Abstract
Acetylation and deacetylation of nucleosomal histones have profound effects on gene transcription in all eukaryotes. In humans, three highly homologous class I and four class II histone deacetylase (HDAC) enzymes have been identified to date. The class I deacetylases HDAC1 and HDAC2 are components of multisubunit complexes, one of which could associate with the nuclear hormone receptor corepressor, N-CoR. N-CoR also interacts with class II deacetylases HDAC4, HDAC5, and HDAC7. In comparison with HDAC1 and HDAC2, HDAC3 remains relatively uncharacterized, and very few proteins have been shown to interact with HDAC3. Using an affinity purification approach, we isolated an enzymatically active HDAC3 complex that contained members of the nuclear receptor corepressor family. Deletion analysis of N-CoR revealed that HDAC3 binds multiple N-CoR regions in vitro and that all of these regions are required for maximal binding in vivo. The N-CoR domains that interact with HDAC3 are distinct from those that bind other HDACs. Transient overexpression of HDAC3 and microinjection of Abs against HDAC3 showed that a component of transcriptional repression mediated by N-CoR depends on HDAC3. Interestingly, data suggest that interaction with a region of N-CoR augments the deacetylase activity of HDAC3. These results provide a possible molecular mechanism for HDAC3 regulation and argue that N-CoR is a platform in which distinct domains can interact with most of the known HDACs.
Keywords: N-CoR, HDAC
The organization of chromatin is a fundamentally important element of gene regulation in all eukaryotic cells. Whether chromatin is transcriptionally active or repressed is determined, at least in part, by the modification of nucleosomal histones. For example, hyperacetylation of histones generally leads to an increase in transcription, whereas hypoacetylation of histones appears to have the opposite effect. A number of histone acetyltransferase (HAT) enzymes have been identified over the last several years. These include GCN5, p300/CBP, P/CAF, TAF250, MOF, and other related proteins, and possibly SRC-1/ACTR (reviewed in refs. 1 and 2). Many of the histone acetyltransferases function as transcriptional coactivators, and, interestingly, some of them can acetylate transcription factors in addition to histones. Almost in parallel with the discovery of histone acetyltransferases, several laboratories identified a number of histone deacetylase (HDAC) enzymes (e.g., refs. 3–8). In humans and in mice, seven HDACs have been cloned to date, and these can be broadly divided into two classes. The class I enzymes bear significant homology to the yeast protein RPD3 and include HDAC1, HDAC2, and HDAC3. The class II HDA1-like enzymes include HDAC4, HDAC5, HDAC6, and HDAC7.
The identification and characterization of HDAC-binding proteins has been tremendously useful in elucidating the mechanisms and functions of the HDACs. Early studies established that HDAC1 and HDAC2 are components of two major multisubunit complexes referred to as the Sin3 and the Mi2 complexes (9–13). The nuclear receptor corepressor N-CoR, for example, interacts with HDAC1/2 through the Sin3 complex (14, 15), whereas other repressors contact HDAC1/2 through the Mi2 complex (10, 16). Whereas previous studies have focused primarily on HDAC1/2-containing complexes, proteins that interact with other members of the HDAC family have also been identified. For example, the myocyte enhancer factor MEF2A associates with HDAC4 and HDAC5 (17, 18), whereas N-CoR and the closely related protein SMRT have been reported to partner with HDAC4, HDAC5, and HDAC7, in addition to HDAC1 and HDAC2 (8, 19).
At present, members of the class I HDACs are distinguished solely on the basis of sequence, with HDAC1 and HDAC2 being more closely related to each other than to HDAC3. This closer relationship suggests that HDAC3 may play unique roles, but the question of whether any HDAC3-specific binding proteins exist remains open.
Using Ab affinity chromatography, we purified an HDAC3-containing histone deacetylase complex from HeLa cell extracts and demonstrated that one of the components in this complex is N-CoR. The N-CoR/HDAC3 complex is suggested to have functional significance, with the interaction modulating HDAC3 enzymatic activity.
Materials and Methods
Plasmids, Immunochemical Reagents, and Recombinant Protein Expression.
pGST-HDAC3, expression plasmids for Gal4-N-CoR fusions and for various HDACs, and reporter plasmids pGal4-tk-luc, pUAS-p36-luc, and Pit-1/RE-LacZ have been previously described (4, 7, 20–22). Additional N-CoR deletion plasmids were similarly constructed as described (20). pcDNA3-F-HDAC3 was constructed by subcloning HDAC3 cDNA downstream of the cytomegalovirus and phage T7 promoters, and in-frame with the FLAG sequence in pcDNA3 (Invitrogen). pCMX-VP16-HDAC3 was constructed by ligating an HDAC3 fragment generated by PCR into the BamHI/EcoRI sites of the pCMX-VP16-N expression vector (23). For pCMX-VP16-HDAC4, pcDNA3.1-HDAC4 was digested with NotI and religated in the presence of the VP16 insert. All constructs were verified by DNA sequencing. Polyclonal anti-N-CoR Ab has been described (14). Monoclonal anti-Flag M2 and polyclonal anti-Gal4 Abs were obtained from Sigma–Aldrich and Santa Cruz Biotechnology, respectively. For production of anti-HDAC3 Ab, a peptide corresponding to amino acids 411 to 428 of HDAC3 (NEFYDGDHDNDKESDVEI) was coupled to keyhole limpet hemacyanin and injected s.c. into New Zealand White rabbits (0.1 mg of peptide per injection). The resulting Abs were immunoaffinity purified on a peptide column. This anti-HDAC3 serum recognizes human and mouse HDAC3 as a single 49-kDa band on Western blots. Recombinant Flag-tagged HDAC1, Flag-tagged HDAC3, and Gal4-N-CoR proteins were produced with the Bac-to-Bac baculovirus expression system (Gibco/BRL).
Protein Purification and Mass Spectrometric Peptide Sequencing.
Affinity purification of the HDAC3 complex with anti-HDAC3 Ab was done according to the protocol used for large-scale purification of an HDAC2 complex (9), except that approximately six times more starting material was used. Purified samples were concentrated, resolved on SDS/PAGE, and stained with Coomassie blue. Bands corresponding to p215, p205, and p195 were excised, and samples were sequenced by peptide MS. The MS/MS spectra were then correlated with known sequences by using the algorithm sequest (24), followed by manual inspection.
HDAC and glutathione S-transferase (GST) Pull-down Assays.
Deacetylase activity was determined by using either hyperacetylated core histones purified from HeLa cells or H4 peptide as described (3, 10), with incubation overnight at room temperature. GST, GST-HDAC3, and various modifications of GST-N-CoR proteins were expressed and purified as described (4, 14). [35S]Met-labeled N-CoR, HDAC3, HDAC4, and HDAC5 proteins were prepared by using the coupled transcription-translation rabbit reticulocyte lysate system (Promega). Equal molar quantities of either GST or GST-fusion proteins on glutathione-Sepharose beads were incubated with labeled proteins. Binding reactions, washing conditions, and analysis by electrophoresis followed by autoradiography were done exactly as described (4, 14).
Transfection and Nuclear Microinjection Assays.
HeLa and 293 cells were cotransfected with plasmids directing the synthesis of various effector proteins plus a luciferase reporter, by using the calcium phosphate method. Each transfection contained 5 μg each of effector and reporter DNAs, and all transfections were normalized to equal amounts of DNA with parental expression vectors. Forty-eight hours after transfection, cells were collected, and luciferase activity was determined by using the Dual Luciferase Reporter Assay System (Promega). Microinjection of reporter plasmids and anti-N-CoR or anti-HDAC3, staining for β-galactosidase activity, and fluorescence microscopy analysis were performed essentially as described (14, 25).
Results
Identification of an Interaction Between N-CoR and HDAC3.
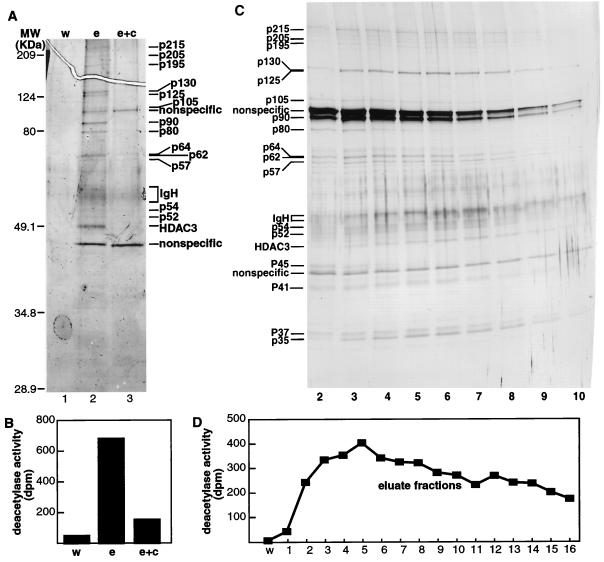
To purify an endogenous HDAC3 complex, extracts from 3 liters of HeLa cells (0.5 × 106 cells/ml) were applied to an anti-HDAC3 immunoaffinity column, and Ab-bound complexes were eluted from the affinity column by using HDAC3 peptide Ag. To control for nonspecific binding, an equal amount of extract was loaded onto an anti-HDAC3 column that had been preincubated with excess peptide Ag. Eluates were concentrated on centricon filters (Millipore) and visualized by silver staining of SDS/polyacrylamide gels (Fig. 1A). In comparison with the negative control (Fig. 1B) and the nonspecific eluate, the material that bound specifically to the anti-HDAC3 column was highly active.
Figure 1.
Purification of the HDAC3 complex. (A and C) Silver-stained SDS/polyacrylamide gel of the HDAC3 complex. (B and D) Histone deacetylase activity assayed from immunoaffinity-purified complexes. Each assay was performed in duplicate, and the values shown are the averages. w = wash, e = eluate, e + c = eluate and competitor.
To determine which proteins specifically associate with HDAC3 activity, we performed a large-scale purification and eluted the Ab-bound complex over multiple fractions. In this purification, we found that, in addition to HDAC3, eight proteins (p215, p205, p195, p130, p125, p54, p52, and p35) specifically coeluted with HDAC activity (Fig. 1 C and D). With the exception of the 35-kDa protein (previously eliminated in the concentration step), these proteins correspond in size to those detected in the smaller scale purification. The 215K, 205K, and 195K polypeptides were subjected to in-gel tryptic digestion, and the resulting peptides were sequenced by microcapillary HPLC ion trap MS. Peptide sequences obtained from these three proteins indicate that they are derivatives of the nuclear receptor corepressors N-CoR/TRAC-1 (T3-associated factor) and SMRT (silencing mediator for retinoic acid and thyroid hormone receptors) (Table 1). Because N-CoR, which has a molecular mass of 270 kDa (20), is highly susceptible to proteolysis (unpublished data), we believe that p215, p205, and p195 represent breakdown products of N-CoR/TRAC-1 and also SMRT.
Table 1.
Sequence of tryptic fragments of p215, p205, and p195
| Protein | Sequence | |
|---|---|---|
| p215: | N-CoR: | SSHLEVSQASQLLQQQQQQQLR |
| SAAVSEQQQLEQK | ||
| TVLSGSIMQGTPR | ||
| NFGLIASYLER | ||
| IMPLPAGGPSISQGLPASR | ||
| VSAAVLPLVHPLPEGLR | ||
| SIVQIIYDENR | ||
| GSITQGTPALPQTGIPTEALVK | ||
| YNTAADALAALVDAAASAPQMDVSK | ||
| ISVESIPSLR | ||
| EQPLGLPYPATR | ||
| GMPPLEIVPENIK | ||
| SPVPGVDPVVSHSPFDPHHR | ||
| p205: | N-CoR: | PIIEGSISQGTPIK |
| PSVGSISLGLPR | ||
| NFGLIASYLER | ||
| SQNSQPEGLLVR | ||
| VELPLYNQPSDTK | ||
| GSITQGTPALPQTGIPTEALVK | ||
| EQPLGLPYPATR | ||
| HNLDNLLQQHK | ||
| SPVPGVDPVVSHSPFDPHHR | ||
| GMPPLEIVPENIK | ||
| N-CoR/TRAC-1: | GIITAVEPSTPTVLR | |
| SMRT/N-CoR/TRAC-1: | HSSSPLSPGGPTHLTK | |
| N-CoR/SMRT: | EEETAAAPPVEEGEEQKPPAAEELAVDTGK | |
| p195: | N-CoR: | VELPLYNQPSDTK |
| SSHLEVSQASQLLQQQQQQQLR | ||
| SQNSQPEGLLVR | ||
| GTAGAIQEGSITR | ||
| GSITQGTPALPQTGIPTEALVK | ||
| LEQVSDSHFQR | ||
| SIVQTTYDENR | ||
| QDILTQESR | ||
| EELIQSMDR | ||
| VEQQILK | ||
| ISVESIPSLR | ||
| AFEGAITK | ||
| GMPPLEIVPENIK | ||
| VEQQILK | ||
| SLITGPSK | ||
| NWAAIAK | ||
| AFEGAITK | ||
| N-CoR isoform 1: | AIEQEAQMHNTAAR | |
| AAPQPDMSAAR | ||
| SMRT: | SYVEAQEDYLR | |
| GSITQGIPR | ||
| N-CoR/SMRT: | SIHEIPR |
Analysis of Interaction between HDAC3 and N-CoR.
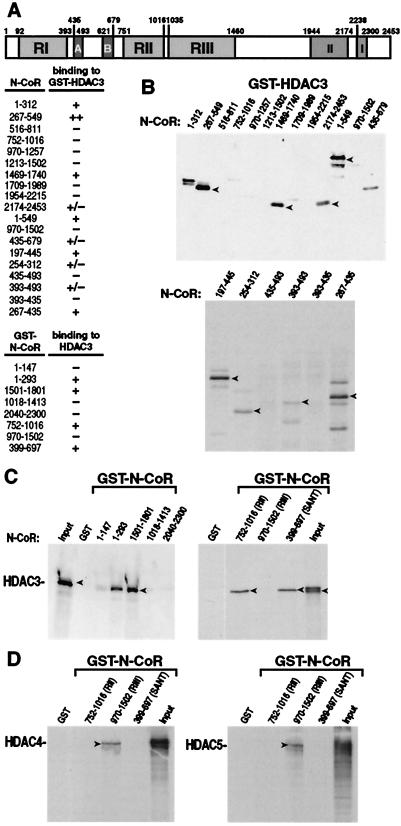
To confirm a physical interaction between HDAC3 and N-CoR and to perform a detailed mapping of N-CoR domains that interact with HDAC3, we used a GST-HDAC3 affinity matrix to capture different deletion derivatives of the N-CoR protein. Bacterially expressed GST-HDAC3 was bound to glutathione-Sepharose beads and incubated with 35S-labeled Gal4-N-CoR produced by in vitro translation in a reticulocyte lysate. The beads were washed, boiled in sample buffer, and analyzed by electrophoresis in an SDS/polyacrylamide gel. As shown in Fig. 2 A and B, an N-CoR fragment containing amino acid residues 267–549 clearly binds HDAC3 (Fig. 2B). Fragments corresponding to N-CoR residues 1–312, 197–445, 267–435, 1–549, and 1469–1740 also bind HDAC3, but less efficiently than does 267–549. Additional fragments can bind HDAC3 minimally. In a reciprocal experiment, 35S-labeled HDAC3 binds GST-N-CoR, emphasizing residues 1–293, 1501–1801, and 399–697 (Fig. 2C). Although 35S-labeled Gal4-N-CoR 752-1016 did not bind GST-HDAC3, the GST-N-CoR 752-1016 fragment, which encompasses repression domain II (RII) consistently binds HDAC3 in this experiment. Taken together, these data suggest that HDAC3 could interact with N-CoR through three distinct regions, one located near the N terminus of N-CoR (amino acid residues 147–516) partially overlapping RI, a second region (residues 1502–1709), and possibly a third region extending over RII (residues 752-1016).
Figure 2.
In vitro association of HDAC3 with N-CoR. (A) Schematic drawing of N-CoR. The ability of each N-CoR fusion protein to bind HDAC3 is indicated (+ or −). Representative autoradiograms of (B) in vitro translated Gal4-N-CoR protein captured by GST-HDAC3 fusion protein, (C) in vitro translated HDAC3 protein captured by GST-N-CoR fusion proteins, and (D) in vitro translated HDAC4 and HDAC5 proteins captured by GST-N-CoR fusion proteins. Several independent experiments yielded consistent results. The input lanes were loaded with one-tenth the amount of 35S-labeled proteins used in the binding reactions.
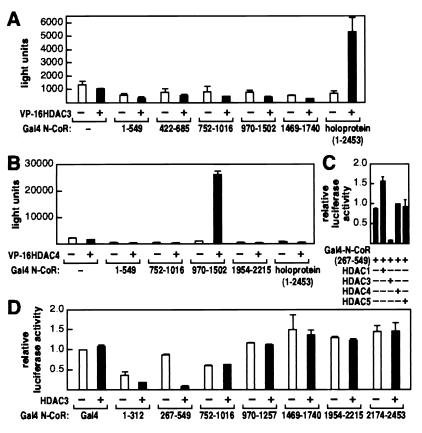
As a second method to confirm the interaction between HDAC3 and N-CoR, we used the mammalian two-hybrid system to ask whether a Gal4-DNA-binding domain-fused N-CoR could support transcriptional activation of a promoter containing Gal4 binding sites by HDAC3 fused to the activation domain of VP16. Fig. 3A shows that neither the Gal4-N-CoR fusion nor the HDAC3-VP16 fusion activated the reporter gene, but the two proteins together activated luciferase activity approximately 5-fold. In contrast, no region of N-CoR, including the N-CoR 1–549 or 1469–1740 region that interacted with HDAC3 in GST pull-down assays, gave sufficiently robust interaction to record in the mammalian two-hybrid experiments. These data suggest that multiple domains in N-CoR must be present together for binding to HDAC3 in vivo.
Figure 3.
In vivo association of HDAC3 with N-CoR. (A and B) Mammalian two-hybrid assays were used to assess the interaction between N-CoR and HDAC3 or HDAC4 in 293 cells. VP16-HDAC3 and VP16-HDAC4 fusion proteins were expressed from pCMX-VP16-HDAC3 and pCMX-VP16-HDAC4, respectively. pUAS-p36-luc was used as the reporter. The results are the averages ± SD from at least two separate experiments. (C and D) HDAC3 enhances repression by N-CoR HDAC3-interacting domain. HDAC1, HDAC3, HDAC4, HDAC5, and various Gal4-N-CoR fusions were expressed by using plasmids described in Materials and Methods. Transfections were done in HeLa cells with the pGal4-tk-luc reporter. The results are the averages ± SD from three separate experiments.
Previous studies showed that HDAC4, HDAC5, and HDAC7 interacted strongly with N-CoR/SMRT RIII (8, 19). To confirm that HDAC3 interacts with N-CoR through distinct domains separate from HDAC4, HDAC5, and HDAC7, we repeated the GST pull-down experiments with three different segments of GST-N-CoR and 35S-labeled HDAC4 or HDAC5. As shown in Fig. 2D, N-CoR RIII, but not RII, interacted with HDAC4 and HDAC5. Similarly, in a mammalian two-hybrid assay, N-CoR RIII (residues 970-1502), but not RII, interacted with HDAC4 (Fig. 3B). Interestingly, whereas the full-length N-CoR interacted with HDAC3, the same holoprotein did not interact with HDAC4 in the two-hybrid experiment (compare Fig. 3 A and B).
HDAC3-Dependent Repression by N-CoR.
Previous studies have shown that N-CoR can repress transcription when directed to the upstream sequence of a minimal promoter (20). To determine the contribution of HDAC3 on N-CoR-induced transcriptional repression, we targeted different regions of N-CoR to promoters containing Gal4-binding sites and cotransfected a plasmid-expressing HDAC3. As shown in Fig. 3D, overexpression of HDAC3 was able to increase repression activity of N-CoR fragments that can interact, with residues 267–549 being most dramatically affected, followed by residues 1–312. Neither the cytomegalovirus promoter used to control production of Gal4-N-CoR nor the Gal4-N-CoR protein level was affected by HDAC3 (data not shown). Transcriptional activity directed by Gal4-N-CoR fragments that did not interact or interacted only minimally with HDAC3 (residues 752-1016, 970-1257, 1954–2215, and 2174–2453) were not affected by overexpression of HDAC3. Interestingly, N-CoR 1469–1740, a region that is outside of any of the repression domains, binds HDAC3 in vitro but did not respond to HDAC3 in this experiment. Repression by Gal4-N-CoR (267–549) was not affected by overexpression of HDAC1, HDAC4, or HDAC5 (Fig. 3C). These results indicate that transcriptional repression by N-CoR RI could be mediated, at least in part, by interaction with the HDAC3 protein.
Requirement of HDAC3 for Repression by Transcription Factor Pit-1.
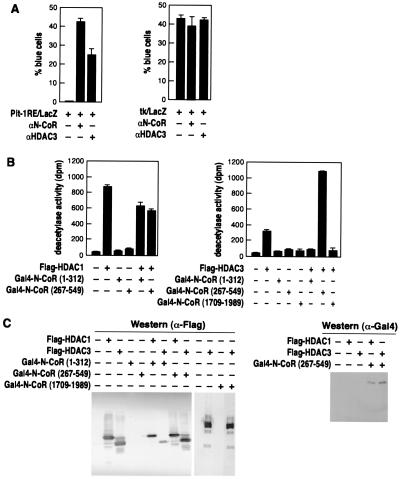
To determine whether HDAC3 is required for N-CoR-mediated repression by specific DNA-binding transcription factors, we chose to test the effect of anti-HDAC3 on repression by the homeodomain protein Pit-1, whose transcriptional activity appears to be regulated by N-CoR (22). Microinjection of anti-N-CoR IgG significantly increased the reporter gene activity from a promoter containing Pit-1 binding sites, confirming that N-CoR can mediate repression by Pit-1 (Fig. 4A Left). More importantly, microinjection of anti-HDAC3 IgG also significantly relieved repression of the same reporter containing Pit-1 response elements, consistent with the premise that HDAC3 is required for N-CoR-mediated repression or inhibition for at least some transcription factors. Neither anti-N-CoR nor anti-HDAC3 had any effect on a promoter lacking Pit-1 binding sites (Fig. 4A Right)
Figure 4.
(A) HDAC3 is involved in N-CoR/Pit-1-mediated repression. A reporter plasmid containing a Pit-1 response element was microinjected into 293 cells in the presence of control IgG, anti-N-CoR, or anti-HDAC3 IgG. The results were quantified as the percentage of injected cells (by rhodamine staining) that also turned blue [by 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) staining] and shown here as the averages ± SD of two experiments performed in triplicate. (B and C) N-CoR HDAC3-interacting domain enhances deacetylase activity by HDAC3. Recombinant Flag-HDACs and Gal4-N-CoR fusion proteins were expressed in Sf9 cells, immunoprecipitated with an anti-Flag Ab. Precipitates were divided into three equal aliquots and used for deacetylase assays (B), analysis by Western blot by using the anti-Flag Ab (C Left), and analysis by Western blot by using an anti-Gal4 Ab (C Right). Deacetylase results are the averages ± SD from four separate experiments.
N-CoR Augments HDAC3 activity.
To test the possibility that the interaction between HDAC3 and N-CoR modifies the deacetylase activity of HDAC3 within a complex, we expressed various combinations of Flag-HDACs and Gal4-N-CoRs in Sf9 insect cells by using baculoviral vectors, immunoprecipitated the proteins with an anti-Flag Ab, and tested their abilities to deacetylate an H4 histone peptide. As shown in Fig. 4B, neither the weaker binding N-CoR fragment (residues 1–312) nor the N-CoR fragment (residues 1709–1989) that does not interact with HDAC3 has any significant effect on the deacetylase activity of HDAC3. However, the N-CoR fragment with highest affinity for HDAC3 in vitro (residues 267–549) significantly increased the deacetylase activity of HDAC3. In contrast, the activity of HDAC1 is not enhanced by the N-CoR 267–549. Further, an HDAC3 mutant with three amino acid substitutions (residues 134–136) that would not have deacetylase activity was not stimulated by N-CoR (data not shown), indicating that the assay reflects increased HDAC3 activity in the presence of N-CoR. The quantity of HDAC3 was not affected by the presence of N-CoR, as indicated by Western blot analysis (Fig. 4C), ruling out the trivial explanation that the increases in deacetylase activity are a consequence of differential expression of HDAC3.
Discussion
Based on sequence homology to the yeast histone deacetylase RPD3, HDAC3 together with HDAC1 and HDAC2 make up the class I HDAC enzymes. Unlike HDAC1 and HDAC2, however, factors that HDAC3 associates with and its biological functions are obscure. Here, we have used immunoaffinity purification and microsequencing to define a complex containing HDAC3 and N-CoR/SMRT. N-CoR and SMRT were originally isolated as proteins that selectively associate with DNA-bound, unliganded, thyroid-hormone/retinoic-acid receptor heterodimers (20, 26). Subsequent studies indicate that these factors serve as effective corepressors for Rev-Erb, COUP-TF, DAX1, MAD, and Pit-1 (14, 22, 27–29).
Understanding of the mechanisms of N-CoR/SMRT-mediated repression came with the identification of its ability to associate with a complex containing the corepressor mSin3, and the histone deacetylases HDAC1 and HDAC2 and, via different domains, with HDAC4, HDAC5, and HDAC7 (8, 14, 15, 19, 30). The results in this manuscript indicate the existence of yet another N-CoR-HDAC complex.
Analysis of N-CoR mutants indicated that interaction with HDAC3 occurs through several regions on N-CoR, all distinct from regions that associate with mSin3, HDAC4, HDAC5, or HDAC7. Taken together, our results confirm and further extend the idea that N-CoR uses distinct domains to engage different HDACs. Our results agree well with the previous observation that HDAC3 does not interact with RIII of SMRT/N-CoR in a yeast two-hybrid assay (19). Indeed, whereas a full-length N-CoR protein consistently interacted with HDAC3 in our mammalian two-hybrid assays, RIII by itself did not interact with HDAC3 in the two-hybrid or the GST pull-down experiments. Surprisingly, in the mammalian two-hybrid assay, although HDAC4 interacted with RIII alone, unlike HDAC3, it did not interact with the full-length N-CoR protein. It is conceivable that the HDAC4 interacting-domain (RIII) is masked by other regions of N-CoR and only on exposure of this domain could N-CoR interact with HDAC4. Thus, it appears that not only do different HDACs bind different specific domains in N-CoR, the mechanisms of their binding may also differ between different members of HDACs.
Our results that overexpression of HDAC3 can enhance the repression activity of RI of N-CoR provide evidence for a simple model in which DNA-bound transcription factors recruit N-CoR/SMRT/HDAC3 to enzymatically modify histones and consequently repress transcription. In the present study, we have also discovered an alternative, non-mutually exclusive, model in which the interaction between HDAC3 and N-CoR enhances the deacetylase activity of HDAC3. Conceivably, by binding N-CoR, HDAC3 could be transposed to a more favorable conformation to interact with substrates or with other proteins in a complex. It will be of particular interest to learn whether similar modification of activity occurs in the case of other associated HDAC proteins.
The observation that anti-HDAC3 IgG stimulates activity of the homeodomain transcription factor Pit-1 supports a biological relevance for the N-CoR-HDAC3 interaction. We favor the concept that HDAC3 regulates only a particular set of repressor actions mediated by N-CoR/SMRT.
Our finding that HDAC3 binds N-CoR adds to the growing list of HDAC proteins that interact with N-CoR. However, it is interesting that HDAC3/N-CoR/SMRT form a particularly stable complex, and suggests that other HDACs may associate only when the conformation of N-CoR is altered by protein–protein interactions. Thus, at least at the biochemical level, N-CoR is the only cellular proteins known to date that can interact with at least five of seven of the known mammalian HDACs. As more novel HDACs are discovered, we predict there will probably be even more HDACs that can be shown to bind N-CoR. To unravel the mechanistic relationship between HDACs and N-CoR, a number of issues will need to be addressed. First, under what situations do HDACs simultaneously associate with N-CoR and under what situations do they interact with N-CoR individually? Second, is the combinatorial binding of HDACs a critical regulatory function of N-CoR? Does binding of specific HDAC3 to N-CoR exclude the binding of other HDACs? And is there cell-type specificity to the interactions between HDACs and N-CoR? It is likely that regulation of these events will prove to exhibit cell and promoter specificity. Finally, the ability of N-CoR to regulate HDAC enzymatic activity is likely to itself be regulated.
Acknowledgments
We thank Christina Grozinger and Stuart Schreiber for their generous gifts of the HDAC4 and HDAC5 cDNAs; Bill Lane and his colleagues at the Harvard Microsequencing Facility for their assistance with protein microsequencing; Yi Zhang and Bill Tsai for their preparation of HDAC substrates; and Nancy Olashaw and Doug Cress for discussion and critical reading of the manuscript. This work was supported by grants from the National Institutes of Health (GM58486, ES09262) to E.S. and from the National Institute of Diabetes and Digestive and Kidney Diseases to M.G.R.
Abbreviations
- HDAC
histone deacetylase
- N-CoR
nuclear receptor corepressor
- R
repression domain
- GST
glutathione S-transferase
- SMRT
silencing mediator for retinoic acid
References
- 1.Kuo M H, Allis C D. BioEssays. 1998;20:615–626. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 2.Kouzarides T. Curr Opin Genet Dev. 1999;9:40–48. doi: 10.1016/s0959-437x(99)80006-9. [DOI] [PubMed] [Google Scholar]
- 3.Taunton J, Hassig C A, Schreiber S L. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 4.Yang W M, Yao Y L, Sun J M, Davie J R, Seto E. J Biol Chem. 1997;272:28001–28007. doi: 10.1074/jbc.272.44.28001. [DOI] [PubMed] [Google Scholar]
- 5.Yang W M, Inouye C, Zeng Y, Bearss D, Seto E. Proc Natl Acad Sci USA. 1996;93:12845–12850. doi: 10.1073/pnas.93.23.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emiliani S, Fischle W, Van L C, Al A Y, Verdin E. Proc Natl Acad Sci USA. 1998;95:2795–2800. doi: 10.1073/pnas.95.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grozinger C M, Hassig C A, Schreiber S L. Proc Natl Acad Sci USA. 1999;96:4868–4873. doi: 10.1073/pnas.96.9.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kao H Y, Downes M, Ordentlich P, Evans R M. Genes Dev. 2000;14:55–66. [PMC free article] [PubMed] [Google Scholar]
- 9.Tong J K, Hassig C A, Schnitzler G R, Kingston R E, Schreiber S L. Nature (London) 1998;395:917–921. doi: 10.1038/27699. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, LeRoy G, Seelig H-P, Lane W S, Reinberg D. Cell. 1998;95:279–289. doi: 10.1016/s0092-8674(00)81758-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Iratni R, Erdjument-Bromage H, Tempst P, Reinberg D. Cell. 1997;89:357–364. doi: 10.1016/s0092-8674(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 12.Laherty C D, Yang W M, Sun J M, Davie J R, Seto E, Eisenman R N. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 13.Hassig C A, Fleischer T C, Billin A N, Schreiber S L, Ayer D E. Cell. 1997;89:341–347. doi: 10.1016/s0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- 14.Heinzel T, Lavinsky R M, Mullen T M, Soderstrom M, Laherty C D, Torchia J, Yang W M, Brard G, Ngo S D, Davie J R, Seto E, Eisenman R N, Rose D W, Glass C K, Rosenfeld M G. Nature (London) 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 15.Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho R A. Nature (London) 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- 16.Ng H H, Zhang Y, Hendrich B, Johnson C A, Turner B M, Erdjument-Bromage H, Tempst P, Reinberg D, Bird A. Nat Genet. 1999;23:58–61. doi: 10.1038/12659. [DOI] [PubMed] [Google Scholar]
- 17.Miska E A, Karlsson C, Langley E, Nielsen S J, Pines J, Kouzarides T. EMBO J. 1999;18:5099–5107. doi: 10.1093/emboj/18.18.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemercier C, Verdel A, Galloo B, Curtet S, Brocard M P, Khochbin S. J Biol Chem. 2000;275:15594–15599. doi: 10.1074/jbc.M908437199. [DOI] [PubMed] [Google Scholar]
- 19.Huang E Y, Zhang J, Miska E A, Guenther M G, Kouzarides T, Lazar M A. Genes Dev. 2000;14:45–54. [PMC free article] [PubMed] [Google Scholar]
- 20.Horlein A J, Naar A M, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass C K, et al. Nature (London) 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 21.Lavinsky R M, Jepsen K, Heinzel T, Torchia J, Mullen T M, Schiff R, Del-Rio A L, Ricote M, Ngo S, Gemsch J, Hilsenbeck S G, Osborne C K, Glass C K, Rosenfeld M G, Rose D W. Proc Natl Acad Sci USA. 1998;95:2920–2925. doi: 10.1073/pnas.95.6.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu L, Lavinsky R M, Dasen J S, Flynn S E, McInerney E M, Mullen T M, Heinzel T, Szeto D, Korzus E, Kurokawa R, Aggarwal A K, Rose D W, Glass C K, Rosenfeld M G. Nature (London) 1998;395:301–306. doi: 10.1038/26270. [DOI] [PubMed] [Google Scholar]
- 23.Perissi V, Staszewski L M, McInerney E M, Kurokawa R, Krones A, Rose D W, Lambert M H, Milburn M V, Glass C K, Rosenfeld M G. Genes Dev. 1999;13:3198–3208. doi: 10.1101/gad.13.24.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eng J K, McCormick A L, Yates J R., III J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 25.Rose D W, McCabe G, Feramisco J R, Adler M. J Cell Biol. 1992;119:1405–1411. doi: 10.1083/jcb.119.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J D, Evans R M. Nature (London) 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 27.Zamir I, Zhang J, Lazar M A. Genes Dev. 1997;11:835–846. doi: 10.1101/gad.11.7.835. [DOI] [PubMed] [Google Scholar]
- 28.Shibata H, Nawaz Z, Tsai S Y, O'Malley B W, Tsai M J. Mol Endocrinol. 1997;11:714–724. doi: 10.1210/mend.11.6.0002. [DOI] [PubMed] [Google Scholar]
- 29.Crawford P A, Dorn C, Sadovsky Y, Milbrandt J. Mol Cell Biol. 1998;18:2949–2956. doi: 10.1128/mcb.18.5.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagy L, Kao H Y, Chakravarti D, Lin R J, Hassig C A, Ayer D E, Schreiber S L, Evans R M. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]