Abstract
Human cytomegalovirus (HCMV) downregulates the class I major histocompatibility complexes (MHCs), HLA-A and -B, in infected fibroblasts to escape from antigen-specific cytotoxic T lymphocytes. The HCMV genes responsible for the downregulation of MHCs are US2, US3, US6, and US11, which encode type I membrane proteins working at the endoplasmic reticulum (ER). However, it is largely unknown whether HCMV downregulates the class I MHC molecules in placental extravillous cytotrophoblasts (EVT), which express HLA-C, -E, and -G to protect a semiallogenic fetus from maternal natural killer (NK) cells at the fetomaternal interface. Here, we report that differentiated EVT prepared from human first-trimester chorionic villi persistently express class I MHC molecules upon HCMV infection. When these US proteins were expressed in uninfected EVT, they were localized at the ER in the entire cytoplasm. However, subsequent HCMV infection resulted in dissociation of these US proteins from the ER, which relocated toward the cell membrane. In fibroblasts, these US proteins were localized at the ER before and after HCMV infection. These results suggest that the US gene products are not integrated into ER of HCMV-infected EVT and fail to downregulate class I MHC molecules.
Human cytomegalovirus (HCMV) is the most common viral agent of intrauterine infection, affecting 1% of all newborns. Five percent of infants with symptomatic congenital HCMV infection die, and most of the survivors as well as 10% of those who are asymptomatic at birth have neurological sequelae, including mental retardation and hearing loss. Maternal viremia and transplacental virus transmission following primary infection or reactivation of the virus are possible causes of congenital HCMV infection.
When HCMV infects fibroblasts, it downregulates the surface expression of class I major histocompatibility complexes (MHCs), HLA-A and -B, to protect the infected cells from attacks by antigen-specific cytotoxic T lymphocytes (4, 44, 48). HCMV US2, US3, US6, and US11 genes are responsible for the HCMV-induced downregulation of the MHCs. US6 inhibits TAP-mediated peptide translocation to endoplasmic reticulum (ER) and subsequent peptide loading of MHC molecules (3, 16, 29). US3 prevents transport of assembled MHC-antigen complexes to the cell surface and causes an accumulation of the complexes at the ER (2, 20). Furthermore, US2 and US11 gene products mediate the rapid dislocation of MHC molecules from the ER to the cytoplasm, where they are degraded by proteasomes (21, 46). However, they show the difference in their abilities to degrade MHC molecules in dendritic cells, suggesting that HCMV has adapted itself to divergent host cell types with its multiple immunoevasive strategies (39).
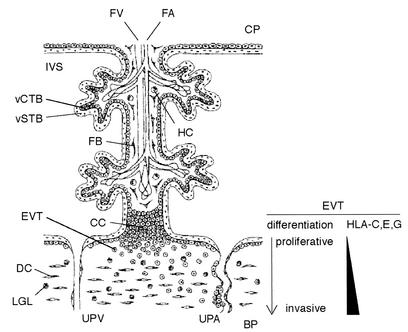
Placenta as the fetomaternal interface facilitates a vast amount of trafficking of the resources required for fetal development, and HCMV is thought to take this pathway to invade the fetus. Placental villi are either floating in the intervillous space filled with maternal blood or are anchored to the basal plate facing the maternal tissue called decidua, which lines the pregnant uterus (6). A villous surface is constituted of two layers of trophoblasts: the villous cytotrophoblast in the inner layer and the villous syncytiotrophoblast in the outer layer facing the intervillous space. Another type of trophoblast residing outside the villi is called extravillous cytotrophoblasts (EVT) (10). EVT differentiate from the stem cells in cell columns of anchoring villi to proliferative and then to invasive phenotypes. They migrate into the uterine interstitium and the maternal vasculature and construct a perfusion system in early pregnancy. Trophoblasts including EVT do not express HLA-A and HLA-B molecules to protect the semiallogeneic embryo from rejection of the maternal immune system (13). However, this might make trophoblasts susceptible to natural killer (NK)-mediated cell lysis. To prevent attack by maternal CD16− CD56bright NK cells abundant in decidua (10), EVT express the nonclassical class I molecule HLA-G (28, 32, 40). HLA-G was first identified in EVT and then found in other types of cells, including monocytes, thymic epithelial cells, and tumor cells (7, 37, 45, 49). Unlike HLA-A and -B, the major function of HLA-G is to protect cells from NK lysis by activating an inhibitory receptor, KIR2DL4 (33, 38). In addition to HLA-G, EVT express HLA-C and -E, which also act as ligands for inhibitory NK cell receptors, KIR2DL and CD94/NKG2A, to downregulate the cytotoxicity of NK cells (5, 23, 24). The expression of class I MHC molecules differs among subgroups of trophoblast cells. Neither villous cytotrophoblasts nor villous syncytiotrophoblasts express any class I MHC molecules (17, 18).
In placental development, EVT invade the endometrium and uterine spiral arteries. Because inefficient invasion of EVT results in infertility, miscarriage, and preeclampsia (34), it has been a great concern whether HCMV infects EVT. Earlier studies (12, 14, 15) showed that trophoblasts are permissive for HCMV infection. Recently, it was demonstrated that HCMV efficiently infects first-trimester villous cytotrophoblast in vitro and in utero (8). These results indicate that the placental trophoblasts form a major pathway of HCMV transmission from mother to fetus. Previous studies also suggested that class I MHC expression in cytotrophoblast may be downregulated by HCMV infection. Overexpression of US3 and US6, but neither US2 nor US11, can induce downregulation of HLA-C and -G in the trophoblast-derived choriocarcinoma cell line, JEG-3 (22, 42). However, JEG-3 is nonpermissive for HCMV, and the effects of viral infection are yet unknown. Another study using indirect immunofluorescence microscopy also showed that HCMV infection reduces HLA-G expression in first-trimester trophoblasts prepared with collagenase, hyaluronidase and trypsin treatment (8), but the surface expression of class I MHC molecules has not been analyzed yet. Thus, it is still unknown whether HCMV infection downregulates class I MHC expression in fully differentiated EVT.
In this study, we report that differentiated EVT, which were prepared from first-trimester placental explants, persistently express class I MHC molecules after HCMV infection. HCMV-infected EVT expressed US gene products, US2, US3, US6, and US11, but class I MHC molecules were expressed on the cell surface at a level similar to that observed in the uninfected cells. When US2, US3, US6, or US11 was overexpressed in uninfected EVT, each US protein was localized at the ER throughout the cytoplasm but was largely dissociated from the ER after HCMV infection. In contrast, in human embryonic lung fibroblasts (HEL), these US proteins were colocalized with the ER after HCMV infection, indicating their tight association. These results suggest that the US gene products does not function in HCMV-infected EVT.
MATERIALS AND METHODS
Cells.
Informed consent was obtained before tissue collections, and the study was approved by the local ethics committee. EVT were isolated and propagated from human first-trimester placentas of women seeking elective termination at 6 to 11 weeks of gestation (11, 47). Chorionic villous fragments were excised with curved scissors and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1 mM sodium pyruvate, penicillin (50 U/ml), and streptomycin (50 μg/ml) under 5% CO2 at 37°C. Adherent explants were cultured for 1 to 2 weeks to allow cells to grow out of the villi. These migrant cells were then replated for propagation. HEL were obtained from the American Type Culture Collection (Manassas, Va.) and maintained in the same medium.
HCMV infection.
HCMV (Towne) was adsorbed by HEL and EVT in DMEM containing 2% FBS for 2 h. These cells were washed five times with serum-free DMEM and then cultured in the medium containing 2% FBS for 1 to 7 days. For titration of HCMV, serial dilutions of culture medium of HCMV-infected cells were used to inoculate subconfluent HEL cultures in eight-well culture slides (Becton Dickinson). These cells were cultured for 1 week and then examined for expression of UL112-113 using M23 monoclonal antibody (MAb) (19).
Indirect immunofluorescence microscopy.
Cells cultured in eight-well slides were fixed with 4% paraformaldehyde in phosphate-buffered saline for 20 min at room temperature. Cells were reacted with primary antibodies in phosphate-buffered saline containing 3% bovine serum albumin for 1 h, and then with fluorescein isothiocyanate-, rhodamine- or Cy3-conjugated secondary antibodies for 1 h. Cells were examined using an Axiophot 2 (Zeiss) fluorescence microscope equipped with a Fujix HC-300 (Fuji) digital camera and an LSM510 (Zeiss) confocal laser scanning microscope.
Flow cytometry.
After trypsinization, cells were incubated with anti-class I MHC MAb (1:100) or an isotype control for 30 min at 4°C and with a fluorescein isothiocyanate-conjugated F(ab′)2 fraction of goat anti-mouse IgG (1:200) for 30 min at 4°C and then were analyzed with the flow cytometer FACScalibur (Becton Dickinson).
Antibodies.
Antibodies used in this study were anti-IE1/2 MAb 810 (Chemicon), anti-UL112-113 MAb M23 (9), anti-pp65 MAb 1-I-11 (ViroGen), anti-class I MHC MAb W6/32 (Dako), anti-FLAG MAb M2 (Stratagene), anticalnexin rabbit polyclonal antibody SPA-860 (StressGen), anticalreticulin goat polyclonal antibody C-17 (Santa Cruz), anticytokeratin MAb MNF116 (Dako), antivimentin MAb V9 (Nichirei), anti-integrin α1 MAb 1973 (Chemicon), anti-integrin β1 MAb 1951 (Chemicon), anti-integrin α6 MAb CBL 458 (Cymbus), and anti-integrin β4 MAb 2058 (Chemicon).
Transient expression of US proteins.
To construct expression plasmids for the US2, US3, US6 and US11 proteins tagged with FLAG epitope at the C-terminal end, the coding regions were amplified by PCR and cloned into pBluescript II KS(+) (Stratagene) containing a FLAG sequence. The US-FLAG cDNAs were then cloned into an expression plasmid, pME18S. These expression plasmids (0.2 μg) were introduced into the cells plated on eight-well culture slides with FuGene 6 (Roche). The efficiency of the plasmid transfection to EVT and HEL in this experiment was about 1%.
RT-PCR.
Total RNA (0.2 μg) of cultured cells was examined with the one-step reverse transcription (RT)-PCR kit (Invitrogen) according to the manufacturer's protocol. The primer sets used for RT-PCR were as follows: 5′-TCGTTAAAGTGGAACGTG-3′ (US2 sense, the nucleotide coordinates in HCMV AD169: 193416 to 193433), 5′-ACTATTGTCCAGGCCACA-3′ (US2 antisense, 193598 to 193615), 5′-CTTACATGGACAGACTGC-3′ (US3 sense, 194353 to 194370), 5′-GCTGAAGGTACCAGTTGA-3′ (US3 antisense, 194554 to 194571), 5′-GCACAGACCCGTTTGTTA-3′ (US6 sense, 195534 to 195551), 5′-TAGCCGACGGACTCGTTG-3′ (US6 antisense, 195703 to 195720), 5′-CCTGCCACCAATGCCAAA-3′ (US11 sense, 200104 to 200121), and 5′-AAAATGTCGGTGCAGCCA-3′ (US11 antisense, 200316 to 200333). The primer set for β-actin mRNA was purchased from TAKARA. The RT reaction was performed at 50°C for 30 min. PCR was repeated for 30 cycles of 15 s at 94°C, 30 s at 55°C, and 1 min at 72°C with a thermal cycler GeneAmp PCR System 9700 (Applied Biosystems).
RESULTS
Differentiated EVT were permissive to the complete replicative cycle of HCMV.
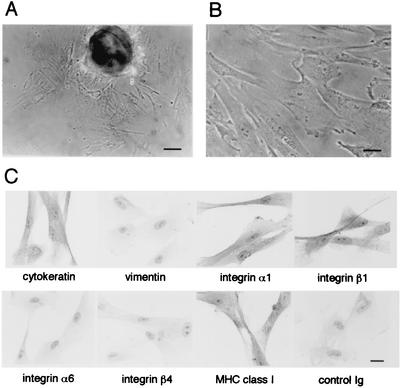
Cytotrophoblasts have been prepared from chorionic villi by enzymatic digestion using collagenase, hyaluronidase, and trypsin (8, 9, 31). However, this cytotrophoblast preparation could contain a mixture of cells at various stages of differentiation, which is initiated from the stem cells in cell columns and completed at the differentiated invasive EVT (Fig. 1). To prepare a substantial amount of first-trimester EVT with the invasive phenotype, we explanted fragments of human first-trimester chorionic villi and collected cells growing out of the tissues (Fig. 2A and B) according to the method described previously (11, 47) and used for examination of adenovirus and herpes simplex virus infection (26, 27). Isolated cells (>95%) showed a phenotype of differentiated EVT: expression of class I MHC molecules, cytokeratin, and integrins α1 and β1 (Fig. 2C) and the ability to invade through the reconstituted basement membrane, Matrigel (data not shown). We also confirmed that they expressed neither a marker of fibroblasts, vimentin, nor those of proliferative cytotrophoblasts, such as integrins α6 and β4 (Fig. 2C).
FIG. 1.
Schematic view of an anchoring chorionic villus in early pregnancy. The EVT express HLA-C, -E, and -G along the differentiation process from the proliferative phenotype in the cell column (CC) to the invasive phenotype in the basal plate (BP). Abbreviations: CP, chorionic plate; DC, decidual cell; FA, fetal artery; FB, fibroblast; FV, fetal vein; HC, Hofbauer cell (placental macrophage); IVS, intervillous space; LGL: large granular lymphocyte (NK cell); UPA, uteroplacental artery; UPV, uteroplacental vein; vCTB, villous cytotrophoblast; vSTB, villous syncytiotrophoblast.
FIG. 2.
HCMV infection of the differentiated EVT. (A) Explant of a first-trimester chorionic villus; (B) isolated EVT; (C) immunocytochemical analysis of EVT. Isolated EVT were stained with specific antibodies indicated in the figure and detected by peroxidase-conjugated secondary antibodies and DAB. Scale bars: 10 μm (A) and 5 μm (B and C).
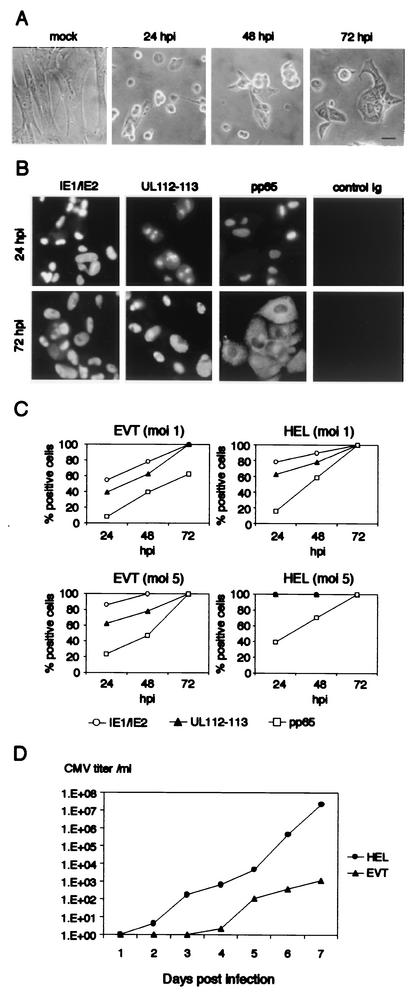
When EVT were infected with HCMV at a multiplicity of infection (MOI) of 5, most of the cells became rounded at 24 h postinfection (hpi) (Fig. 3A). At 72 hpi, these cells spread again but did not recover the original morphology of uninfected cells. The expression of major immediate-early proteins, IE1 and IE2, was detected in nuclei at 24 hpi, and the staining pattern at 72 hpi showed large and irregular structures (Fig. 3B). Early gene products, UL112-113 proteins, were found at several spots in nuclei at 24 hpi and had diffused throughout the nuclei at 72 hpi. An early-late gene product, pp65, was also found in nuclei at 24 hpi and then moved to the cytoplasm at 72 hpi. The subcellular localization of these viral gene products was similar to those reported for HCMV-infected fibroblasts (1, 41). More than 80% of EVT expressed IE1 or IE2 protein at 24 hpi, while UL112-113- and pp65-positive cells accounted for about 60 and 20% of the population, respectively (Fig. 3C). All EVT expressed pp65 by 72 hpi. As a control, HEL were infected with HCMV at the same MOI. The percentages of IE1/IE2-, UL112-113-, and pp65-expressing HEL at 24 hpi were 100, 100, and 40%, respectively. We performed a similar infection experiment at an MOI of 1. At 24 hpi, the percentages of EVT positive for IE and UL112-113 were about 20 percentile points less than those of HEL. About 60% of EVT expressed pp65 at 72 hpi when most of HEL had already expressed it (Fig. 3C).
FIG. 3.
Viral gene expression and production of infectious virions in HCMV-infected EVT. (A) EVT infected with HCMV at an MOI of 5. Scale bar; 5 μm. (B) Expression of HCMV genes in infected EVT. EVT were infected with HCMV at an MOI of 5, and the expression of immediate-early (IE1/IE2), early (UL112-113), and early-late (pp65) proteins was examined by indirect immunofluorescence microscopy (C) Expression of IE1/IE2, UL112-113, and pp65 in HCMV-infected EVT and HEL. EVT and HEL were infected with HCMV at an MOI of 1 or 5 and were examined by the indirect immunofluorescence method at 24, 48, and 72 hpi. (D) Production of infectious virions in EVT and HEL. The single-step growth curve of HCMV in EVT and HEL after infection of HCMV at an MOI of 5.
We next examined whether HCMV-infected EVT produced infectious virions. EVT were infected at an MOI of 5 and the culture medium was collected for the titration of released virions after 1 to 7 days of incubation (Fig. 3D). In 3 days after infection, HCMV-infected EVT did not produce a detectable amount of virions. A substantial amount of infectious virions was first detected in the culture medium at 4 days postinfection (dpi), and then the amount gradually accumulated. A similar experiment using HEL was performed in parallel. Infectious virions released from HEL were first detected as early as 2 dpi.
HCMV infection did not downregulate surface expression of class I MHC molecules in EVT.
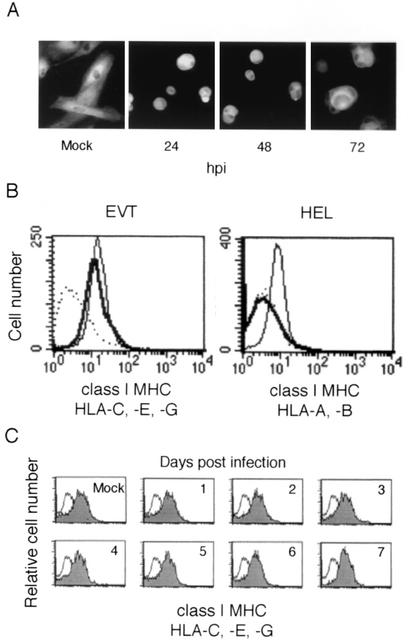
EVT express the class I MHC molecules HLA-C, -E, and -G but not HLA-A and -B (13). It was previously shown by an indirect immunofluorescence method that HCMV infection suppresses expression of HLA-G in cultured first-trimester villous cytotrophoblasts (8). We first examined the expression of class I MHC molecules in HCMV-infected EVT by a similar method. EVT were infected at an MOI of 5, and their expression of the class I MHC molecules was examined using the MAb W6/32 that recognizes HLA-A, -B, -C, -E, and -G. Use of a relatively high MOI ensured that most cells (>95%) were infected with HCMV (Fig. 3C). As shown in Fig. 4A, HCMV-infected EVT expressed the class I MHC molecules at a level similar to that observed in uninfected cells. Then, we examined the surface expression of the class I MHC molecules (HLA-C, -E, and -G) by flow cytometry and found that HCMV infection did not affect their expression at 72 hpi (Fig. 4B). As a control experiment, we examined HCMV-infected HEL and confirmed that HCMV infection completely suppressed the surface expression of the class I MHC molecules (HLA-A and -B) at 72 hpi as reported previously (4, 44, 48). We also examined class I MHC expression at later stages of infection, and found that the expression was maintained 7 days after HCMV infection (Fig. 4C).
FIG. 4.
Expression of class I MHC molecules in HCMV-infected EVT. (A) Indirect immunofluorescence analysis. Expression of class I MHC molecules in HCMV-infected EVT. EVT were infected with HCMV at an MOI of 5 and examined at 72 hpi using the MAb W6/32, which reacts with HLA-A, -B, -C, -E, and -G. (B) Flow cytometric analysis of the surface expression of class I MHC molecules at 72 hpi. EVT express HLA-C, -E, and -G. HEL express HLA-A and -B. These class I MHC molecules of HCMV-infected cells (thick line) and mock-infected cells (thin line) were detected using the MAb W6/32 or isotype control (dotted line). (C) Expression of class I MHC molecules in HCMV-infected EVT on later days. The flow cytometry of EVT was performed for 7 days after infection. Shaded curve, W6/32 antibody; open curve, isotype control antibody.
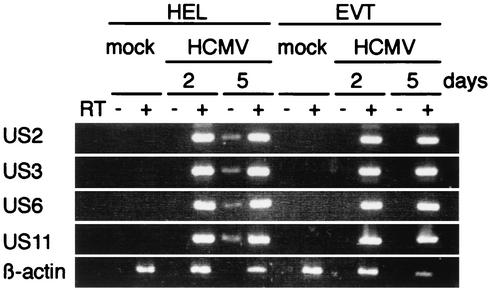
HCMV-infected EVT expressed US2, US3, US6, and US11 mRNA.
HCMV US2, US3, US6, and US11 genes are responsible for the downregulation of class I MHC molecules in fibroblasts. Because the surface expression of class I MHC molecules was not downregulated in HCMV-infected EVT, we examined the expression of these US genes by RT-PCR. Total RNA was prepared from mock- or HCMV-infected EVT at 1, 2, and 5 dpi. US2, US3, US6, and US11 mRNA were detected in HCMV-infected EVT as early as 1 dpi (data not shown) and their expression was maintained by 5 dpi (Fig. 5). There were no differences in the expression of these US gene mRNAs in HCMV-infected EVT and HEL.
FIG. 5.
Expression of US2, US3, US6, and US11 mRNA in HCMV-infected EVT and HEL. Total RNA (0.2 μg) was prepared from mock- or HCMV-infected (MOI = 5) cells and examined by RT-PCR.
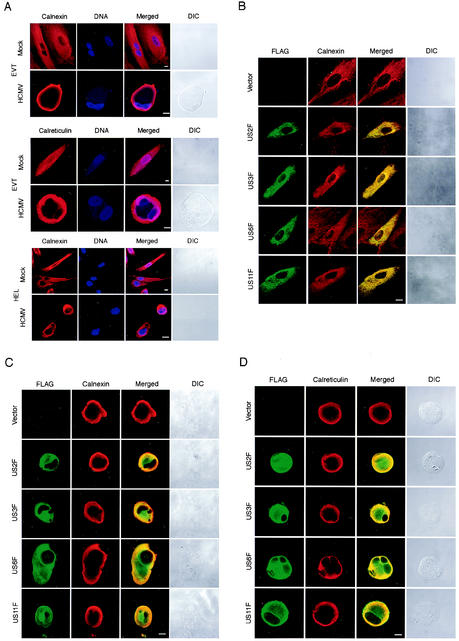
ER relocated toward the cell membrane in HCMV-infected EVT.
US2, US3, US6, and US11 were previously shown to function at the ER (2, 3, 16, 46). ER, the polygonal lattice of interconnected membrane tubules and cisternae, associates with microtubules and behaves dynamically in living cells (25, 30). Because the ER structure in HCMV-infected cells was largely unknown, we examined the subcellular localization of ER in HCMV-infected EVT by using a MAb against calnexin, an ER-resident chaperone (Fig. 6A). In uninfected EVT, the ER was distributed in the entire cytoplasm, and the ER network was well developed. HCMV infection, surprisingly, induced a relocation of the ER toward the cell membrane at 24 hpi in all infected cells we examined (100%). We also noticed that ER tubules, which were abundant in uninfected cells, mostly disappeared in the HCMV-infected cells. We also examined the cells using the antibody against calreticulin located in the ER lumen and confirmed that HCMV infection induced the relocation of ER (Fig. 6A). As a control experiment, we examined the ER structure of HCMV-infected HEL and found that it did not relocate toward either the cell membrane or the nucleus. Relocation of the ER was not a fixation artifact because it was observed by examining unfixed living EVT using an ER-specific dye, ER-Tracker (data not shown). These results indicate that the EVT ER and the HEL ER behave differently in response to HCMV infection.
FIG. 6.
Subcellular localization of ER in HCMV-infected EVT. (A) The localization of ER in EVT and HEL was examined using anticalnexin antibody and anticalreticulin antibody. DNA was stained with Hoechst 33258. Cells were infected at an MOI of 5 and examined at 24 hpi. (B) Localization of FLAG-tagged US proteins in EVT was examined. EVT were transfected with the expression plasmids for FLAG-tagged US proteins, US2F, US3F, US6F, and US11F. The localization of FLAG-tagged proteins was detected at 24 h after transfection using anti-FLAG antibody. The localization of the ER in the same cells was examined with anticalnexin antibody. (C) Localization of FLAG-tagged US proteins and ER (calnexin) in HCMV-infected EVT was examined. EVT were transfected with the expression plasmids for FLAG-tagged US proteins and then infected with HCMV at an MOI of 5 at 24 h after transfection. The localization of FLAG-tagged proteins was detected at 24 hpi using anti-FLAG antibody. The localization of the ER in the same cells was examined with anticalnexin antibody. (D) Localization of FLAG-tagged US proteins and ER (calreticulin) in HCMV-infected EVT. EVT were transfected with the expression plasmids and then infected with HCMV as described in Fig. 6C. The localization of FLAG-tagged proteins and the ER were detected at 24 hpi using anti-FLAG antibody and anticalreticulin antibody. All cells were examined using a confocal laser scanning microscope. DIC, differential interference contrast microscopy. Scale bar, 10 mm.
US2, US3, US6, and US11 gene products were displaced from ER in HCMV-infected EVT.
US2, US3, US6, and US11 gene products are type I membrane proteins whose integration with the ER membrane is essential for their functions. To know the localization of these US proteins in EVT, we expressed the FLAG-tagged versions, US2F, US3F, US6F, and US11F, and examined their subcellular localization using the anti-FLAG peptide antibody. The ER of these transfected cells was also detected with the anticalnexin antibody to examine their colocalization with US proteins. As shown in Fig. 6B, US2F, US3F, US6F, and US11F were located at the ER in the entire cytoplasm of most transfected cells, and the structure of the ER showed no differences from that of the vector-transfected EVT. US3F induced small aggregates of ER at the perinuclear domain, but most of the protein was localized at the ER network. Furthermore, ectopic expression of these US genes did not induce the ER relocation, which was observed after HCMV infection. We also examined the expression of cytokeratin in these transfected cells and confirmed that the US-expressing cells were EVT. These results indicated that the US gene products associated with the ER but did not affect their subcellular localization. We next examined the localization of the US proteins in HCMV-infected EVT (Fig. 6C). At first we introduced the expression plasmid for US2F, US3F, US6F, and US11F into EVT and then infected these cells with HCMV at 24 h after the transfection. The localization of the ER and US gene products in these cells was examined at 24 hpi. As described above, HCMV infection induced a relocation of the ER toward the cell membrane, but significant amounts of US2F, US3F, US6F, and US11F proteins were detected in the middle of the cytoplasm where the ER was mostly absent. Different localization of the US proteins and the ER was evident in most of the plasmid-transfected cells (73 to 83%). We also examined the localization of the ER of these cells using anticalreticulin antibody and confirmed that the ER was absent in the middle of the cytoplasm (Fig. 6D). These results indicated that these US gene products were displaced from the ER in HCMV-infected EVT.
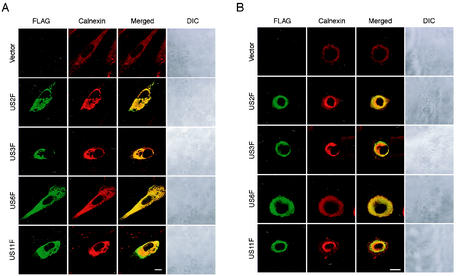
US2, US3, US6, and US11 proteins were colocalized with ER in HCMV-infected HEL.
As a control experiment, we expressed US2F, US3F, US6F, and US11F and examined their localization in HEL, in which class I MHC is known to be downregulated by HCMV infection. In the uninfected HEL, US2F, US3F, and US11F were colocalized with the ER in the perinuclear domain (Fig. 7A). Overexpression of these US proteins resulted in the aggregation of ER at the perinuclear region because it was not observed in HCMV-infected HEL (Fig. 6A). US6F also colocalized with the ER, but it did not induce aggregation. We then examined the localization of the FLAG-tagged US proteins in the plasmid-transfected HEL after subsequent HCMV infection (Fig. 7B). In contrast to EVT, these US proteins were colocalized with the ER in most of the HCMV-infected HEL (77 to 86%), indicating that they were tightly associated with or integrated into the ER membrane.
FIG. 7.
Subcellular localization of ER in HCMV-infected HEL. (A) Localization of FLAG-tagged US proteins in HEL was examined. HEL were transfected with the expression plasmids for FLAG-tagged US proteins, i.e., US2F, US3F, US6F, and US11F, and FLAG-tagged proteins were detected at 24 h after transfection. (B) Localization of FLAG-tagged US proteins in HCMV-infected HEL was examined at 24 hpi. HEL were transfected with the expression plasmids for FLAG-tagged US proteins as described for panel A and then infected with HCMV at an MOI of 5 at 24 h after transfection. The localization of FLAG-tagged proteins and the ER was detected using anti-FLAG antibody and anticalnexin antibody. These cells were examined using a confocal laser scanning microscope. DIC; differential interference contrast microscopy. Scale bar, 10 mm.
DISCUSSION
In early pregnancy, differentiated EVT invade the basal plate of a placenta to replace maternal spiral arteries and protect the embryo from maternal immune cells in the decidua (10). For this specialized purpose, EVT express class I MHC HLA-C, -E, and -G, which protect them from attack by NK cells, the principal component of decidual leukocytes. In this study we demonstrated that EVT are resistant to the HCMV-mediated downregulation of these class I MHC molecules (Fig. 4).
We showed that EVT continued to express class I MHC molecules after HCMV infection. However, this result does not imply that the HLA-C, -E, and -G molecules themselves are resistant to the HCMV-mediated downregulation, because the surface expression of HLA-C and -G was suppressed by US3 and US6 in JEG-3 cells (22). The difference between HEL and EVT in expression levels of US2, US3, US6, and US11 mRNA was too small to account for the resistance of EVT to the downregulation of class I MHC (Fig. 5). It is thus likely that these US proteins are expressed but do not function in EVT. US6 inhibits the TAP-mediated peptide translocation to ER (3, 16, 29), and US3 prevents the transport of assembled MHC-antigen complexes (2, 20). US2 and US11 mediate a rapid dislocation of MHC molecules from the ER to the cytoplasm (21, 46). All these US products are type I transmembrane glycoproteins, their integration into the ER membrane being essential for their functions. In this study, we found that these US gene products were largely displaced from ER in HCMV-infected EVT (Fig. 6C and D). This indicates that the products do not effectively associate with or integrate into the ER, thus allowing the surface expression of class I MHC molecules.
In a previous study suggesting downregulation of HLA-G in HCMV-infected cytotrophoblasts, cells were prepared with enzymatic digestion of chorionic villi (8). We examined differentiated EVT that had grown out of villous explants by immunofluorescence microscopy and flow cytometer analysis and found that HCMV infection did not reduce expression of class I MHC molecules (Fig. 4). It has been widely accepted that EVT differentiate through several stages after leaving the cell columns (10). The EVT located on and near the basal lamina show a proliferative phenotype, and those left there gradually become invasive. It is likely that the cytotrophoblasts prepared by enzymatic digestion include a wide spectrum of cells at various stages, to which the difference of MHC class I sensitivity may be attributed. We noticed that the morphology was also significantly different among these trophoblast cells. The cytotrophoblasts and JEG-3 cells sensitive to the MHC downregulation show a typical polygonal morphology of epithelial cells with a small cytoplasm and a large nucleus. In contrast, the differentiated EVT used in our study have a large and extended cytoplasm relative to the nucleus (Fig. 2 and 6A). A subset of cytotrophoblasts, presumably with the proliferative phenotype, may be sensitive to the HCMV-mediated MHC downregulation, but the differentiated EVT are not. Although we demonstrated that the total amount of the class I MHC molecules in EVT was not affected by HCMV infection, we did not use specific antibodies against either HLA-C, -E, or -G in this study. Therefore, it is possible that the ratio of HLA-C, -E, or -G molecules expressed in EVT may change after HCMV infection.
The ER is known to associate directly with microtubules but not with actin filaments (25, 30, 43). Disruption of microtubules by nocodazole induces retraction of the ER network toward the cell center, indicating that the ER is delivered by microtubule-dependent machinery. Overexpression of the US2, US3, or US11 gene in HEL induced an aggregation of the ER (Fig. 7A) similar to that caused by microtubule disruption (43), suggesting that an excess of US proteins affects the interaction between the ER and microtubules. Interestingly, HCMV infection of EVT induced ER relocation toward the cell membrane in the opposite direction to that induced by the US proteins in HEL (Fig. 6A). Microtubules are oriented with their minus ends anchored in the centrosome and their plus ends toward the cell membrane, and they transport membrane vesicles and organelles in either direction using motor proteins such as kinesin and dynein. Therefore, EVT ER may associate with microtubules via molecular machinery different from that used in fibroblasts.
The first-trimester EVT infected with HCMV face maternal NK cells, which dominate the leukocytes of decidua by 70% (10). If HCMV downregulates HLA-C, -E, and -G on EVT, the infected cells should be targets of decidual NK cells, and this may trigger a full-scale response of the maternal immune system against the placenta. Therefore, the persistent expression of these MHC molecules in HCMV-infected EVT may help the virus to survive in the fetus and the placenta. Expression of HLA-G was first detected in EVT (28) and then was also discovered in monocytes where HCMV established a latent and persistent infection (49). Interestingly, HLA-G is upregulated during HCMV reactivation in macrophages differentiated from latently infected monocytes (35, 36). These results suggest that HCMV may have adapted itself to survive in HLA-G-expressing cells, taking advantage of HLA-G to evade the host immune system.
Acknowledgments
We are grateful to Kwangseog Ahn for US2, US3, US6, and US11 cDNAs, to Kazuo Ichimiya for his kind cooperation, and to members of T. Aso's and Y. Yamanashi's laboratories for discussions.
This work is supported by a grant-in-aid for scientific research of the Japan Society for the Promotion of Science (JSPS).
REFERENCES
- 1.Ahn, J.-H., W.-J. Jang, and G. S. Hayward. 1999. The human cytomegalovirus IE2 and UL112-113 proteins accumulate in viral DNA replication compartments that initiate from the periphery of promyelocytic leukemia protein-associated nuclear bodies (PODs or ND10). J. Virol. 73:10458-10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, K., A. Angulo, P. Ghazal, P. A. Petersen, Y. Yang, and K. Früh. 1996. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc. Natl. Acad. Sci. USA 93:10990-10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn, K., A. Gruhler, B. Galocha, T. R. Jones, E. J. H. J. Wiertz, H. L. Ploegh, P. A. Petersen, Y. Yang, and K. Früh. 1997. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6:613-621. [DOI] [PubMed] [Google Scholar]
- 4.Beersma, M. F. C., M. J. E. Bijlmakers, and H. L. Ploegh. 1993. Human cytomegalovirus down-regulates HLA class I expression by reducing the stability of class I H chains. J. Immunol. 151:4455-4464. [PubMed] [Google Scholar]
- 5.Borrego, F., M. Ulbrecht, E. H. Weiss, J. E. Coligan, and A. G. Brooks. 1998. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J. Exp. Med. 187:813-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellucci, M., and P. Kaufmann. 1999. Basic structure of the villous trees, p. 50-115. In K. Benirschke and P. Kaufmann (ed.), Pathology of the human placenta, 4th ed. Springer-Verlag, New York, N.Y.
- 7.Crisa, L., M. T. McMaster, J. K. Ishii, S. J. Fisher, and D. R. Salomon. 1997. Identification of a thymic epithelial cell subset sharing expression of the class Ib HLA-G molecule with fetal trophoblasts. J. Exp. Med. 186:289-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher, S., O. Genbacev, E. Maidji, and L. Pereira. 2000. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J. Virol. 74:6808-6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher, S. J., T.-Y. Cui, L. Zhang, L. Hartman, K. Grahl, Z. Guo-Yang, J. Tarpey, and C. H. Damsky. 1989. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J. Cell Biol. 109:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank, H. G., and P. Kaufmann. 1999. Nonvillous parts and trophoblast invasion, p. 171-272. In K. Benirschke and P. Kaufmann (ed.), Pathology of the human placenta, 4th ed. Springer-Verlag, New York, N.Y.
- 11.Graham, C. H., T. S. Hawley, R. G. Hawley, J. R. MacDougall, R. S. Kerbel, N. Khoo, and P. K. Lala. 1993. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 206:204-211. [DOI] [PubMed] [Google Scholar]
- 12.Halwachs-Baumann, G., M. Wilders-Truschnig, G. Desoye, T. Hahn, L. Kiesel, K. Klingel, P. Rieger, G. Jahn, and C. Singer. 1998. Human trophoblast cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Virol. 72:7598-7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammer, A., H. Hutter, and G. Dohr. 1997. HLA class I expression on the materno-fetal interface. Am. J. Reprod. Immunol. 38:150-157. [DOI] [PubMed] [Google Scholar]
- 14.Hemmings, D. G., R. Kilani, C. Nykiforuk, J. Preiksaitis, and L. J. Guilbert. 1998. Permissive cytomegalovirus infection of primary villous term and first trimester trophoblasts. J. Virol. 72:4970-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmings, D. G., and L. J. Guilbert. 2002. Polarized release of human cytomegalovirus from placental trophoblasts. J. Virol. 76:6710-6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hengel, H., J. Koopmann, T. Flohr, W. Muranyi, E. Goulmy, G. J. Hämmerling, U. H. Koszinowski, and F. Momburg. 1997. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity 6:623-632. [DOI] [PubMed] [Google Scholar]
- 17.Hunt, J. S., J. L. Fishback, G. Chumbley, and Y. W. Loke. 1990. Identification of class I MHC mRNA in human first trimester trophoblast cells by in situ hybridization. J. Immunol. 144:4420-4425. [PubMed] [Google Scholar]
- 18.Hunt, J. S., B.-L. Hsi, C. R. King, and J. L. Fishback. 1991. Detection of class I MHC mRNA in subpopulations of first trimester cytotrophoblast cells by in situ hybridization. J. Reprod. Immunol. 19:315-323. [DOI] [PubMed] [Google Scholar]
- 19.Iwayama, S., T. Yamamoto, T. Furuya, R. Kobayashi, K. Ikuta, and K. Hirai. 1994. Intracellular localization and DNA-binding activity of a class of viral early phosphoproteins in human fibroblasts infected with human cytomegalovirus (Towne strain). J. Gen. Virol. 75:3309-3318. [DOI] [PubMed] [Google Scholar]
- 20.Jones, T. R., E. J. H. J. Wiertz, L. Sun, K. N. Fish, J. A. Nelson, and H. L. Ploegh. 1996. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex. Proc. Natl. Acad. Sci. USA 93:11327-11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones, T. R., and L. Sun. 1997. Human cytomegalovirus US2 destabilizes major histocompatibility complex class I heavy chains. J. Virol. 71:2970-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jun, Y., E. Kim, M. Jin, H. C. Sung, H. Han, D. E. Geraghty, and K. Ahn. 2000. Human cytomegalovirus gene products US3 and US6 down-regulate trophoblast class I MHC molecules. J. Immunol. 164:805-811. [DOI] [PubMed] [Google Scholar]
- 23.King, A., C. Boocock, A. M. Sharkey, L. Gardner, A. Beretta, A. G. Siccardi, and Y. W. Loke. 1996. Evidence for the expression of HLA A-C class I mRNA and protein by human first trimester trophoblast. J. Immunol. 156:2068-2076. [PubMed] [Google Scholar]
- 24.King, A., D. S. J. Allan, M. Brown, S. J. Powis, S. Joseph, S. Verma, S. E. Hiby, A. J. McMichael, Y. W. Loke, and V. M. Braud. 2000. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur. J. Immunol. 30:1623-1631. [DOI] [PubMed] [Google Scholar]
- 25.Klopfenstein, D. R. C., F. Keppeler, and H.-P. Hauri. 1998. A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J. 17:6168-6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koi, H., J. Zhang, A. Makrigiannakis, S. Getsios, C. D. MacCalman, G. S. Kopf, J. F. Strauss III, and S. Parry. 2001. Differential expression of the coxsackievirus and adenovirus receptor regulates adenovirus infection of the placenta. Biol. Reprod. 64:1001-1009. [DOI] [PubMed] [Google Scholar]
- 27.Koi, H., J. Zhang, A. Makrigiannakis, S. Getsios, C. D. MacCalman, J. F. Strauss III, and S. Parry. 2002. Syncytiotrophoblast is a barrier to maternal-fetal transmission of herpes simplex virus. Biol. Reprod. 67:1572-1579. [DOI] [PubMed] [Google Scholar]
- 28.Kovats, S., E. K. Main, C. Librach, M. Stubblebine, S. J. Fisher, and R. DeMars. 1990. A class I antigen, HLA-G, expressed in human trophoblasts. Science 248:220-223. [DOI] [PubMed] [Google Scholar]
- 29.Kyritsis, C., S. Gorbulev, S. Hutschenreiter, K. Pawlitschko, R. Abele, and R. Tampé. 2001. Molecular mechanism and structural aspects of transporter associated with antigen processing inhibition by the cytomegalovirus protein US6. J. Biol. Chem. 276:48031-48039. [DOI] [PubMed] [Google Scholar]
- 30.Lee, C., and L. B. Chen. 1988. Dynamic behavior of endoplasmic reticulum in living cells. Cell 54:37-46. [DOI] [PubMed] [Google Scholar]
- 31.Librach, C. L., Z. Werb, M. L. Fitzgerald, K. Chiu, N. M. Corwin, R. A. Esteves, D. Grobelny, R. Galardy, C. H. Damsky, and S. J. Fisher. 1991. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J. Cell Biol. 113:437-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMaster, M. T., C. L. Librach, Y. Zhou, K.-H. Lim, M. J. Janatpour, R. DeMars, S. Kovats, C. Damsky, and S. J. Fisher. 1995. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J. Immunol. 154:3771-3778. [PubMed] [Google Scholar]
- 33.Münz, C., N. Holmes, A. King, Y. W. Loke, M. Colonna, H. Schild, and H.-G. Rammensee. 1997. Human histocompatibility leukocyte antigen (HLA)-G molecules inhibit NKAT3 expressing natural killer cells. J. Exp. Med. 185:385-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norwitz, E. R., D. J. Schust, and S. J. Fisher. 2001. Implantation and the survival of early pregnancy. N. Engl. J. Med. 345:1400-1408. [DOI] [PubMed] [Google Scholar]
- 35.Onno, M., G. Le Friec, C. Pangault, L. Amiot, V. Guilloux, B. Drénou, S. Caulet-Maugendre, P. André, and R. Fauchet. 2000. Modulation of HLA-G antigens expression in myelomonocytic cells. Hum. Immunol. 61:1086-1094. [DOI] [PubMed] [Google Scholar]
- 36.Onno, M., C. Pangault, G. Le Friec, V. Guilloux, P. André, and R. Fauchet. 2000. Modulation of HLA-G antigens expression by human cytomegalovirus: specific induction in activated macrophages harboring human cytomegalovirus infection. J. Immunol. 164:6426-6434. [DOI] [PubMed] [Google Scholar]
- 37.Paul, P., N. Rouas-Freiss, I. Khalil-Daher, P. Moreau, B. Riteau, F. A. Le Gal, M. F. Avril, J. Dausset, J. G. Guillet, and E. D. Carosella. 1998. HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc. Natl. Acad. Sci. USA 95:4510-4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponte, M., C. Cantoni, R. Biassoni, A. Tradori-Cappai, G. Bentivoglio, C. Vitale, S. Bertone, A. Moretta, L. Moretta, and M. C. Mingari. 1999. Inhibitory receptors sensing HLA-G1 molecules in pregnancy: decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-G1-specific receptor. Proc. Natl. Acad. Sci. USA 96:5674-5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rehm, A., A. Engelsberg, D. Tortorella, I. J. Körner, I. Lehmann, H. L. Ploegh, and U. E. Höpken. 2002. Human cytomegalovirus gene products US2 and US11 differ in their ability to attack major histocompatibility class I heavy chains in dendritic cells. J. Virol. 76:5043-5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rouas-Freiss, N., R. M.-B. Gonçalves, C. Menier, J. Dausset, and E. D. Carosella. 1997. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc. Natl. Acad. Sci. USA 94:11520-11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schust, D. J., D. Tortorella, J. Seebach, C. Phan, and H. L. Ploegh. 1998. Trophoblast class I major histocompatibility complex (MHC) products are resistant to rapid degradation imposed by the human cytomegalovirus (HCMV) gene products US2 and US11. J. Exp. Med. 188:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terasaki, M., L. B. Chen, and K. Fujiwara. 1986. Microtubules and the endoplasmic reticulum are highly interdependent structures. J. Cell Biol. 103:1557-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warren, A. P., D. H. Ducroq, P. J. Lehner, and L. K. Borysiewicz. 1994. Human cytomegalovirus-infected cells have unstable assembly of major histocompatibility complex class I complexes and are resistant to lysis by cytotoxic T lymphocytes. J. Virol. 68:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiendl, H., M. Mitsdoerffer, V. Hofmeister, J. Wischhusen, A. Bornemann, R. Meyermann, E. H. Weiss, A. Melms, and M. Weller. 2002. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J. Immunol. 168:4772-4780. [DOI] [PubMed] [Google Scholar]
- 46.Wiertz, E. J. H. J., T. R. Jones, L. Sun, M. Bogyo, H. J. Geuze, and H. L. Ploegh. 1996. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84:769-779. [DOI] [PubMed] [Google Scholar]
- 47.Yagel, S., R. F. Casper, W. Powell, R. S. Parhar, and P. K. Lala. 1989. Characterization of pure human first-trimester cytotrophoblast cells in long-term culture: growth pattern, markers, and hormone production. Am. J. Obstet. Gynecol. 160:938-945. [DOI] [PubMed] [Google Scholar]
- 48.Yamashita, Y., K. Shimokata, S. Saga, S. Mizuno, T. Tsurumi, and Y. Nishiyama. 1994. Rapid degradation of the heavy chain of class I major histocompatibility complex antigens in the endoplasmic reticulum of human cytomegalovirus-infected cells. J. Virol. 68:7933-7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang, Y., W. Chu, D. E. Geraghty, and J. S. Hunt. 1996. Expression of HLA-G in human mononuclear phagocytes and selective induction by IFN-γ. J. Immunol. 156:4224-4231. [PubMed] [Google Scholar]