. G. Collman, T. R. Fouts, J. Y. Zhou, M. Bilska, J. A. Hoxie, J. P. Moore, and D. P. Bolognesi. 1998. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 by sera from infected individuals is independent of coreceptor usage. J. Virol. 72:1886-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore, J. P., Y. Cao, L. Qing, Q. J. Sattentau, J. Pyati, R. Koduri, J. Robinson, C. F. Barbas III, D. R. Burton, and D. D. Ho. 1995. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J. Virol. 69:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore, J. P., and D. C. Montefiori. 1997. Neutralization assays using the BZ167 strain of human immunodeficiency virus type 1. J. Infect. Dis. 176:1410.. [DOI] [PubMed] [Google Scholar]
- 26.Nable, G. J. 2001. Challenges and opportunities for the development of an AIDS vaccine. Nature 410:1002-1007. [DOI] [PubMed] [Google Scholar]
- 27.Parren, P. W. H. I., M. Wang, A. Trkola, J. M. Binley, M. Purtscher, H. Katinger, J. P. Moore, and D. R. Burton. 1998. Antibody neutralization-resistant primary isolates of human immunodeficiency virus type 1. J. Virol. 72:10270-10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parren, P. W. H. I., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13:S137-S162. [PubMed] [Google Scholar]
- 29.Polonis, V. R., M. de Souza, P. Chanbancherd, S. Chantakulkij, A. Jugsudee, L. D. Loomis-Price, T. C. Vancott, R. Garner, L. E. Markowitz, A. E. Brown, and D. L. Birx. 2001. HIV type 1 subtype E infected patients with broadened, dual (B/E) V3 loop serology have increased cross-neutralizing antibodies. AIDS Res. Hum. Retrovir. 17:69-79. [DOI] [PubMed] [Google Scholar]
- 30.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 100:4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson, D. L., J. P. Anderson, J. A. Bradac, J. K. Carr, B. Foley, R. K. Funkhouser, F. Gao, B. H. Hahn, M. L. Kalish, C. Kuiken, G. H. Learn, T. Leitner, F. McCutchan, S. Osmanov, M. Peeters, D. Pieniazek, M. Salminen, P. M. Sharp, S. Wolinsky, and B. Korber. 2000. HIV-1 nomenclature proposal. Science 288:55-56. [DOI] [PubMed] [Google Scholar]
- 32.Tersmette, M., J. M. Lange, R. E. Y. de Goede, F. de Wolf, J. K. Eeftink Schattenkerk, P. T. Schellekens, R. A. Coutinho, J. G. Huisman, J. Goudsmit, and F. Miedema. 1989. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet i:983-985. [DOI] [PubMed]
- 33.Trkola, A., W. A. Paxton, S. P. Monard, J. A. Hoxie, M. A. Siani, D. A. Thompson, L. Wu, C. R. Mackay, R. Horuk, and J. P. Moore. 1998. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J. Virol. 72:396-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trkola, A., T. Ketas, V. N. Kewalramani, F. Endorf, J. M. Binley, H. Katinger, J. Robinson, D. R. Littman, and J. P. Moore. 1998. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J. Virol. 72:1876-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.UNAIDS. 2000. Report on the global HIV/AIDS epidemic. Report 13E, Joint United Nations Programme on HIV/AIDS. UNAIDS, Geneva, Switzerland.
- 36.VanCott, T. C., F. R. Bethke, A. W. Artenstein, F. E. McCutchan, J. G. McNeil, J. R. Mascola, R. R. Redfield, and D. L. Birx. 1994. Serotyping international HIV-1 isolates by V3 peptides and whole gp160 proteins using BIAcore. Methods Enzymol. 6:188-198. [Google Scholar]
- 37.Van der Groen, G., P. N. Nyambi, E. Beirnaert, D. Davis, K. Fransen, L. Heyndrickx, P. Ondoa, G. van der Auwera, and W. Janssens. 1998. Genetic variation of HIV type 1: relevance of interclade variation to vaccine development. AIDS Res. Hum. Retrovir. 14:S211-S221. [PubMed] [Google Scholar]
- 38.Verrier, F., S. Burda, R. Belshe, A. M. Duliege, J.-L. Excler, M. Klein, and S. Zolla-Pazner. 2000. A human immunodeficiency virus prime-boost immunization regimen in humans induces antibodies that show interclade cross-reactivity and neutralize several X4-, R5-, and dualtropic clade B and C primary isolates. J. Virol. 74:10025-10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei, Q., and P. N. Fultz. 2002. Differential selection of specific human immunodeficiency virus type 1/JC499 variants after mucosal and parenteral inoculation of chimpanzees. J. Virol. 76:851-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 41.Wolfs, T. F. W., G. Zwart, M. Bakker, and J. Goudsmit. 1992. HIV-1 genomic RNA diversification following sexual and parenteral virus transmission. Virology 189:103-110. [DOI] [PubMed] [Google Scholar]
- 42.York, J., K. E. Follis, M. Trahey, P. N. Nyambi, S. Zolla-Pazner, and J. H. Nunberg. 2001. Antibody binding and neutralization of primary and T-cell line-adapted isolates of human immunodeficiency virus type 1. J. Virol. 75:2741-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, L. Q., P. MacKenzie, A. Cleland, E. C. Holmes, A. J. Leigh-Brown, and P. Simmonds. 1993. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J. Virol. 67:3345-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, Y. J., T. Dragic, Y. Cao, L. Kostrikis, D. S. Kwon, D. R. Littman, V. N. Kewalramani, and J. P. Moore. 1998. Use of coreceptors other than CCR5 by non-syncytium-inducing adult and pediatric isolates of human immunodeficiency virus type 1 is rare in vitro. J. Virol. 72:9337-9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu, T. F., H. M. Mo, N. Wang, D. S. Nam, Y. Z. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]
- 46.Zolla-Pazner, S., C. Alving, R. Belshe, P. Berman, S. Burda, P. Chigurupati, M. L. Clements, A.-M. Duliege, J.-L. Excler, J. Kahn, M. J. McElrath, S. Sharpe, F. Sinangil, K. Steimer, M. C. Walker, N. Wassef, and S. Xu. 1997. Neutralization of clade B primary isolates by sera from human immunodeficiency virus-uninfected recipients of candidate AIDS vaccines. J. Infect. Dis. 175:764-774. [DOI] [PubMed] [Google Scholar]
- 47.Zolla-Pazner, S., S. Xu, S. Burdqa, A.-M. Duliege, J.-L. Excler, and M. L. Clements-Mann. 1998. Neutralization of syncytium-inducing primary isolates by sera from human immunodeficiency virus (HIV)-uninfected recipients of candidate HIV vaccines. J. Infect. Dis. 178:1502-1506. [DOI] [PubMed] [Google Scholar]
- 48.Zolla-Pazner, S., K. G. Miroslaw, and P. N. Nyambi. 1999. The implications of antigenic diversity for vaccine development. Immunol. Lett. 66:159-164. [DOI] [PubMed] [Google Scholar]
- 49.Zwart, G., N. K. Back, C. Ramautarsing, M. Valk, L. van der Hoek, and J. Goudsmit. 1994. Frequent and early HIV-1MN neutralizing capacity in sera from Dutch HIV-1 seroconverters is related to antibody reactivity to peptides from the gp120 V3 domain. AIDS Res. Hum. Retrovir. 10:245-251. [DOI] [PubMed] [Google Scholar]