Abstract
The mechanisms of immune evasion and the role of the early immune response in chronic infection caused by hepatitis C virus (HCV) are still unclear. Here, we present evidence for a cascade of molecular events that the virus initiates to subvert the innate immune attack. The HCV core protein induced p53-dependent gene expression of TAP1 (transporter associated with antigen processing 1) and consecutive major histocompatibility complex (MHC) class I upregulation. Moreover, in p53-deficient liver cell lines, only reconstitution with wild-type p53, but not mutated p53 lacking DNA binding capacity, showed this effect. As a consequence of increased MHC class I expression, a significantly downregulated cytotoxic activity of natural killer (NK) cells against HCV core-transfected liver cells was observed, whereas lysis by HCV-specific cytotoxic T cells was not affected. These results demonstrate a way in which HCV avoids recognition by NK cells that may contribute to the establishment of a chronic infection.
Hepatitis C virus (HCV) acts as a major causative agent of chronic hepatitis, cirrhosis, and hepatocellular carcinoma. To date, HCV infection is one of the primary causes of liver transplantation in the United States and other countries. More than 170 million individuals have been reported to be seropositive worldwide (40). There is no sufficient prophylaxis by vaccination, and antiviral therapy with a combination of alpha interferon (IFN-α) and ribavirin is effective only in selected patients. This underlines an urgent need to improve our knowledge of virus-cell interactions and immune escape mechanisms. Elucidation of the mechanisms by which HCV interacts with and regulates the immune response, especially in the early stages of infection, is likely to lead to productive insights into how to combat chronic infection and prevent its serious complications.
HCV has been classified as the sole member of a distinct genus called Hepacivirus in the family Flaviviridae. The characteristic all of these viruses have in common is a positive-stranded RNA that, in the case of HCV, consists of a genome of approximately 10 kb containing one large open reading frame (ORF) that is initially translated into one single polyprotein and subsequently cleaved into the functional proteins. The putative organization of the HCV genome includes the 5′-untranslated region, four structural proteins, six nonstructural proteins, and a 3′-untranslated region (41). Among the structural proteins of HCV, the basic 19-kDa core protein exhibits pleiotropic features. It may be involved in nucleocapsid formation, and it plays a role in the switch between viral polyprotein synthesis and subsequent viral RNA replication. In addition, HCV core protein was shown to transcriptionally regulate several cellular and viral genes (28). Furthermore, several—although in part contradictory—reports suggest an influence of the HCV core protein on the transcriptional activity of p53 and the regulation of p53-dependent genes. Both suppression and upregulation of p53-dependent gene transcription by interaction with the HCV core protein have been described (34, 37). However, the effects of viral proteins on the function of HCV-infected cells are still poorly understood. Both the lack of tissue culture systems permissive for HCV infection and replication and the lack of a suitable animal model of HCV infection are the major obstacles to our understanding of HCV pathogenesis.
The infected liver is the primary site of HCV replication and displays substantial infiltration of cells of the innate immune response like natural killer (NK) cells (47). In general, NK cells are quite abundant in the liver (9, 18, 53) and provide a critical first line of defense against viral infection (5). Nevertheless, a role for NK cells in the course of infection has not been established.
Several lines of evidence have revealed a critical role for major histocompatibility complex (MHC) class I molecules in the regulation of NK cell activity (30, 36). Multiple types of negative receptors are expressed on NK cells, and the killer cell immunoglobulin (Ig)-like receptors are the best-characterized family in humans. Interactions between these receptors and MHC class I ligands on target cells result in an inhibitory signal that prevents the lysis of target cells. Thus, the level of MHC class I surface expression regulates NK cell activity, and any modulation of MHC class I molecules by viral proteins will have consequences for recognition by NK cells.
MHC class I molecules assemble with antigenic peptides in the endoplasmic reticulum (ER), and MHC class I-peptide complexes are transported to the cell surface, where they can be recognized by cytotoxic T lymphocytes (CTL) (49). These peptides are generated in the cytosol and are translocated into the ER by the transporter associated with antigen processing (TAP). TAP is composed of two polypeptide chains, TAP1 and TAP2, and is a member of the ATP-binding cassette transporter family (38). It has been shown that TAP-mediated peptide transport is kinetically coordinated with the binding of peptides to MHC class I molecules. The subsequent release of the MHC class I molecule from the loading complex is linked to conformational signals arising predominantly from TAP1. Thus, the presence of TAP1 in the ER membrane is the main factor responsible for MHC class I cell surface expression (32). Moreover, it has previously been shown that TAP1 transcription is strongly induced by the tumor suppressor protein p53 through a p53-responsive element in the gene for TAP1 (54).
In order to escape the T-cell response, viruses have developed survival mechanisms, most often mediated by viral nonstructural proteins that subvert the antigen processing and presentation machinery. Several viruses target specific components of the MHC class I pathway, leading to diminished cell surface expression of MHC class I molecules. Other viruses block the transport of MHC class I molecules through the ER, inhibit TAP-mediated translocation of cytoplasmic peptides into the ER, or interfere with proteasomal degradation of their own proteins (42). Other viruses, like human cytomegaloviruses, escape CD8+ T-cell recognition by downregulating MHC class I molecules (13) and may simultaneously induce the expression of virus-encoded MHC class I homologues capable of engaging inhibitory receptors that give a negative signal blocking NK cell function (48). Flaviviruses can upregulate MHC class I cell surface expression by increased peptide supply to the ER (29, 31). Viruses may use these strategies to evade and counteract a potential NK cell attack.
The aim of this study was to unravel the molecular interference of the HCV core protein with antigen presentation and immune response mechanisms. We present here the first evidence that the HCV core protein impairs NK cell activity via p53-dependent upregulation of TAP1 and consequently MHC class I surface expression in liver cells.
MATERIALS AND METHODS
Cell lines.
HepG2, Hep3B, Huh7, and Hek293 cells were obtained from the American Type Culture Collection (Manassas, Va.). The liver cell lines were maintained in Dulbecco's modified Eagle's medium (Invitrogen, San Diego, Calif.) supplemented with 10% heat-inactivated fetal calf serum (FCS; Invitrogen), 10 mM HEPES (Invitrogen), 5 mM l-glutamine (Invitrogen), and 100 μg of gentamicin (Invitrogen) per ml in 5% CO2. Hek293 cells were maintained in Dulbecco's modified Eagle's medium supplied with 10% heat-inactivated FCS, 10 mM HEPES, 5 mM l-glutamine, and 100 μg of penicillin (Invitrogen) per ml in 5% CO2.
Isolation and culture of primary human hepatocytes.
Primary human hepatocytes were isolated from healthy liver tissue obtained from patients receiving partial liver resections with a two-step perfusion technique as described and modified from the initial procedure established by Berry and Friend (3). The isolation procedure was approved by the Ethics Committee, Medical Faculty, University of Heidelberg. Briefly, a blood vessel of the resected liver tissue was cannulated and perfused with Ca2+- and Mg2+-free Hanks balanced salt solution (Invitrogen) containing 0.5 mM EGTA (Invitrogen) and 50 mM HEPES for 15 to 20 min. The perfusion was continued with Williams medium E (WME; Invitrogen) containing 0.05% collagenase type IV (Sigma, Taufkirchen, Germany) and 5 mM CaCl2 for 15 to 25 min. Cells were mechanically separated from the liver capsule, and the resulting cell suspension was filtered and washed in ice-cold, serum-free WME. To separate hepatocytes from nonparenchymal cells, we performed a centrifugation in Percoll (adjusted to a density of 1.065 g/ml; Biochrom, Berlin, Germany) for 10 min at 50 × g as described previously (44). Cells were washed twice in WME and seeded in maintenance medium at a density of 1.0 × 105 to 1.5 × 105 viable cells/cm2 on collagen-coated culture plates (collagen type I; Serva Biochemicals, Heidelberg, Germany). Viability was determined by trypan blue dye exclusion. The maintenance medium was changed 2 to 4 h after seeding and every 24 to 48 h thereafter. As maintenance medium we used WME supplemented with 5 mM l-glutamine, 0.6% glucose (Serva), 20 mM HEPES, 50 μg of gentamicin per ml, 100 μg of penicillin per ml, 100 μg of streptomycin (Invitrogen) per ml, 37 μM inosine (Serva), 1.5% dimethyl sulfoxide (Merck, Darmstadt, Germany), and 0.14 U of insulin (Serva) per ml. On days 1 and 2, the maintenance medium was supplemented with 10% FCS. Cells were incubated at 37°C in 5% CO2.
Plasmid constructs.
The TAP1 reporter construct was described previously (45). Reporter plasmid pG13-Luc, containing 13 copies of the p53 consensus site (22), was obtained from B. Vogelstein (Johns Hopkins University, Baltimore, Md.), and expression plasmids WTp53 and p53(H175) were kindly provided by M. Oren (Weizmann Institute, Rehovot, Israel).
As a source of viral genes, a plasmid designated pBRTM/HCV1-3011, covering the full-length ORF of HCV strain H77 genotype 1a (19), was used as a template for cloning into expression vectors. The HCV core protein-encoding gene fragment was PCR cloned after insertion of engineered start and stop codons, as well as restriction enzyme sites, with the primers 5′-CCG GAA TTC CGG ATG AGC ACG AAT CCT AAA CCT-3′ (EcoRI) and 5′-G CTC TAG AGC GAT GGC TGA AGC GGG CAC AGT C-3′ (XbaI) (21). Subsequently, the fragment was cloned into the pcDNA3 expression vector (Invitrogen). Correct insertion of the cDNA was verified by sequence analysis by standard methods. All of the other HCV protein-encoding gene fragments used were cloned as previously described (21).
Adenovirus constructs.
Recombinant adenoviruses were generated with the AdEasy system (20) kindly provided by B. Vogelstein. In brief, the EcoRI/XbaI HCV core fragment was cloned into the KpnI/XbaI multiple cloning site of the shuttle vector pAdShuttle-CMV. The resulting construct was linearized by digestion with restriction endonuclease PmeI and subsequently cotransformed into Escherichia coli BJ5183 cells with the adenovirus backbone plasmid pAdEasy-1. Recombinants were selected for kanamycin resistance, and recombination was confirmed by multiple restriction endonuclease analysis. The linearized recombinant plasmid was transfected into the adenovirus packaging cell line Hek293. Recombinant adenoviruses were harvested 8 days after transfection, further propagated by serial infection of Hek293 cells, and used for infection of primary human hepatocytes.
RT-PCR.
RNA was isolated with the RNeasy system (Qiagen) in accordance with the instructions of the manufacturer. One microgram of RNA was incubated with oligo(dT)12-18 primer (Roche), deoxynucleoside triphosphates (Gibco BRL), and 2 U of Moloney murine leukemia virus reverse transcriptase (RT; Gibco BRL) at 37°C for 1 h to generate cDNA. The cDNA was used as a template for PCR amplification of the gene products HLA-A (5′-GAC AGC GAC GCC GCG AGC CA-3′ and 5′-GGC AGC GAC CAC AGC TCC AG-3′; 817 bp), HLA-B (5′-GAC AGC GAC GCC GCG AGT CC-3′ and 5′-AGT AGC GAC CAC AGC TCC GA-3′; 798 bp), HLA-C (5′-GAG ATC ACA CTG ACC TGG CA-3′ and 5′-GAA CAC AGT CAA TGT GGG G-3′; 630 bp), TAP1 (5′-ACG TCC ACC CTG AGT GAT TC-3′ and 5′-AGC TTT TCC CTA AAC TTC TGG G-3′; 381 bp), TAP2 (5′-TAC CTG CTC ATA AGG AGG GTG C-3′ and 5′-ATT GGG ATA TGC AAA GGA GAC G-3′; 311 bp), tapasin (5′-AGT TCA ACC CTT TCA GGA GGG CA-3′ and 5′-GAA AGG CAG ACA GGA AAA GGC-3′; 385 bp), LMP2 (5′-TTG TGA TGG GTT CTG ATT CCC G-3′ and 5′-CAG AGC AAT AGC GTC TGT GG-3′; 347 bp), LMP7 (5′-TCG CCT TCA AGT TCC AGC ATG G-3′ and 5′-CCA ACC ATC TTC CTT CAT GTG G-3′; 241 bp), β-actin (5′-GTG GGG CGC CCC AGG CAC CA-3′ and 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′; 484 bp), and p53 (5′-ACA CAA TGC AGG ATT CCT CC-3′ and 5′-AAG CTC AAA ACT TTT AGC GCC-3′; 321 bp). PCR products were amplified with 25 cycles (60°C), separated by 2% agarose gel electrophoresis, and visualized by ethidium bromide staining.
Quantitative real-time PCR.
Quantitative PCR was performed with a LightCycler system in accordance with the manufacturer's (Roche Diagnostics, Mannheim, Germany) instructions with the following primers: 5′-GGTCACGACCCCTCATCC-3′ and 5′-GCCTAAACTGAAAATGAAACCG-3′ for a 349-bp fragment of HLA-A, 5′-GTGGGCTACGTGGACGAC-3′ and 5′-AGATCTGTGTGTTCCGGTCC-3′ for a 298-bp fragment of HLA-B, and 5′-ACGACGGCAAGGATTACATC-3′ and 5′-ACAGGCCCTCCAGGTAGG-3′ for a 356-bp fragment of HLA-C. For all other primer sequences, see the description of the RT-PCR procedure above. All primers are located within nonpolymorphic regions of the HLA-A, -B, and -C molecules.
Transient transfection and luciferase assay.
Transient transfections of Hep3B and Huh7 cells were performed with Lipofectamine reagent (Gibco BRL), whereas HepG2 cells were transfected with FuGene6 reagent (Roche) in accordance with the manufacturer's instructions.
For luciferase assays, cells were plated on six-well plates. The reporter constructs were cotransfected with the control vector pcDNA3 or the expression plasmid for the HCV core protein and the TAP1 reporter with an expression vector for wild-type p53 (WTp53) or p53(R175H) as indicated. One hundred nanograms of Renilla vector (Promega, Madison, Wis.) was cotransfected to determine transfection efficiencies. Cells were harvested after 36 h, and the assay was performed in accordance with the manufacturer's instructions. Luciferase activity was measured with a DuoLumat LB9507 (Berthold, Wildbach, Germany). Duplicate measurements were performed for all experiments.
Protein determination was performed with the bicinchoninic acid assay (Bio-Rad GmbH, Munich, Germany).
Flow cytometry.
The antibodies specific for MHC classes I (W6/32) and II/HLA-DR (L243) were obtained from the American Type Culture Collection. A goat anti-mouse F(ab′)2 fragment labeled with phycoerythrin, used as the secondary reagent, was obtained from Jackson ImmunoResearch Laboratories Inc., as were the isotype controls.
Cells were rinsed twice with phosphate-buffered saline (PBS) 48 h after transfection, detached from culture dishes with 20 mM EDTA, washed, and resuspended in PBS supplemented with 5% FCS. Approximately 5 × 105 cells were incubated with primary and secondary antibodies for 30 min at 4°C and then washed twice with PBS-5% FCS after each incubation. Analysis was performed on a Becton Dickinson fluorescence-activated cell sorter (FACS) flow cytometer with CellQuest software.
Western blotting.
Cells were washed twice with PBS and lysed in buffer containing 20 mM Tris-HCl (pH 7.4), 137 mM NaCl, 10% (wt/vol) glycerin, 1% Triton X-100, 2 mM EDTA, and a protein inhibitor cocktail (Roche) for 30 min on ice. Lysates were cleared by centrifugation at 5,000 × g for 20 min at 4°C. Protein concentrations were determined with the bicinchoninic acid assay (Bio-Rad). A 15-μg aliquot of total cellular protein per sample was subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and transferred to an Immobilon (Millipore) polyvinylidene difluoride membrane. The membrane was blocked with 5% milk powder diluted in PBS containing 0.05% Tween 20 at room temperature for 30 min and then subjected to the immune reaction with the respective antibody diluted 1:1,000 in blocking solution for 1 h at room temperature. Before and after 1 h of incubation with a horseradish peroxidase-conjugated secondary antibody (Dianova), the membrane was washed for 40 min in four changes of PBS-0.05% Tween 20. The proteins were detected with the ECL detection system (Pierce). Subsequently, the membranes were washed in 20 mM glycine (pH 2.3) and subjected to an anti-tubulin antibody (Sigma) to verify for equal protein loading.
The HCV core antigen was detected with the monoclonal antibody (MAb) MA1-080, obtained from ABR (Golden, Colo.). The MAb recognizing p53 (Do-1) was obtained from Calbiochem (Bad Soden, Germany), and the polyclonal antibody against TAP1 was from Biomol (Golden, Colo.).
The HCV NS3 and NS5 antigens were detected with polyclonal rabbit antisera kindly provided by R. Bartenschlager (University of Heidelberg).
Cytotoxic effector cells.
Human NK cells were generated by allogeneic stimulation as previously described (14). Briefly, peripheral blood lymphocytes obtained from a normal healthy donor (donor B: HLA-A1, B8, B57, Cw∗0602, Cw∗0702) were used for the generation of cytotoxic effector cells by allogeneic stimulation with irradiated (20 Gy) peripheral blood lymphocytes (donor CP41: HLA-A∗0201, A∗0301, B∗3501, B∗3701, Cw∗0401, Cw∗0602) in RPMI 1640 medium supplemented with 2 mM l-glutamine, 1 mM pyruvate, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 15% heat-inactivated pooled human serum. Effector cells were restimulated every 7 to 12 days with allogeneic B-lymphoblastoid cells and cultured in medium supplemented with 300 U of recombinant IL-2 (Proleukin; Cetus, Emeryville, Calif.) per ml. B.3 NK cells were isolated by depletion of T cells with CD4- and CD3-coated immunomagnetic beads (Dynal AS, Oslo, Norway) that were incubated for 45 min on ice and then removed through magnetic adherence as previously described (14). The human NK leukemic line NKL was kindly provided by M. Lopez-Botet (Madrid, Spain) (39).
Peripheral blood mononuclear cells of an HLA-A2 healthy donor were stimulated several times with peptide (NS31073-1081)-pulsed mature dendritic cells. The HLA-A2-NS3 tetramer-specific CD8+ T-cell line was cloned by limiting dilution (generated by A. Ulsenheimer). Specificity for the NS3-derived peptide (NS31073-1081) in the context of HLA-A2 was identified by extended analysis with peptide-pulsed T2 cells (data not shown). The CTL clone was expanded by restimulation with irradiated allogeneic peripheral blood mononuclear cells of an HLA-A2-matched healthy donor in phytohemagglutinin-supplemented medium every 14 days.
Cell-mediated cytotoxicity assay.
Cell-mediated lysis was quantitated with standard 4-h chromium-51 release assays (43). Spontaneous release was determined by incubating target cells alone, and total release was determined by directly counting labeled cells. Percent cytotoxicity was calculated as follows: % specific lysis = (experimental cpm − spontaneous cpm/total cpm − spontaneous cpm) × 100. Duplicate measurements of four-step titrations of effector cells were used for all experiments.
To mask MHC class I molecules, the anti-MHC class I-specific MAb W6/32 (IgG2a) was added to target cells 30 min prior to incubation with effector cells. MAb W6/32 was used as ascites fluid diluted 1:100, and purified Ig (MOPC21, IgG2a; Sigma) was used as the isotype control at a concentration of 10 μg/ml. Mean values of percent specific lysis ± standard deviations were calculated from triplicate samples.
For analysis of the NS3-specific CTL clone, HepG2 mock-, HCV core-, and HCV ORF-transfected cells were labeled with chromium in the presence of 1 μg of NS31073-1081 peptide per ml. After being washed twice and counted, 2,000 cells per well were preincubated with 1 μg of peptide per ml for 45 min at room temperature, and then T cells were added and the mixture was incubated for an additional 4 h.
RESULTS
MHC class I is upregulated in HepG2 cells upon expression of the HCV core protein.
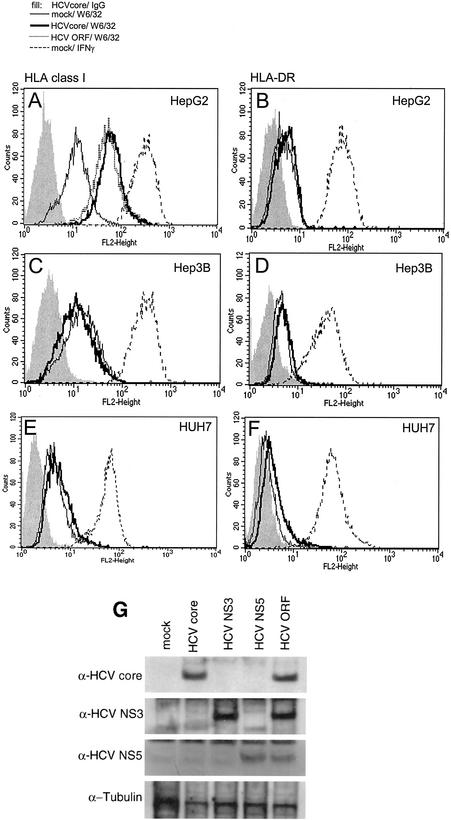
To study the effect of the HCV core protein on antigen presentation in liver cells, several different liver cell lines were transfected with an expression plasmid encoding the cDNA of the structural protein alone and with a vector containing the entire ORF of the virus. Surface expression of MHC classes I and II was analyzed 48 h after transfection with antibodies specific for pan-HLA class I and HLA-DR, respectively. The liver cell lines Huh7 and Hep3B did not show any changes in cell surface expression of MHC class I or II (Fig. 1C-1F). In contrast, HepG2 cells showed increased MHC class I (Fig. 1A), but not class II (Fig. 1B), expression. This was observed upon expression of the HCV core protein alone or that of the whole ORF of the HCV genome (Fig. 1A). Expression of all viral proteins was proven by Western blot analysis and is demonstrated for the HCV core, NS3, and NS5 proteins (which is indicative of the expression of all viral proteins, as the HCV genome is translated into one single polyprotein and subsequently cleaved) in Fig. 1G, as well as in reference 19. Individual expression of other HCV proteins derived from the ORF did not result in increased MHC class I expression (data not shown).
FIG. 1.
HCV core protein influences HLA class I expression in liver cell lines. HepG2 (A and B), Hep3B (C and D), and Huh7 (E and F) cells were transfected with an HCV core protein expression plasmid (bold line), a plasmid containing the complete ORF of the HCV genome (A, scattered line), or the empty vector as a negative control (thin line) or were stimulated with 500 U of IFN-γ per ml as a positive control (dashed line). At 48 h after treatment, cells were harvested, incubated with the antibodies W6/32 (pan-HLA class I specific) (A, C, and E) and L243 (HLA-DR) (B, D, and F) or the appropriate control IgG (grey fill) and a phycoerythrin-coupled secondary antibody and subjected to FACS analysis. Results of representative experiments are shown. HepG2 cells were transfected with expression plasmids for the HCV core, NS3, or NS5 protein or a plasmid containing the complete ORF of the HCV genome, and cell lysates were subjected to Western blot analysis for investigation of expression of the HCV proteins indicated (G).
Functional integrity of MHC class I- and II-linked antigen presentation could be shown by exposure of cells to IFN-γ. All cell lines were responsive to IFN-γ and showed upregulation of MHC class I, as well as induction of class II, upon stimulation for 48 h (Fig. 1A to F).
These results show that expression of the HCV core protein causes an increase in MHC class I expression in HepG2 cells but not in Huh7 and Hep3B cells. Importantly, MHC class I upregulation occurs also in the context of all viral proteins. This difference in HCV-mediated MHC class I upregulation in the liver cell lines analyzed may be explained by differences in their p53 status. HepG2 cells express WTp53, whereas Hep3B and Huh7 cells do not express functional p53.
Expression of the HLA complex is not directly influenced on the transcriptional level.
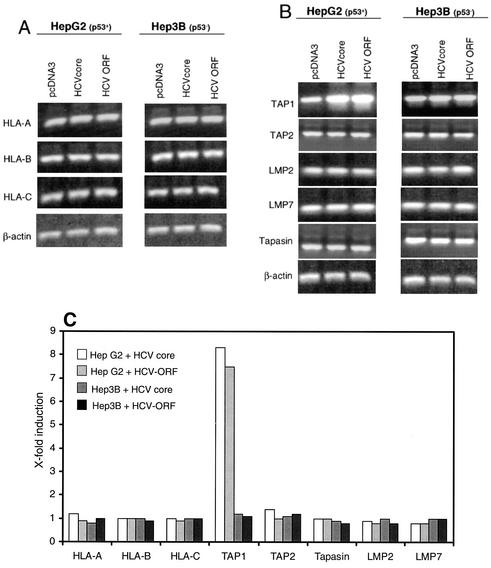
In order to elucidate the underlying mechanism of MHC class I upregulation by the HCV core protein, mRNA expression of HLA class I and of several components of the antigen processing and presentation pathway was analyzed. Thus, we performed RT-PCRs 24 and 48 h, and quantitative real-time PCRs 48 h, after transfection of HepG2 and Hep3B cells with either empty pcDNA3 vector or HCV core cDNA. As shown in Fig. 2A and C, neither HLA-A mRNA levels nor HLA-B or -C mRNA levels were increased upon expression of the HCV core protein. The other members of the antigen presentation cascade investigated, TAP1, TAP2, tapasin, LMP2, and LMP7, were constitutively expressed in both cell lines, but only TAP1 was induced up to eightfold after HCV core expression or expression of a plasmid containing the complete ORF of the HCV genome. Interestingly, this effect was observed only in WTp53-expressing HepG2 cells and not in p53-negative Hep3B (Fig. 2B and C) and Huh7 (data not shown) cells. These results suggest that the HCV core protein mediates increased TAP1 transcription, which, in turn, was previously reported to induce elevated MHC class I surface expression.
FIG. 2.
TAP1, but not HLA, transcription is enhanced by the HCV core protein. HepG2 and Hep3B cells were harvested 24 or 48 h after transfection with an HCV core protein expression plasmid, a plasmid containing the complete ORF of the HCV genome, or the empty expression vector as a negative control. RNA was isolated and reverse transcribed. PCR was performed with primers for HLA-A, -B, and -C (A); central components of the antigen presentation machinery (TAP1, TAP2, tapasin, LMP2, and LMP7) (B); and β-actin as a standard. Results of a representative of four independent experiments are shown. mRNA levels for data in panels A and B were determined by quantitative PCR with β-actin for normalization (C). A representative of three independent experiments is shown.
HCV core protein-induced TAP1 upregulation is dependent on functional p53.
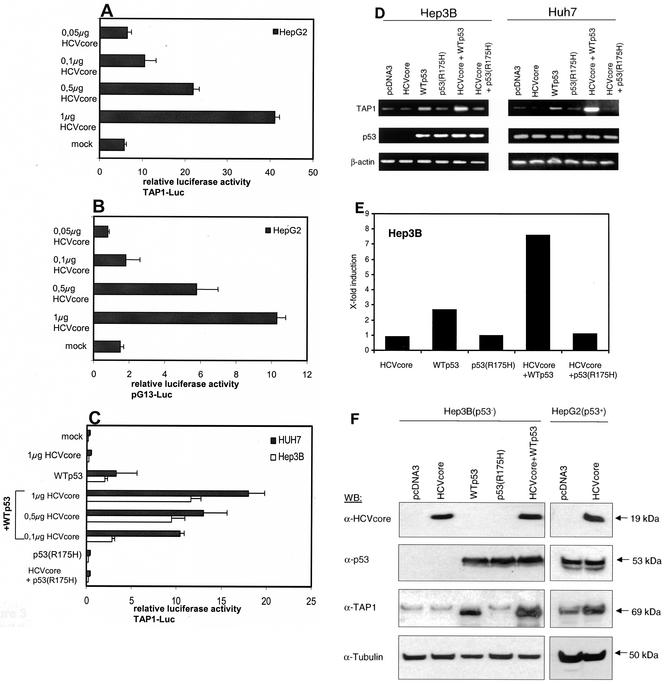
IFN-γ treatment increased MHC class I and II expression in all of the cell lines tested, indicating that antigen presentation was functional (Fig. 1). However, elevated HLA class I cell surface expression in response to HCV core protein expression was only detected in HepG2 cells. As already mentioned, the three cell lines used differ with respect to their p53 status. HepG2 cells express WTp53, whereas Huh7 cells express mutant p53 and Hep3B cells are p53 deficient. Since TAP1 was previously reported to be encoded by a p53-responsive gene, we further investigated whether the molecular mechanism of HCV core protein-induced TAP1 upregulation requires p53-dependent transcriptional events. To determine whether the transcriptional effect of p53 on TAP1 expression is direct, we used a luciferase reporter construct containing the TAP1 promoter. Luciferase activity was markedly increased upon expression of the HCV core protein in a dose-dependent manner only in p53-positive HepG2 cells (Fig. 3A) but not in Hep3B and Huh7 cells (Fig. 3C). To determine whether the increase in TAP1 promoter activity by the HCV core protein depends on p53-responsive elements, a reporter plasmid containing multiple copies of the p53 binding site, pG13-Luc, was used for the luciferase assay. In WTp53-containing HepG2 cells, HCV core protein enhanced the transcriptional ability of p53 in a dose-dependent manner (Fig. 3B). The increase in luciferase activity was not observed in Hep3B and Huh7 cells without exogenous p53 (data not shown). However, transfection of WTp53 into Hep3B and Huh7 restored activation of the TAP1 reporter similar to that observed for WTp53-expressing HepG2 cells. TAP1 promoter activity was significantly increased upon cotransfection with HCV core protein (Fig. 3C). This suggests that the TAP1 promoter is activated by HCV core protein only in the presence of functional p53. Luciferase activity was not increased upon cotransfection of mutant p53(R175H), which contains a defect in the DNA-binding domain (Fig. 3C).
FIG. 3.
TAP1 expression is dependent on p53. HepG2 cells were cotransfected with a luciferase reporter construct controlled by the TAP1 promoter (TAP1-Luc), decreasing amounts of HCV core expression vector, and the empty expression vector to adjust for equal amounts of DNA or as a negative control (A). HepG2 cells were cotransfected with a luciferase reporter construct controlled by multimers of intact p53 consensus sites (pG13-Luc) and decreasing amounts of HCV core expression plasmid and the empty expression vector to adjust for equal amounts of DNA or as negative control (B). Hep3B and Huh7 cells (both p53−) were cotransfected with TAP1-Luc, decreasing amounts of HCV core expression vector, the empty expression vector to adjust for equal amounts of DNA, WTp53, or p53(R175H), as indicated. Cells were lysed and luciferase activity was determined 36 h after transfection. Transactivation by the empty expression vector was arbitrarily set as basal activation. Bars in all experiments indicate standard deviations of at least three independent experiments (C). Hep3B and Huh7 cells were transfected with expression plasmids for WTp53, p53(R175H), or the HCV core protein alone or in combination as indicated. At 36 h after transfection, RNA was isolated, reverse transcribed, and subjected to PCR in order to analyze TAP1 and p53 mRNA expression levels (D). mRNA levels for data in panel D were determined by quantitative PCR with β-actin for normalization (E). Hep3B and HepG2 cells were transfected with the constructs indicated, and cell lysates were subjected to Western blot (WB) analysis for investigation of TAP1, p53, and HCV core protein expression (F). Results of representative experiments are shown.
p53-dependent HCV core protein-induced upregulation of TAP1 was further assessed by RT-PCR (Fig. 3D) and Western blot (Fig. 3F) analysis in Hep3B and Huh7 cells. Differential expression of TAP1 mRNA levels quantitated by real-time PCR (Fig. 3E). Consistent with the results of the reporter gene assay, WTp53, but not mutant p53, increased TAP1 mRNA and protein levels. Again, this effect was further enhanced more than seven-fold upon transfection of the HCV core expression plasmid but not upon transfection with the vector control. In contrast to WTp53-expressing HepG2 cells (Fig. 3E), cells expressing HCV core protein alone in the absence of p53 did not lead to higher TAP1 protein expression. p53 mRNA and protein expression levels did not change upon HCV core expression (Fig. 3D and E). Taken together, these data indicate that the HCV core protein induces TAP1 expression in liver cell lines, dependent on a functional p53 status.
Lack of HLA class I upregulation by HCV core protein in p53 mutant cells can be reconstituted by WTp53.
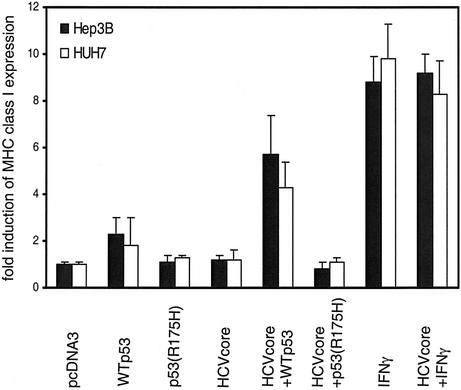
To assess whether not only TAP1 upregulation but also elevated MHC class I cell surface levels are dependent on WTp53, HLA class I cell surface expression on Hep3B and Huh7 cells was determined after reconstitution with WTp53. In line with the observed upregulation of TAP1, also MHC class I expression was increased in response to HCV core protein in the presence of WTp53 but not in the presence of p53(R175H) (Fig. 4).
FIG. 4.
HLA class I upregulation upon HCV core protein expression is dependent on p53. Hep3B and Huh7 cells were transfected with expression constructs as described in the legend to Fig. 3D or treated with 500 U of IFN-γ per ml for 48 h and subjected to FACS analysis. HLA class I expression was detected with the MAb W6/32. Results are given as fold induction of mean fluorescence intensity. Mean values of three experiments are shown with standard deviations.
Thus, the p53-dependent induction of TAP1 upon HCV core protein expression was associated with increased HLA class I expression on the cell surface. Therefore, one can postulate the following sequence of events. After expression of the HCV core protein, p53 transcriptional activity is induced, which causes upregulation of TAP1 expression and subsequently increased HLA class I surface expression.
HCV core protein-induced MHC class I upregulation occurs also in primary human hepatocytes.
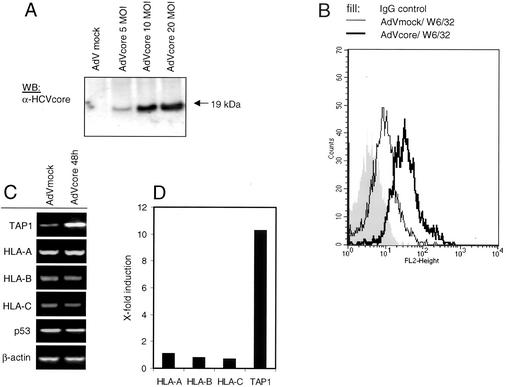
Intracellular effects in tumor cell lines do not necessarily reflect conditions in primary liver cells. Therefore, we investigated the observed effects of the HCV core protein also in primary human liver cells. Because conventional transfection methods do not lead to sufficient transfection efficiencies in primary nonproliferating cells, we inserted the HCV core protein-encoding gene into an adenovirus vector under control of the cytomegalovirus promoter. Because of deletions in the E1 and E3 regions, this virus is replication deficient and requires propagation in Hek293 cells to complement the defect in the E1 gene. The recombinant adenovirus was used for transduction of the HCV core protein-encoding gene into primary human hepatocytes. To examine the expression of the HCV core protein by this construct and its transduction efficiency, lysates of infected cells were tested by immunoblotting (Fig. 5A) and immunofluorescence assay (data not shown).
FIG. 5.
HLA class I and TAP1 are upregulated in primary human hepatocytes upon expression of HCV core protein. Cell lysates of primary human hepatocytes were tested 48 h after infection with either adeno-core virus (AdVcore) or empty adenovirus (AdVmock) for expression of HCV core protein by Western immunoblotting (WB) (A). Primary human hepatocytes were infected with an adeno-core virus or empty adenovirus multiplicity of infection (MOI) of 10. At 48 h after infection, cells were harvested and subjected to FACS analysis with the pan-HLA-specific MAb W6/32 (B). In parallel, RNA was isolated from HCV core-expressing versus mock control hepatocytes and levels of TAP1; HLA-A, -B, and -C; p53; and β-actin mRNAs were analyzed by RT-PCR. Representative results of three experiments performed are shown (C). mRNA levels for data in panel C were determined by quantitative PCR with β-actin for normalization (D). Representative results of two experiments performed are shown.
As demonstrated in Fig. 5B, HLA class I surface expression is increased in primary liver cells upon HCV core protein expression, in comparison to that in cells infected with the control virus (Fig. 5B), whereas HLA class II expression did not change (data not shown). Moreover, TAP1 mRNA was induced upon HCV core expression up to 10-fold also in primary hepatocytes, whereas p53 mRNA levels were unaltered, as assessed by RT-PCR analysis (Fig. 5C) and further quantified by real-time PCR (Fig. 5D). Therefore, one can conclude that the effect of the HCV core protein on HLA class I expression in liver cell lines is also found in normal liver cells.
Cytotoxic NK cell activity is impaired upon HCV core protein-induced HLA class I upregulation.
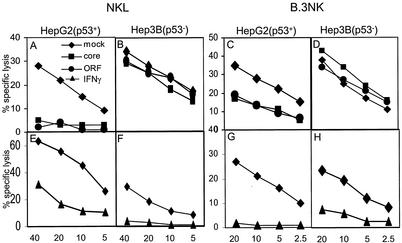
Since HLA class I molecules are known to inhibit NK cell activity, we investigated whether increased MHC class I expression in HCV core protein-expressing cells affects the cytotoxic activity of NK cells. HepG2 cells expressing the HCV core protein or a plasmid containing the complete ORF of the HCV genome, compared to mock-transfected control HepG2 cells, were less susceptible to lysis by NKL cells (Fig. 6A) and by purified primary human B.3 NK cells (Fig. 6C). The impaired cytotoxicity toward HCV core protein-expressing HepG2 cells was also observed with polyclonal NK cell populations (data not shown). In contrast, Hep3B cells that do not show increased MHC class I cell surface expression in response to the HCV core protein or the entire ORF, respectively, did not reduce the cytotoxicity of NKL (Fig. 6B) and B.3 NK (Fig. 6D) cells, respectively. However, treatment of both Hep3B and HepG2 cells with IFN-γ induced MHC class I upregulation and concomitant reduction of the lytic activity (Fig. 6E to H).
FIG. 6.
NK cell cytotoxicity is impaired against HepG2 cells expressing the HCV core protein. HepG2 (p53+) (A, C, E, and G) and Hep3B (p53−) (B, D, F, and H) cells were used as target cells in standard chromium release assays 48 h after transfection with an HCV core expression plasmid (▪), a plasmid containing the complete ORF of the HCV genome (•), or the respective empty control vector as a negative control (mock) (⧫) or after treatment with 500 U of IFN-γ per ml as a positive control (▴). Two NK cell lines were compared for cytotoxic activity against these target cells. NKL (A, B, E, and F) represents a leukemic NK cell line. B.3 NK (C, D, G, and H) represents a primary human NK cell line displaying a uniform phenotype and specificity. Mean values of duplicate samples from four-step titrations of one of three representative experiments are shown.
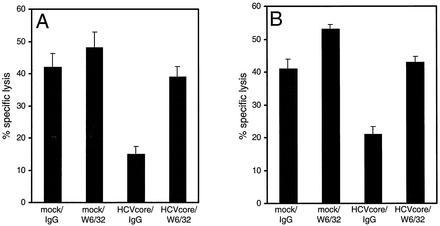
To determine if the impaired lysis of HCV core protein-expressing HepG2 cells is mediated by MHC class I molecules, target cells were preincubated with the pan-class I-specific MAb W6/32. In the presence of the masking antibody, but not with an appropriate control IgG, specific lysis of core-expressing HepG2 cells by NKL (Fig. 7A) and B. 3 NK (Fig. 7B) cells was substantially increased. The possibility of a contribution of CD16-mediated antibody-dependent cell-mediated cytotoxicity could be excluded for both NK lines. NKL cells showed minimal CD16 expression, as previously described for long-term-cultured cells (13), and no cytotoxic activity could be induced by redirected lysis experiments with anti-CD16 MAb (data not shown). B.3 NK cells do not express CD16 at all (data not shown).
FIG. 7.
Impairment of NK cell activity is directly mediated by HLA class I expression. HLA class I molecules of HepG2 target cells, either mock-transfected control or expressing the HCV core protein, were masked with pan-class I-specific MAb W6/32 or an appropriate control IgG by 30 min of preincubation. Specific lysis of NKL (A) is shown at an effector-to-target cell ratio of 20:1. B.3 NK cells (B) are shown at an effector-to-target ratio of 10:1. Data represent mean values of triplicate samples.
Therefore, our data suggest that HCV core protein induces immune escape from NK cells by a p53-dependent, TAP1-mediated increase in cell surface MHC class I that finally reduces cytotoxic NK cell activity.
Cytotoxic activity of HCV-specific CTL is not changed upon HCV core protein-induced HLA class I upregulation.
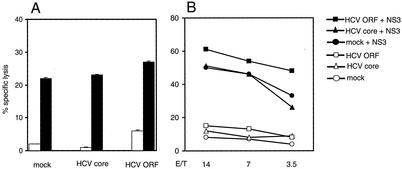
To test if HCV core-induced MHC class I expression influences the cytotoxic activity of HCV-specific CTLs, a CTL clone that specifically recognizes the HCV NS31073-1081 peptide in the context of HLA-A2 was used as the effector cell. HepG2 cells transfected with constructs containing the complete HCV ORF or the coding region for the HCV core protein alone or mock-transfected cells were used as targets that were either pulsed with peptide or not pulsed. CTL recognition of cells transfected with expression constructs containing the complete HCV ORF was only slightly enhanced despite expression of NS3 (verified by RT-PCR; data not shown) (Fig. 8A, open bars, and B, empty symbols). Lysis of peptide-pulsed HepG2 cells was identical in the absence or presence of HCV core protein (Fig. 8A, black bars, and B, filled symbols). This indicates that endogenous expression of NS3 may not be sufficient to generate appropriate HLA-A2-peptide complexes for recognition by specific CTL. Lysis of HepG2 cells transfected with constructs containing the complete HCV ORF or the coding region for the HCV core protein alone pulsed with the NS3 peptide was not significantly different from that of mock-transfected control cells, indicating that the amount of exogenous HLA-A2-peptide complexes of untreated HepG2 cells was sufficient for the induction of cytotoxicity (Fig. 8A, black bars, and B, filled symbols). However, definition of CTL epitopes by peptide loading does not guarantee that these peptides can be generated by the endogenous peptide processing and presentation machinery. At least for the experimental situation of exogenous peptide loading, HCV core expression did not influence recognition by HCV-specific CTL.
FIG. 8.
HCV core-mediated enhancement of MHC class I expression has no effect on recognition by HCV-specific CTL. The cytotoxicity of an HCV NS3-specific CTL clone (peptide 1073-1081) against mock-transfected control and HCV core- and ORF-transfected HepG2 target cells in two experiments was analyzed (A and B). Target cells were left untreated (white bars) or preincubated with 1 μg of the NS31073-1081 peptide per ml (black bars). The NS3-specific clone was used at an effector-to-target (E/T) cell ratio of 5:1. Mean values ± standard deviations of triplicate samples are shown (A). Titration of the NS3-specific CTL clone against untreated (open symbols) and NS3-pulsed (1 μg/ml) HepG2 mock-transfected control or HCV core- and ORF-transfected cells (B). The data shown are mean values of triplicate samples.
DISCUSSION
Modulation of MHC class I expression is a known mechanism of immune escape for some viruses and tumors. In this report, we demonstrate that expression of the HCV core protein causes a substantial increase in MHC class I expression on HepG2 cells. Importantly, this increase was also observed in the context of the entire HCV polyprotein (Fig. 1A). Further investigation of downstream events revealed upregulation of the antigen transporter protein TAP1 (Fig. 3). TAP1 is the only p53-responsive component of the antigen presentation cascade (54). We could demonstrate that both TAP1 and MHC class I were induced by the HCV core protein only in cells expressing WTp53 (Fig. 3). Furthermore, TAP1 has previously been shown to be crucial for enhanced MHC class I cell surface expression. Thus, the strategy of HCV could be described as follows. Upon infection with HCV, the HCV core protein enhances the transcriptional activity of p53, leading to TAP1 upregulation and then to enhanced MHC class I expression. As the liver is the predominant site of HCV infection and replication, we concentrated on liver cells to study the effect of the HCV protein. The HCV core protein-mediated increase in MHC class I expression in primary human liver cells clearly demonstrated that this effect was not limited to tumor cell lines but rather represented the course of infection in normal tissue (Fig. 4).
Our data suggest that the HCV core protein and p53 act in synergy to defend the integrity of the host cell, to evade diverse non-antigen-specific effector mechanisms against HCV-infected liver cells, and to compromise viral replication. We observed that the HCV core protein did not increase either p53 mRNA or protein levels (Fig. 3D and E). The HCV core protein has been reported to not only enhance p53 DNA binding and transcriptional activity but to also induce the mdm2 promoter (34). Together, these data suggest that p53 protein levels may be kept at a steady state between stabilization and degradation. However, the mechanism by which the HCV core protein influences and controls p53 transcriptional activity remains to be fully elucidated. Further investigations focusing on this question are under way.
Inhibition of NK cell cytotoxicity was identified as a major functional consequence of HCV core protein-induced MHC class I upregulation (Fig. 6). Elevated MHC class I levels following HCV core protein expression induce negative signals that lead to inhibition of NK cytotoxicity. In contrast, recognition by HCV-specific CTL was not enhanced. Although expression of the entire HCV ORF enhances MHC class I surface expression (Fig. 1A), the appropriate peptide may not be generated and presented properly. Recognition of peptide-pulsed hepatoma cells was the same in the presence or absence of the HCV core protein, indicating that in this experimental situation of exogenous peptide loading, HCV core protein expression does not influence recognition by HCV-specific CTL. Without a natural infection system, it is difficult to prove suggestions that may explain this phenomenon. The expression of costimulatory molecules may be influenced by the viral proteins. Furthermore, the generation of altered peptide repertoires by HCV-infected cells may additionally hamper CTL recognition and may represent a CTL escape mechanism but would not affect impairment of NK cell cytotoxicity. The high error rate of the viral polymerase during viral RNA replication (2) results in a high frequency of HCV quasispecies detection in HCV-infected patients (15, 16). Rapid mutation of viral species within an individual causes the natural selection of escape mutants. Mutations leading to loss of T-cell recognition have been observed in acute and chronic disease states (8). Highly replicating viruses like HCV may need a strong specific humoral and cellular immune response to allow development of escape mutants and to establish persistence. Thus, the immense genetic diversity and rapid replication of HCV may crucially contribute to the failure of CTLs to control the infection. This effect may even be supported by enhanced MHC class I antigen presentation.
Interestingly, MHC class I expression on hepatocytes was shown to be upregulated during chronic HCV infection. This correlated negatively with susceptibility to IFN-α treatment (51). If this effect already occurs early during the acute phase of HCV infection, it may represent a viral strategy by which to avoid lysis by NK cells. Moreover, several lines of evidence indicate that NK cells are also able to regulate responses of the adaptive immune system and are even necessary for optimal priming of virus-specific T cells (12, 23, 33, 46). It has been shown that elimination of NK cells before infection leads to, among other things, inhibition of liver injury, as well as inhibition of the induction of a virus-specific T-cell response (27). Therefore, it can be suggested that NK cells constitute an important component required for optimal responses to viral liver infections and, thereby, may be of high importance for a successful cell-mediated immune response in this organ. Thus, negative regulation of NK cell function in HCV infection by HCV core protein-induced MHC class I expression may lead to insufficient induction of the adaptive immune response and, as a consequence, may contribute to persistence and establishment of a chronic infection. Nevertheless, it is well established that control of virus infection by the immune response normally requires virus-specific cytotoxic T cells. Also in HCV infection, all components of the immune system appear to function normally (25, 26). Therefore, HCV infection is a paradoxical infection because chronicity develops in the majority of cases despite humoral and cellular responses to HCV proteins. However, both the early and the adaptive immune responses must efficiently be overruled. It is still an unsolved question which strategies HCV has developed to evade the first line of defense to establish a chronic infection. This is primarily due to the fact that a direct study of HCV is hampered by many technical obstacles, such as the lack of in vitro culture systems and small-animal models.
Still, activated NK cells provide a critical first line of defense against a number of viral infections (4). In addition, NK cells have been demonstrated to respond to chemokines and concentrate antiviral activity in particular tissues, predominantly in the liver (5). NK cell depletion and deficiency in animal models result in a significant increase in sensitivity to a number of viruses, and a crucial role of NK cells in the control of murine cytomegalovirus infection has been established (1, 7, 11, 52). This underscores the importance of NK cells in early viral defense. In humans, NK cells may be important in infections with various viruses, including herpes simplex virus, Epstein-Barr virus, human cytomegalovirus, and human immunodeficiency virus (17, 35). Recently, two studies have addressed the issue of a role for NK cells in the anti-HCV immune response, showing that engagement of CD81 on NK cells by the major envelope protein of HCV (HCV E2) leads to inhibition of NK cells (10, 50).
It remains to be determined if differences exist between the HCV quasispecies with respect to NK cell regulation. In this context, it is known that the outcome of infection and the response to IFN-α treatment are strikingly different, depending on the viral genotype. Some HCV genotypes cause chronic infection significantly more often than others (6, 24). This may, in part, be due to differences in the ability to impair the innate immune response. Genotype-dependent differences in the regulation of MHC expression may be involved in this phenomenon and remain to be determined.
Taken together, our data provide new experimental evidence of how HCV may impair NK cell activity in the liver. The understanding of how this virus interacts with and regulates the host immune system, especially at an early stage of infection, will lead to insight into how chronic infection may eventually be prevented. Finally, we may be able to take advantage of this knowledge for vaccine development or specific immunotherapy.
Acknowledgments
We are grateful to M. Oren for providing expression plasmids WTp53 and p53(R175H), to B. Vogelstein for pG13-Luc and tools for adenovirus constructs, to E. Klar and T. Lehnert for making human liver tissue available, to R. Bartenschlager and T. Pietschmann for providing antibodies, to D. Koppenhöfer, M. Pach, and B. Mosetter for excellent technical assistance, and to A. Krueger and M. Sprick for critical review of the manuscript.
This work was partially supported by grants from the Deutsche Krebshilfe and the Deutsche Forschungsgemeinschaft (SFB 571 and 405).
Footnotes
This report is dedicated to Harald zur Hausen on the occasion of his retirement as head of the German Cancer Research Center with gratitude and appreciation for 20 years of leadership.
REFERENCES
- 1.Arase, H., E. S. Mocarski, A. E. Campbell, A. B. Hill, and L. L. Lanier. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296:1323-1326. [DOI] [PubMed] [Google Scholar]
- 2.Bartenschlager, R., and V. Lohmann. 2000. Replication of hepatitis C virus. J. Gen. Virol. 81 Pt7:1631-1648. [DOI] [PubMed] [Google Scholar]
- 3.Berry, M. N., and D. S. Friend. 1969. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J. Cell Biol. 43:506-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biron, C. A., and L. Brossay. 2001. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13:458-464. [DOI] [PubMed] [Google Scholar]
- 5.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 6.Brechot, C. 1994. Hepatitis C virus genetic variability: clinical implications. Am. J. Gastroenterol. 89:S41-S47. [PubMed] [Google Scholar]
- 7.Brown, M. G., A. O. Dokun, J. W. Heusel, H. R. Smith, D. L. Beckman, E. A. Blattenberger, C. E. Dubbelde, L. R. Stone, A. A. Scalzo, and W. M. Yokoyama. 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 292:934-937. [DOI] [PubMed] [Google Scholar]
- 8.Chang, K. M., B. Rehermann, J. G. McHutchison, C. Pasquinelli, S. Southwood, A. Sette, and F. V. Chisari. 1997. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J. Clin. Investig. 100:2376-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crispe, I. N., and W. Z. Mehal. 1996. Strange brew: T cells in the liver. Immunol. Today 17:522-525. [DOI] [PubMed] [Google Scholar]
- 10.Crotta, S., A. Stilla, A. Wack, A. D'Andrea, S. Nuti, U. D'Oro, M. Mosca, F. Filliponi, R. M. Brunetto, F. Bonino, S. Abrignani, and N. M. Valiante. 2002. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J. Exp. Med. 195:35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniels, K. A., G. Devora, W. C. Lai, C. L. O'Donnell, M. Bennett, and R. M. Welsh. 2001. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 194:29-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doolan, D. L., and S. L. Hoffman. 1999. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J. Immunol. 163:884-892. [PubMed] [Google Scholar]
- 13.Falk, C. S., M. Mach, D. J. Schendel, E. H. Weiss, I. Hilgert, and G. Hahn. 2002. NK cell activity during human cytomegalovirus infection is dominated by US2-11-mediated HLA class I down-regulation. J. Immunol. 169:3257-3266. [DOI] [PubMed] [Google Scholar]
- 14.Falk, C. S., E. Nossner, B. Frankenberger, and D. J. Schendel. 2000. Non-MHC-restricted CD4+ T lymphocytes are regulated by HLA-Cw7-mediated inhibition. Hum. Immunol. 61:1219-1232. [DOI] [PubMed] [Google Scholar]
- 15.Farci, P., A. Shimoda, A. Coiana, G. Diaz, G. Peddis, J. C. Melpolder, A. Strazzera, D. Y. Chien, S. J. Munoz, A. Balestrieri, R. H. Purcell, and H. J. Alter. 2000. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288:339-344. [DOI] [PubMed] [Google Scholar]
- 16.Forns, X., R. H. Purcell, and J. Bukh. 1999. Quasispecies in viral persistence and pathogenesis of hepatitis C virus. Trends Microbiol. 7:402-410. [DOI] [PubMed] [Google Scholar]
- 17.Garzetti, G. G., A. Ciavattini, G. Goteri, M. De Nictolis, S. Menso, M. Muzzioli, and N. Fabris. 1995. HPV DNA positivity and natural killer cell activity in the clinical outcome of mild cervical dysplasia: integration between virus and immune system. Gynecol. Obstet. Investig. 39:130-135. [DOI] [PubMed] [Google Scholar]
- 18.Goossens, P. L., H. Jouin, G. Marchal, and G. Milon. 1990. Isolation and flow cytometric analysis of the free lymphomyeloid cells present in murine liver. J. Immunol. Methods 132:137-144. [DOI] [PubMed] [Google Scholar]
- 19.Grakoui, A., C. Wychowski, C. Lin, S. M. Feinstone, and C. M. Rice. 1993. Expression and identification of hepatitis C virus polyprotein cleavage products. J. Virol. 67:1385-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heintges, T., J. Encke, J. zu Putlitz, and J. R. Wands. 2001. Inhibition of hepatitis C virus NS3 function by antisense oligodeoxynucleotides and protease inhibitor. J. Med. Virol. 65:671-680. [DOI] [PubMed] [Google Scholar]
- 22.Kern, S. E., J. A. Pietenpol, S. Thiagalingam, A. Seymour, K. W. Kinzler, and B. Vogelstein. 1992. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science 256:827-830. [DOI] [PubMed] [Google Scholar]
- 23.Kos, F. J., and E. G. Engleman. 1996. Role of natural killer cells in the generation of influenza virus-specific cytotoxic T cells. Cell. Immunol. 173:1-6. [DOI] [PubMed] [Google Scholar]
- 24.Large, M. K., D. J. Kittlesen, and Y. S. Hahn. 1999. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J. Immunol. 162:931-938. [PubMed] [Google Scholar]
- 25.Lechner, F., N. H. Gruener, S. Urbani, J. Uggeri, T. Santantonio, A. R. Kammer, A. Cerny, R. Phillips, C. Ferrari, G. R. Pape, and P. Klenerman. 2000. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur. J. Immunol. 30:2479-2487. [DOI] [PubMed] [Google Scholar]
- 26.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, Z. X., S. Govindarajan, S. Okamoto, and G. Dennert. 2000. NK cells cause liver injury and facilitate the induction of T cell-mediated immunity to a viral liver infection. J. Immunol. 164:6480-6486. [DOI] [PubMed] [Google Scholar]
- 28.McLauchlan, J. 2000. Properties of the hepatitis C virus core protein: a structural protein that modulates cellular processes. J. Viral Hepatitis 7:2-14. [DOI] [PubMed] [Google Scholar]
- 29.Momburg, F., A. Mullbacher, and M. Lobigs. 2001. Modulation of transporter associated with antigen processing (TAP)-mediated peptide import into the endoplasmic reticulum by flavivirus infection. J. Virol. 75:5663-5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moretta, L., R. Biassoni, C. Bottino, M. C. Mingari, and A. Moretta. 2000. Human NK-cell receptors. Immunol. Today 21:420-422. [DOI] [PubMed] [Google Scholar]
- 31.Mullbacher, A., and M. Lobigs. 1995. Up-regulation of MHC class I by flavivirus-induced peptide translocation into the endoplasmic reticulum. Immunity 3:207-214. [DOI] [PubMed] [Google Scholar]
- 32.Natarajan, K., H. Li, R. A. Mariuzza, and D. H. Margulies. 1999. MHC class I molecules: structure and function. Rev. Immunogenet. 1:32-46. [PubMed] [Google Scholar]
- 33.Orange, J. S., B. Wang, C. Terhorst, and C. A. Biron. 1995. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 182:1045-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otsuka, M., N. Kato, K. Lan, H. Yoshida, J. Kato, T. Goto, Y. Shiratori, and M. Omata. 2000. Hepatitis C virus core protein enhances p53 function through augmentation of DNA binding affinity and transcriptional ability. J. Biol. Chem. 275:34122-34130. [DOI] [PubMed] [Google Scholar]
- 35.Pietra, G., C. Semino, F. Cagnoni, L. Boni, G. Cangemi, G. Frumento, and G. Melioli. 2000. Natural killer cells lyse autologous herpes simplex virus infected targets using cytolytic mechanisms distributed clonotypically. J. Med. Virol. 62:354-363. [PubMed] [Google Scholar]
- 36.Raulet, D. H., R. E. Vance, and C. W. McMahon. 2001. Regulation of the natural killer cell receptor repertoire. Annu. Rev. Immunol. 19:291-330. [DOI] [PubMed] [Google Scholar]
- 37.Ray, R. B., R. Steele, K. Meyer, and R. Ray. 1997. Transcriptional repression of p53 promoter by hepatitis C virus core protein. J. Biol. Chem. 272:10983-10986. [DOI] [PubMed] [Google Scholar]
- 38.Ritz, U., and B. Seliger. 2001. The transporter associated with antigen processing (TAP): structural integrity, expression, function, and its clinical relevance. Mol. Med. 7:149-158. [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson, M. J., K. J. Cochran, C. Cameron, J. M. Le, R. Tantravahi, and J. Ritz. 1996. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp. Hematol. 24:406-415. [PubMed] [Google Scholar]
- 40.Rosen, H. R., and D. R. Gretch. 1999. Hepatitis C virus: current understanding and prospects for future therapies. Mol. Med. Today 5:393-399. [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg, S. 2001. Recent advances in the molecular biology of hepatitis C virus. J. Mol. Biol. 313:451-464. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg, W. 1999. Mechanisms of immune escape in viral hepatitis. Gut 44:759-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schendel, D. J., R. Wank, and B. Dupont. 1979. Standardization of the human in vitro cell-mediated lympholysis technique. Tissue Antigens 13:112-120. [DOI] [PubMed] [Google Scholar]
- 44.Schroder, A. J., R. A. Blaheta, M. Scholz, A. Encke, and B. H. Markus. 1994. Isolation and separation of human adult hepatocytes from resected liver: cellular yield and purity using different methods. Zentbl. Chir. 119:127-138. [PubMed] [Google Scholar]
- 45.Seliger, B., S. Hammers, A. Hohne, R. Zeidler, A. Knuth, C. D. Gerharz, and C. Huber. 1997. IFN-gamma-mediated coordinated transcriptional regulation of the human TAP-1 and LMP-2 genes in human renal cell carcinoma. Clin. Cancer Res. 3:573-578. [PubMed] [Google Scholar]
- 46.Su, H. C., R. Ishikawa, and C. A. Biron. 1993. Transforming growth factor-beta expression and natural killer cell responses during virus infection of normal, nude, and SCID mice. J. Immunol. 151:4874-4890. [PubMed] [Google Scholar]
- 47.Takahashi, M., K. Ogasawara, K. Takeda, W. Hashimoto, H. Sakihara, K. Kumagai, R. Anzai, M. Satoh, and S. Seki. 1996. LPS induces NK1.1+ αβ T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J. Immunol. 156:2436-2442. [PubMed] [Google Scholar]
- 48.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 49.Townsend, A., and H. Bodmer. 1989. Antigen recognition by class I-restricted T lymphocytes. Annu. Rev. Immunol. 7:601-624. [DOI] [PubMed] [Google Scholar]
- 50.Tseng, C. T., and G. R. Klimpel. 2002. Binding of the hepatitis C virus envelope protein e2 to CD81 inhibits natural killer cell functions. J. Exp. Med. 195:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Thiel, D. H., X. Zhang, N. Baddour, H. I. Wright, L. Friedlander, and J. S. Gavaler. 1994. Intrahepatic mononuclear cell populations and MHC antigen expression in patients with chronic hepatitis C: effect of interferon-alpha. Dig. Dis. Sci. 39:970-976. [DOI] [PubMed] [Google Scholar]
- 52.Vivier, E., and C. A. Biron. 2002. A pathogen receptor on natural killer cells. Science 296:1248-1249. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe, H., K. Ohtsuka, M. Kimura, Y. Ikarashi, K. Ohmori, A. Kusumi, T. Ohteki, S. Seki, and T. Abo. 1992. Details of an isolation method for hepatic lymphocytes in mice. J. Immunol. Methods 146:145-154. [DOI] [PubMed] [Google Scholar]
- 54.Zhu, K., J. Wang, J. Zhu, J. Jiang, J. Shou, and X. Chen. 1999. p53 induces TAP1 and enhances the transport of MHC class I peptides. Oncogene 18:7740-7747. [DOI] [PubMed] [Google Scholar]