Abstract
No reliable cell culture assay is currently available for monitoring human influenza virus sensitivity to neuraminidase inhibitors (NAI). This can be explained by the observation that because of a low concentration of sialyl-α2,6-galactose (Sia[α2,6]Gal)-containing virus receptors in conventional cell lines, replication of human virus isolates shows little dependency on viral neuraminidase. To test whether overexpression of Sia(α2,6)Gal moieties in cultured cells could make them suitable for testing human influenza virus sensitivity to NAI, we stably transfected MDCK cells with cDNA of human 2,6-sialyltransferase (SIAT1). Transfected cells expressed twofold-higher amounts of 6-linked sialic acids and twofold-lower amounts of 3-linked sialic acids than parent MDCK cells as judged by staining with Sambucus nigra agglutinin and Maackia amurensis agglutinin, respectively. After transfection, binding of a clinical human influenza virus isolate was increased, whereas binding of its egg-adapted variant which preferentially bound 3-linked receptors was decreased. The sensitivity of human influenza A and B viruses to the neuraminidase inhibitor oseltamivir carboxylate was substantially improved in the SIAT1-transfected cell line and was consistent with their sensitivity in neuraminidase enzyme assay and with the hemagglutinin (HA) receptor-binding phenotype. MDCK cells stably transfected with SIAT1 may therefore be a suitable system for testing influenza virus sensitivity to NAI.
The neuraminidase (NA) of influenza A and B viruses cleaves the α-glycosidic linkages between sialic acid and the adjacent sugar and thus destroys virus receptors on the cell surface, extracellular inhibitors, and viral glycoproteins (reviewed in references 2 and 8). The NA activity is believed to be particularly important at the late stages of infection by preventing hemagglutinin (HA)-mediated self-aggregation and facilitating release of progeny virions from cells. Interaction of virions with cell-associated and soluble sialylglycoconjugates of the host is mediated by HA and NA in an antagonistic manner, which has to be carefully balanced to allow efficient virus replication (reviewed in reference 36).
With increasing use of neuraminidase inhibitors (NAI) for influenza treatment, there is a need for a suitable methodology to monitor for emergence of virus resistance (32, 34, 38). In cell culture experiments, resistance to NAI results from mutation of either HA, NA, or both glycoproteins. Mutations in HA usually precede NA mutations and reduce virus affinity for sialic acid-containing receptors, thereby decreasing the dependency of the virus on NA function, whereas mutations in NA decrease the binding affinity of the inhibitor to the catalytic site (reviewed in references 19, 29, and 30). In a clinical setting, NA-mediated resistance seems to be the primary mechanism of resistance to NAI and can be easily and reliably monitored using an in vitro enzyme inhibition assay (32, 34, 38). Since the possibility cannot be excluded that the loss of sensitivity to NAI in humans occurs also as a result of HA mutations (18, 20), it is necessary to develop techniques to study this type of resistance in low-passage-number clinical isolates.
The method of choice for testing virus sensitivity to NAI would be a virus neutralization assay in cell culture that accounts for both HA- and NA-mediated resistance. However, there is no good correlation between virus sensitivity to NAI in vivo and in laboratory cell cultures. The sensitivity of clinical virus isolates to NA inhibitors can vary in cell culture assays dramatically (up to complete insensitivity) despite a uniform high sensitivity of the enzyme in NA-inhibition tests (1, 3,37). This problem is likely due to a mismatch between virus receptors in humans and in available cell culture systems. The target cells for virus replication in human airway epithelium express high concentrations of Sia(α2,6)Gal-containing receptors and small amounts of Sia(α2,3)Gal-containing receptors (below abbreviated to 6-linked and 3-linked sialic acid receptors, respectively) (4, 9). Clinical isolates of human influenza viruses bind strongly to 6-linked sialic acids but do not bind to 3-linked sialic acids (references 13 and 21 and references therein). It is therefore believed that in order to adequately assay human influenza virus sensitivity to NAI, a cell line is required which supports efficient growth of clinical influenza virus isolates and expresses large amounts of 6-linked sialic acids and small amounts of 3-linked sialic acids (38). Unfortunately, the concentration of 6-linked sialic acids in continuous cell lines used for propagation of influenza viruses in the laboratory (such as MDCK and VERO cells) is relatively low and is comparable to the concentration of 3-linked sialic acids (16, 21, 33).
In this study, we wished to test whether performance of standard laboratory cells in the NAI sensitivity assay can be improved by purposefully changing the concentration of virus receptors on the cell surface. To this end, we permanently transfected MDCK cells with the gene of the human CMP-N-acetylneuraminate beta-galactoside α-2,6-sialyltransferase (SIAT1) [EC 2.4.99.1; other acronyms used in the literature are ST6Gal I and ST6(N)], an enzyme that catalyzes the α-2,6-sialylation of N-acetyllactosamine moieties of glycoproteins and glycolipids (see references 11 and 35 for a review). Based on previous reports on successful overexpression of SIAT1 in mammalian cells (6, 10, 12, 24, 25), we expected that transfection with SIAT1 would increase α-2,6-sialylation of the cellular and viral glycoconjugates and, thus, would increase a virus dependency on a neuraminidase activity and, hence, sensitivity to NA inhibitors. Furthermore, we hoped that transfection would decrease α-2,3-sialylation in the cells due to a competition between SIAT1 and 2,3-specific sialyltransferases for the same precursor substrate [Gal(β1,4)GlcNAc-R] (6, 24).
MATERIALS AND METHODS
Viruses.
Clinical isolates in MDCK cells of A/Memphis/14/96 (H1N1) and B/Memphis/25/99 at passage level 2 were kindly provided by Robert Webster (St. Jude Children's Hospital). The Memphis/14/96 virus was plaqued in MDCK cells; two plaques of different size were picked, amplified in MDCK cells, and designated variants M and M1. In parallel, the original passage 2 virus was passaged twice in embryonated chicken eggs. The virus progeny from two different eggs were plaque purified in MDCK cells, amplified in MDCK cells, and designated variants E and E1. The complete HA genes of the variants were sequenced by the dideoxy method, using an automatic sequencer (Perkin-Elmer). The GenBank accession numbers for the sequences are from AY282756 to AY282759.
A clinical isolate of A/Sydney/5/97-like virus (H3N2) isolated and propagated in MDCK cells and its oseltamivir-resistant variant with an R292K mutation in NA obtained from a patient on the treatment arm of a phase III efficacy study for oseltamivir phosphate were described previously (7). A clinical isolate of A/Wuhan/359/95-like virus (H3N2) and its oseltamivir-resistant variant with mutation E119V in NA were described previously (22).
MDCK cells.
The MDCK cells used were originally obtained from Alan Hay at the National Institute of Medical Research, London, United Kingdom, and passaged in Frederick Hayden's Laboratory at the University of Virginia Health Sciences Center, using standard laboratory procedures prior to distribution to Roche Discovery Welwyn. In this study, the original and SIAT1-transfected cells were passaged in Dulbecco's modified Eagle medium (Gibco BRL) supplemented with 10% fetal calf serum.
Expression vector pIRESneo-SIAT1 and stable transfection of MDCK cells.
The cDNA clone of human SIAT1 in the pcDNA3.1GS expression vector was obtained from the GeneStorm human clone collection (GeneStorm clone H-X17247 M; Invitrogen). The full-length open reading frame of SIAT1 was PCR amplified with primers 5′GGCGCAATAGCGGCCGCGCCACCATGATTCACACCAACCTGAAG-3′ and 5′GGCCGATATGGATCCTTAGCAGTGAATGGTCCGGAAGCCAG-3′, containing restriction sites for NotI and BamHI, respectively. The PCR product was cloned into the pIRESneo bicistronic expression vector (Clontech) at 5′ NotI and 3′ BamHI. Sequencing of the initially prepared construct revealed a single-nucleotide deletion in the coding region of SIAT1, leading to a frame shift (plasmid pIRESneo-SIAT1-del). The deletion was located in the region complementary to the 5′-end PCR primer used and was most likely caused by an error during primer synthesis. We corrected the deletion by using a QuikChange site-directed mutagenesis kit (Stratagene).
MDCK cells were transfected with pIRESneo-SIAT1 and pIRESneo-SIAT1-del plasmids using Lipofectamine 2000 (Life Technologies) according to the manufacturer's instructions. Transfected cultures were cultivated in the presence of 1.5 mg of antibiotic G418 sulfate (Promega)/ml. After 2 weeks, cells surviving the selection were pooled, passaged three times in 1.5 mg of G418/ml, and frozen in aliquots. Serial passages of stably transfected cells were done in the presence of 1 mg of G418/ml. Terminal plating of the cells for the infection experiments, isolation of plasma membranes, and lectin staining were performed without G418. The cells in these experiments were used at passage levels from 5 to 30 starting from the frozen cell stock.
Detection of 6-linked and 3-linked sialic acids.
Cell surface expression of sialic acids in two different types of linkages was quantified by using digoxigenin (DIG)-labeled lectins Sambucus nigra agglutinin (SNA) specific for 6-linked sialic acids, Maackia amurensis agglutinin (MAA) specific for 3-linked sialic acids, and either fluorescein isothiocyanate-labeled or peroxidase-labeled anti-DIG antibodies from the DIG-glycan differentiation kit (Boehringer Mannheim, Mannheim, Germany). Fluorescence-activated cell sorter (FACS) analysis of the cells stained with lectins was performed as described previously (17) using a FACScan fluorospectrometer (Becton Dickinson). For the solid-phase assay of lectin binding, plasma membranes were isolated from MDCK and MDCK-SIAT1 cells as described previously (14). Membrane preparations were suspended in phosphate-buffered saline (PBS) to a final protein concentration of 2 μg/ml, and 0.05-ml aliquots were incubated in the wells of a polystyrene 96-well microplate overnight at 4°C. Wells incubated with PBS served as a control of the background binding. The plate was washed with PBS and blocked with 0.1 ml of a 0.04% solution of bovine serum albumin (BSA) in PBS for 1 h at 37°C. Solutions (0.05 ml each) of DIG-labeled lectins (either SNA [2 μg/ml] or MAA [5 μg/ml]) in lectin-binding buffer (LBB) (0.2% BSA, 1 mM Mg2+, Ca2+, and Mn2+ in Tris-buffered saline [pH 7.2]) were incubated in the wells for 2 h at 4°C. After washing with PBS, the plate was incubated with 0.05 ml of peroxidase-labeled anti-DIG antibodies/well in LBB for 1 h at 4°C. O-phenylenediamine was used as a chromogenic substrate.
Binding of virus to cell membranes.
Virus attachment to cell membranes was assayed by using the microplate adsorption method (14). In brief, viruses were clarified by low-speed centrifugation, pelleted by high-speed centrifugation through a 25% sucrose cushion, and resuspended in 0.1 M Tris-HCl buffer (pH 7.3). Ninety-six-well plates coated with the cell membranes and blocked as described above were incubated with 0.05 ml of serially diluted viruses in 0.5% BSA-PBS for 30 min at 37°C. The plates were washed with ice-cold washing solution (PBS-T) (0.02% Tween 80 in PBS) and incubated with sheep antiserum against influenza virus A/Taiwan/1/86 (H1N1) (Centers for Disease Control and Prevention, Atlanta, Ga.) for 1 h at 4°C, followed by washing (PBS-T) and incubation with peroxidase-labeled anti-sheep antibodies (DAKO) for an additional hour at 4°C. PBS containing 1% BSA and 0.05% Tween 80 was used for the preparation of working dilutions of immunoreagents. The amount of bound conjugate, which reflected the amount of the virus present in the wells, was quantified using o-phenylenediamine as a substrate. The absorbency at 490 nm was measured, and the data were converted to Scatchard plots (A490 versus A490/C), where the concentration of the viruses (C) was expressed in hemagglutination units. The background virus binding to the noncoated blocked wells did not exceed 0.02 optical units and was disregarded.
Peroxidase-labeled resialylated fetuin.
To prepare monospecific fetuin-horseradish peroxidase (HRP) conjugates containing either 6-linked- or 3-linked sialic acid moieties, bovine asialofetuin (Sigma, St. Louis, Mo.) was first conjugated with HRP by using the periodate method (5). Solutions in phosphate buffer (0.1 M, pH 7.0) containing 8 mg of asialofetuin-HRP conjugate/ml, 1.5 mM CMP-Neu5Ac (Boehringer), 2 mM MgCl2, and either 80 mU of α-2,6-sialyltransferase from rat liver (WAKO Chemicals)/ml or 8.5 mU of rat recombinant α-2,3-(N)-sialyltransferase (Calbiochem)/ml were incubated at 37°C for 20 h. Resialylated conjugates were dialyzed against 0.1 M Tris-HCl buffer (pH 7.3), diluted with an equal volume of glycerol, and stored at −20°C.
Binding affinity assay.
The binding of the HRP-labeled fetuins to the viruses was studied using a solid-phase assay, as described previously (27). In brief, viruses adsorbed in the wells of 96-well EIA microplates (Greiner) were incubated with serial dilutions of HRP-labeled resialylated fetuins in PBS supplemented with 0.02% BSA, 0.02% Tween 80, and a 2 μM concentration of the neuraminidase inhibitor oseltamivir carboxylate. After incubation for 1 h at 4°C, the plates were washed with ice-cold PBS-T, and the amount of bound labeled fetuin-HRP was quantified by the peroxidase activity present in the wells. The binding data were converted to A490/C versus A490 Scatchard plots, arbitrarily taking the concentration of fetuin-HRP stocks for 1,000 U/ml. The association constants of the virus-fetuin complexes were determined from the slopes of these plots.
Focus reduction assay.
Cell cultures in 96-well plates (Greiner) were inoculated with 0.05 ml of either infection medium (IM) (0.1% BSA in Dulbecco's modified Eagle medium) or serial 10-fold dilutions of oseltamivir carboxylate in IM (concentration range from 2 nM to 200 μM)/well. After that, 50 μl/well of serial twofold virus dilutions in IM were added to cover a range of multiplicities of infection (MOIs) from about 5 to 100 focus-forming units per well. TPCK-trypsin (final concentration, 1 μg/ml) was added simultaneously with the virus to provide for multicycle replication. After 24 to 48 h of incubation at 35°C, the cells were fixed with 4% paraformaldehyde for 1 h and permeabilized with 0.2% solution of Triton X-100 in PBS for 20 min. These and all subsequent treatments of the cells were performed at room temperature. Fixed cultures were immunostained for the expression of viral nucleoprotein by incubating for 1 h with monoclonal antibodies specific for the nucleoprotein of either influenza A virus (kindly provided by Alexander Klimov at Centers for Disease Control) or influenza B virus (Serotec) followed by 1 h of incubation with peroxidase-labeled anti-mouse antibodies (Sigma) and 30 min of incubation with precipitate-forming peroxidase substrate (True Blue; KPL). Ten-percent horse serum plus 0.05% Tween 80 in PBS was used for the preparation of working dilutions of immunoreagents. Stained plates were scanned on a flatbed scanner with the data acquired by Adobe Photoshop 7.0 software. To compensate for some occasional variation in the size and number of foci in individual wells, we used two to four replicate wells per experimental point. Ninety percent inhibitory concentrations presented in Table 1 were estimated by visual observation of cultures infected at a MOI of 20 to 40 focus-forming units per well. These values correspond to the lowest of the drug concentrations used, which reduced the stained area in the wells at least 10-fold.
TABLE 1.
Receptor-binding characteristics and sensitivities to oseltamivir carboxylate of HA variants of A/Memphis/14/96 (H1N1)
| Variant | Amino acid substitution in the HAa | Binding affinityc, Kass (ml/U)
|
Sensitivity to oseltamivir carboxylate, IC90 (μM)d
|
||
|---|---|---|---|---|---|
| 2,6-Fetuin-HRP | 2,3-Fetuin-HRP | 24 h | 48 h | ||
| M | 3.3 ± 0.10 | 0.046 ± 0.021 | 0.1 | 1.0 | |
| M1 | E156K | 4.9 ± 0.58 | 0.70 ± 0.30 | NDe | 0.01 |
| E | N129Kb | 4.8 ± 0.60 | 0.48 ± 0.20 | 0.01 | NTf |
| E1 | Q226R | 0.09 ± 0.02 | 0.85 ± 0.31 | >100 | >100 |
With respect to HA sequence of variant M. H3 numbering system.
The mutation destroys a glycosylation sequon in the HA.
Affinity of virus binding to HRP-labeled resialylated fetuins containing either 6-linked or 3-linked sialic acid moieties. The higher values of association constants (Kass) reflect higher affinities.
Focus reduction assay in MDCK-SIATI cells was performed on two different occasions, with infection times of 24 and 48 h, respectively (see the text). IC90, 90% inhibitory concentration.
ND, not determined. IC90 of MI variant could not be determined due to a poor virus spread 24 h postinfection.
NT, not tested.
RESULTS
Transfection of MDCK cells with SIAT1 alters surface expression of 6-linked and 3-linked sialic acids and enhances binding of a human influenza virus.
For the permanent expression of 2,6-sialyltransferase, we cloned human SIAT1 cDNA into the bicistronic mammalian expression vector pIRESneo. Because this vector expresses the gene of interest and an antibiotic resistance marker from the same mRNA, nearly all colonies surviving the selective pressure must stably express the gene of interest (31). Therefore, after transfection of MDCK cells with the pIRESneo-SIAT1 plasmid, we did not isolate individual resistant clones but pooled several clones which survived the selection with G418 sulfate.
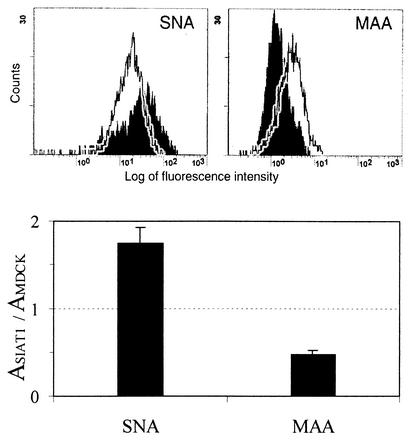
To compare the expression of 3-linked and 6-linked sialic acids on the surface of transfected and parent cells, we used the linkage-specific lectins SNA and MAA. FACS analysis indicated that transfection with the pIRESneo-SIAT1 plasmid enhanced expression of 6-linked sialic acids and simultaneously lowered expression of 3-linked sialic acids (Fig. 1, top panel). In a solid-phase lectin binding assay, the binding of SNA to the plasma membrane of transfected cells increased about twofold, and the binding of MAA decreased by about 50% (Fig. 1, bottom panel). This pattern of reactivity with lectins remained unchanged after 25 passages of MDCK-SIAT1 cells in the presence of G418 (data not shown), indicating that the sialylation phenotype of the cells was stable. As a control, we used MDCK cells permanently transfected with a pIRESneo-SIAT1-del plasmid which contained a frame shift deletion in the SIAT1 gene (see Materials and Methods). In this case, as expected, the lectin binding assay revealed no differences between parent and transfected cells (data not shown).
FIG. 1.
Binding of the linkage-specific lectins SNA and MAA to cell surface receptors of SIAT1-transfected and parent MDCK cells. Top panel, MDCK-SIAT1 cells (shaded profiles) and MDCK cells (open profiles) were incubated with DIG-labeled lectins followed by incubation with fluorescein isothiocyanate-labeled anti-DIG antibodies and subjected to FACS analysis. Bottom panel, preparations of cell plasma membranes adsorbed in the wells of a 96-well plate were incubated with DIG-labeled lectins followed by incubation with peroxidase-labeled anti-DIG-antibodies. The data represent ratios of absorbencies in the wells coated with membranes of MDCK-SIAT1 cells to those in wells coated with membranes of MDCK cells.
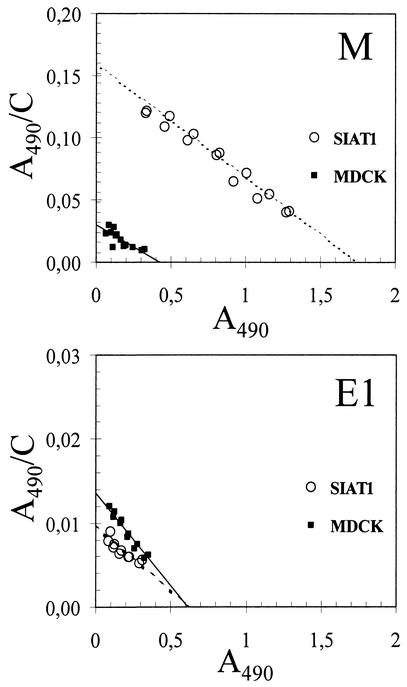
To test whether changes in sialylation of SIAT1-transfected cells affected the strength of virus binding to these cells, we used two influenza viruses with distinct receptor-binding phenotypes. The clinical A/Memphis/14/96-M (H1N1) isolate displayed a preference for 6-linked sialic acids and did not bind to 3-linked sialic acids as is typical for human viruses. The egg-adapted variant E1 of this virus had the opposite receptor specificity due to a point mutation Q226R in the HA (see Table 1). The M virus bound to membranes isolated from MDCK-SIAT1 cells significantly better than it bound to membranes of parent MDCK cells (Fig. 2, top panel). On the contrary, the egg-adapted mutant bound to transfected cells more weakly than to MDCK cells (Fig. 2, bottom panel), although the difference in binding was less pronounced than in the case of M virus.
FIG. 2.
Scatchard plots for the binding of A/Memphis/14/96-M (H1N1) (top panel) and its egg-adapted mutant E1 (bottom panel) to plasma membranes from MDCK cells (closed boxes, solid line) and from MDCK-SIAT1 cells (open circles, dotted line). Data points represent results of two replicate experiments performed on the same plate as described in Materials and Methods.
Transfection of MDCK cells with SIAT1 increases sensitivity of influenza viruses to oseltamivir carboxylate in a cell culture neutralization assay.
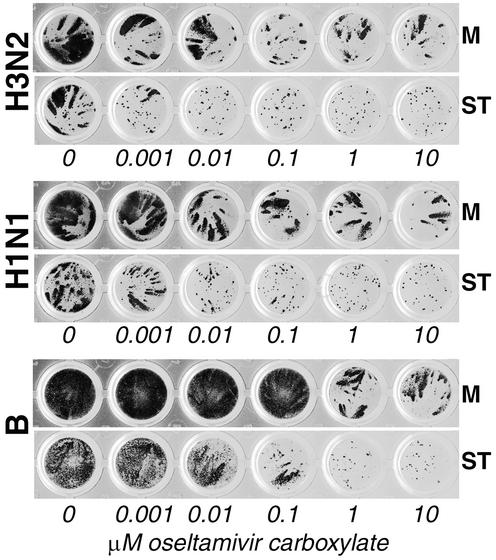
Because influenza viruses did not produce clearly visible plaques in MDCK-SIAT1 cells, we used a focus reduction assay to compare virus sensitivity to NA inhibitors in the parent MDCK cells and in the SIAT1-transfected cells. Parallel cultures of two cell lines in 96-well plates were infected with serial virus dilutions in the presence or absence of oseltamivir carboxylate and immunostained for the expression of virus nucleoprotein. The effect of the drug on virus spread in parent and transfected cells was compared at MOIs which resulted in growth of about 15 to 50 foci of infected cells per well. In the first series of experiments, three clinical virus isolates, A/Sydney/5/97 (H3N2), A/Memphis/14/96-M (H1N1), and B/Memphis/25/99, were tested. All three viruses displayed a higher sensitivity to oseltamivir carboxylate in MDCK-SIAT1 cells than in nontransfected cells. This pattern of sensitivity was reproducible in replicate assays performed on different days. Results of one representative experiment are shown in Fig. 3. In MDCK-SIAT1 cells, 0.001, 0.01, and 0.1 μM concentrations of the drug markedly decreased the size of infectious foci formed by H3N2, H1N1, and type B viruses, respectively. A further 10-fold increase in the respective drug concentrations completely abolished virus spread in transfected cells, as judged by the disappearance of the comet-like foci of infection. By contrast, even the highest drug concentrations did not completely inhibit formation of foci in MDCK cells. Thus, MDCK-SIAT1 cells provide a more sensitive system than MDCK cells for testing the susceptibility of human influenza A and B viruses to NAI.
FIG. 3.
Effect of oseltamivir carboxylate on the formation of virus foci in parallel cultures of MDCK (M) and MDCK-SIAT1 cells (ST) infected with the same virus multiplicity. Experiments were performed in 96-well plates as described in Materials and Methods using clinical virus isolates A/Sydney/5/97 (H3N2) (top panel), A/Memphis/14/96-M (H1N1) (middle panel), and B/Memphis/25/99 (bottom panel). The infection time was 24 h for the type A viruses and 48 h for the type B virus.
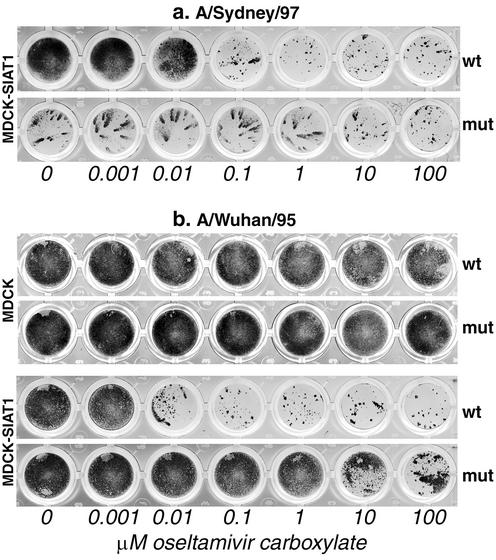
To check whether a focus reduction assay with MDCK-SIAT1 cells could clearly identify viruses with drug-resistant mutations in NA, we compared two drug-sensitive clinical virus isolates with their oseltamivir treatment-selected drug-resistant mutants (Fig. 4). A 0.1 μM concentration of oseltamivir carboxylate substantially inhibited spread of a wild-type A/Sydney/97-like virus (Fig. 4a). By contrast, a 100-fold-higher concentration of the drug (10 μM) was required for a detectable inhibition of the drug-resistant variant with an R292K mutation in NA. Interestingly, the infectious foci of the mutant in the absence of the drug were smaller that those of the wild-type virus, suggesting that the NA mutation impaired spread of the virus in MDCK-SIAT1 cells. This effect is consistent with the reported compromised infectivity of the R292K NA mutant in vivo (7). Because of a slower spread of the NA mutant, this virus produced very small foci 24 h postinfection, making it impossible to assess a reduction in focus size in the presence of NA inhibitor (data not shown). To solve this problem, we used longer infection times for comparison of wild-type viruses and NA mutants. It has to be mentioned that an increase in the incubation time resulted in an apparent decrease in the virus sensitivity to the inhibitor. For example, 0.001 and 0.1 μM concentrations of the drug were required, respectively, for a substantial inhibition of wild-type virus spread during 24 h (Fig. 3) and 40 h of infection (Fig. 4a). Thus, standardization of the assay time will be important for routine drug sensitivity monitoring using a focus reduction assay.
FIG. 4.
Comparison of clinical isolates (wt) and their drug-resistant variants with NA mutations (mut) by focus reduction assay. (a) A/Sydney/5/97-like virus (H3N2) and its variant with NA-substitution R292K assayed in MDCK-SIAT1 cells. Infection time was 40 h. (b) A/Wuhan/359/95-like virus (H3N2) and its variant with NA mutation E119V assayed in parallel in MDCK cells (two upper rows) and in MDCK-SIAT1 cells (two lower rows). Infection time was 48 h.
Figure 4b shows the results obtained with another pair of pre- and posttreatment H3N2 viruses which were tested in parallel in two cell lines. In MDCK cells, neither virus is drug sensitive, making it impossible to discriminate between resistant mutant and the original virus. However, in MDCK-SIAT1 cells, the wild-type virus can be clearly differentiated from its drug-resistant mutant with an E119V mutation in the NA.
Thus, experiments with two pre- and posttreatment virus pairs tested suggested that NA-mediated drug resistance can be reliably detected in SIAT1-transfected cells.
Sensitivity to NA inhibitor in MDCK-SIAT1 cells correlates with virus affinity for 6-linked sialic acids.
To address the question of whether HA-mediated drug resistance could be adequately assayed in MDCK-SIAT1 cells, we needed a panel of virus variants which differ by the receptor-binding characteristics of their HAs. To generate such variants, we selected two receptor-binding mutants (E and E1) of the clinical H1N1 virus isolate A/Memphis/14/96 by adaptation of the virus to embryonated hen's eggs. Furthermore, during the plaque purification of the original human virus in MDCK cells, two MDCK-derived variants were obtained, M and M1. The complete HA genes of four virus variants were sequenced, and their receptor-binding characteristics were determined using resialylated fetuin, which carried either 3- or 6-linked sialic acids (Table 1).
Among both MDCK-derived viruses, only variant M displayed a typical receptor-binding phenotype of clinical human isolates, namely, a high affinity for 6-linked sialic acids and a lack of binding to 3-linked sialic acids (13, 15). Variant M1 differed from variant M by the amino acid substitution E156K, which increased a positive charge of the globular head of HA by two units of charge and enhanced the virus binding affinity for both 3-linked and 6-linked sialic acids. Analysis of the HA sequences in the Influenza Sequence Database (26) indicated that the A/Memphis/14/96 strain used in this study was phylogenetically closely related to H1N1 viruses isolated in 1995 to 1996. Among about 30 H1 HA sequences from this time period, three sequences carry 156G and the rest carry 156E. Furthermore, none of more than a hundred human H1N1 viruses isolated between 1995 and 2001 contains 156K. Based on the results of sequence comparison and on receptor-binding characteristics, we conclude that variant M represents the original clinical human virus, whereas M1 is a receptor-binding mutant selected in MDCK cells.
Egg adaptation of human viruses is based on selection of HA mutants with enhanced affinity for 3-linked sialic acids and, as a rule, leads to accompanying changes in the affinity for 6-linked sialic acids (13, 15, 21). Accordingly, the egg-adapted variants E and E1 demonstrated significantly enhanced binding to fetuin carrying 3-linked sialic acids compared to the binding of the original M variant (Table 1). In the case of variant E, the HA mutation N129K, responsible for the adaptation, increased the positive charge of HA, destroyed a glycosylation sequon near the receptor binding site, and enhanced the virus affinity for both 3-linked and 6-linked sialic acids. By contrast, substitution Q226R in E1 almost completely destroyed the ability of the virus to bind to 6-linked receptors. Interestingly, the amino acid in position 226 is crucial for the recognition of the Sia-Gal linkage by H2 and H3 subtype HAs (reference 28 and references therein). The E1 variant described here provides the first example of a similar role of the 226 position in an H1N1 human virus.
We next compared four receptor-binding variants for their sensitivity to oseltamivir carboxylate in MDCK-SIAT1 cells (Table 1). In the first experiment, the cells were fixed 24 h after infection. Under these conditions, 0.1 and 0.01 μM concentrations of the drug were required to inhibit the spread of M and E variants by 90%, respectively, whereas E1 virus was not inhibited to any significant extent at the highest concentration of oseltamivir carboxylate tested (100 μM). Because the M1 variant produced very tiny foci 24 h postinfection, precluding unambiguous detection of inhibition, the assay was repeated for M, M1, and E1 viruses using a 48-h incubation time. In this case, the M1 variant was 100-fold more sensitive to the NA inhibitor than the original M virus. Together these results indicated that the sensitivity of the viruses to the drug in MDCK-SIAT1 cells was mainly dependent on virus affinity for 6-linked rather than for 3-linked sialic acid receptors. Namely, E and M1 mutants with enhanced binding affinity for 6-linked receptors were more sensitive to oseltamivir carboxylate than the wild-type virus, whereas the E1 variant was completely resistant, in correlation with its inability to bind to 6-linked sialic acids and despite its highest affinity for 3-linked sialic acids. It seems, however, that the virus affinity for 3-linked receptors could somewhat modify its susceptibility to the drug. Thus, a high sensitivity to oseltamivir of the M1 variant compared to that of the E variant correlated with a higher affinity of the former virus for 2,3-fetuin.
DISCUSSION
Low-passage-number clinical influenza virus isolates often appear insensitive to NAI when tested in MDCK or other laboratory cell lines. This precludes the detection of changes in viral drug sensitivity (resistance) between pre- and posttreatment isolates and can give rise to false-positive identification of resistance in population screening. This study was undertaken to test whether a genetically engineered increase in the density of human influenza virus receptors on the surface of MDCK cells can make the virus more sensitive to NAI in these cells. To address this question, we permanently transfected MDCK cells with the gene of human 2,6-sialyltransferase.
Stable transfection with the SIAT1 gene increased the concentration of 6-linked sialic acids on the surface of MDCK cells about twofold. This effect is consistent with enhanced α-2,6-sialylation in several other mammalian cell lines after their transfection with SIAT1 (6, 10, 12, 24, 25). We found that a typical non-egg-adapted human H1N1 influenza virus bound more strongly to transfected cells than to parent MDCK cells. This result confirmed that expression of SIAT1 enhanced the concentration of 6-linked sialic acid receptors accessible to the virus on a cell surface. SIAT1 catalyses synthesis of 6′-sialyl(N-acetyllactosamine) [Neu5Ac(α2,6)Gal(β1,4)GlcNAc], the common high-affinity receptor determinant of human influenza A and B viruses (13). We speculate, therefore, that all human influenza viruses irrespective of their type and subtype will likely display enhanced binding to MDCK-SIAT1.
Representative clinical isolates of H1N1 and H3N2 influenza A viruses and type B viruses were substantially more sensitive to the NA inhibitor oseltamivir carboxylate in SIAT1-transfected cells than in parent cells. These data support the hypothesis that the well-known low sensitivity of clinical isolates of human viruses to NAI in MDCK cells is due to a low expression of 6-linked virus receptors in these cells (19, 30, 38). There are at least two possible mechanisms by which increased α-2,6-sialylation in the cells enhances virus sensitivity to NAI. First, due to an increased density of 6-linked cell-surface sialic acids, progeny virions attach more strongly to the surface of MDCK-SIAT1 cells and hence require a higher neuraminidase activity for receptor cleavage and virus release. Second, enhanced activity of SIAT1 in the cells can lead to an increased α-2,6-sialylation of the viral glycoproteins. As a consequence, a higher level of NA activity would be needed to desialylate viral HA and NA to a degree that prevents self-aggregation of the progeny virions. Thus, our results suggest that transfection with SIAT1 may solve or at least reduce the major problem which hampers utilization of MDCK cells for a neutralization assay of virus sensitivity to NAI (38).
In addition to the low sensitivity of clinical human viruses to NAI in standard laboratory cells potentially giving false-positive resistance results (3, 37), false-negative resistance results in MDCK cells were also reported (18, 20). For example, an influenza B virus isolate recovered from an immunocompromised child after 12 days of treatment with zanamivir was highly sensitive to the drug in MDCK cells despite a drug-resistant phenotype in the NA enzyme inhibition assay (18). Compared to the pretreatment isolate, this virus had a drug-resistant mutation in the NA catalytic site (R152K) and a mutation in the region of the HA receptor-binding site (T198I). The HA mutation increased the virus binding to 3-linked receptors and, as a result, to MDCK cells which express high quantities of 3-linked sialic acids (21, 33). Enhanced binding to cells increased the virus dependence on the NA activity, thereby masking the drug-resistant effect of the NA mutation. These data suggested that laboratory cells do not adequately mimic virus infection of airway epithelium in vivo, not only because of a lower level of expression of 6-linked sialic acids but also because of a higher concentration of 3-linked receptors. If this hypothesis is correct, a reduced expression of 3-linked sialic acids in laboratory cells would be desirable for adequate testing of virus sensitivity to NAI. In view of this hypothesis, we hoped that permanent expression of the SIAT1 gene in MDCK cells could decrease α-2,3-sialylation in these cells due to competition between SIAT1 and α-2,3-sialyltransferases which utilize the same substrate [Gal(β1,4)GlcNAc] (for example, ST3Gal III and ST3Gal IV [35]). The MAA which we used to determine changes in expression of 3-linked sialic acids selectively binds to Neu5Ac/Gc(α2,3)Gal(β1,4)GlcNAc moieties synthesized by ST3Gal III and ST3Gal IV (23). As we expected, the concentration of these moieties in the SIAT1-transfected cells decreased by about 50%. However, because one cannot expect a competition between SIAT1 and α-2,3-sialyltransferases with different substrate specificities [such as ST3Gal I and ST3Gal II, which sialylate Gal(β1,3)GalNAc], the total decrease in expression of 3-linked sialic acids in all possible contexts was certainly less than that determined with MAA. This notion may explain why we observed a relatively marginal decrease in binding of an egg-adapted human virus to MDCK-SIAT1 cells.
Although NA mutations appear to be the major mechanism of influenza virus resistance to NAI in a clinical setting (32, 34, 38), the possibility that resistance emerges due to mutations in HA cannot be completely excluded. Thus, in a study cited above, the HA mutation T198I in influenza B virus was first observed after 8 days of treatment with zanamivir, at least 4 days before the emergence of a drug resistance mutation in NA (18). The substitution T198I was found to lower substantially the HA affinity for 6-linked sialic acid receptors. The authors suggested that this mutation resulted in a decreased virus binding to human airway epithelial cells (where 6-linked receptors predominate) and thus reduced dependence of virus replication on the NA function in humans (HA-mediated resistance to NAI). Support to this concept came from the study in which volunteers who were experimentally infected with egg-adapted human H1N1 virus and treated with oseltamivir were monitored for the emergence of drug-resistant viruses by phenotypic assays and sequencing of HA and NA (20). Due to its passaging in eggs, the original virus inoculum represented a heterogeneous mixture of receptor-binding variants differing by amino acid substitutions in HA. In the last-day isolates from volunteers, the ratio of the variants changed from that in the inoculum. In the placebo group, variants with a higher affinity for 6-linked receptors predominated over the egg-derived low-affinity variants with typical egg adaptation mutations in HA. By contrast, in drug-treated groups reversion of the egg-derived variants to the non-egg-adapted genotype was inhibited. These data suggested that viruses with a lower affinity for 6-linked sialic acid receptors, such as egg-adapted variants of clinical human isolates, can be less sensitive to NAI in vivo than original non-egg-adapted viruses. Based on these results, it is currently believed that HA mutations decreasing virus affinity for 6-linked sialic acids may be a primary mechanism of the HA-mediated resistance to NA inhibitors in humans.
To study HA-mediated virus resistance to NAI in MDCK-SIAT1 cells, we compared a clinical human virus with its three laboratory-derived receptor binding variants, which differed from the parent virus by single amino acid substitutions in HA. The sensitivity of the viruses to oseltamivir carboxylate correlated with their affinity for 6-linked sialic acids rather than for 3-linked sialic acids. This result suggests that neutralization assays in MDCK-SIAT1 have the potential to adequately account for the HA-mediated resistance in humans. However, viruses with known HA-mediated resistance in vivo have to be tested in this assay to check whether this assumption is correct.
In summary, by stable transfection of the SIAT1 gene we were able to make the spectrum of the sialic acid receptors in MDCK cells more similar to that of human airway epithelium. The sensitivity of human influenza A and B viruses to neuraminidase inhibitor in MDCK-SIAT1 cells was increased and was consistent with their sensitivity in the NA enzyme assay and with the HA receptor-binding phenotype. These features make MDCK-SIAT1 cells a promising system for testing sensitivity of influenza virus to NAI.
Acknowledgments
M.M., T.M., and H.-D.K. were supported by Roche Products Ltd., Welwyn Garden City, United Kingdom.
We thank Robert Webster for providing clinical virus isolates, Matthias Dobbelstein for his advice on cloning, and Andreas Kaufmann for assistance with FACS analysis.
REFERENCES
- 1.Abed, Y., A. M. Bourgault, R. J. Fenton, P. J. Morley, D. Gower, I. J. Owens, M. Tisdale, and G. Boivin. 2002. Characterization of 2 influenza A(H3N2) clinical isolates with reduced susceptibility to neuraminidase inhibitors due to mutations in the hemagglutinin gene. J. Infect. Dis. 186:1074-1080. [DOI] [PubMed] [Google Scholar]
- 2.Air, G. M., and W. G. Laver. 1989. The neuraminidase of influenza virus. Proteins 6:341-356. [DOI] [PubMed] [Google Scholar]
- 3.Barnett, J. M., A. Cadman, D. Gor, M. Dempsey, M. Walters, A. Candlin, M. Tisdale, P. J. Morley, I. J. Owens, R. J. Fenton, A. P. Lewis, E. C. Claas, G. F. Rimmelzwaan, R. De Groot, and A. D. Osterhaus. 2000. Zanamivir susceptibility monitoring and characterization of influenza virus clinical isolates obtained during phase II clinical efficacy studies. Antimicrob. Agents Chemother. 44:78-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baum, L. G., and J. C. Paulson. 1990. Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem. Suppl. 40:35-38. [PubMed] [Google Scholar]
- 5.Boorsma, D. M., and J. G. Streefkerk. 1979. Periodate or glutaraldehyde for preparing peroxidase conjugates? J. Immunol. Methods 30:245-255. [DOI] [PubMed] [Google Scholar]
- 6.Breen, K. C., A. Potratz, N. Georgopoulou, and K. Sandhoff. 1998. The generation and characterization of a rat neural cell line overexpressing the alpha2,6(N) sialyltransferase. Glycoconj. J. 15:199-202. [DOI] [PubMed] [Google Scholar]
- 7.Carr, J., J. Ives, L. Kelly, R. Lambkin, J. Oxford, D. Mendel, L. Tai, and N. Roberts. 2002. Influenza virus carrying neuraminidase with reduced sensitivity to oseltamivir carboxylate has altered properties in vitro and is compromised for infectivity and replicative ability in vivo. Antivir. Res. 54:79-88. [DOI] [PubMed] [Google Scholar]
- 8.Colman, P. M. 1998. Structure and function of the neuraminidase, p. 65-73. In K. G. Nicholson, R. G. Webster, and A. J. Hay (ed.), Textbook of influenza. Blackwell Science, London, United Kingdom.
- 9.Couceiro, J. N., J. C. Paulson, and L. G. Baum. 1993. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 29:155-165. [DOI] [PubMed] [Google Scholar]
- 10.Dall'Olio, F., M. Chiricolo, P. Lollini, and J. T. Lau. 1995. Human colon cancer cell lines permanently expressing alpha 2,6-sialylated sugar chains by transfection with rat beta-galactoside alpha 2,6 sialyltransferase cDNA. Biochem. Biophys. Res. Commun. 211:554-561. [DOI] [PubMed] [Google Scholar]
- 11.Dall'Olio, F. 2000. The sialyl-α2,6-lactosaminyl structure: biosynthesis and functional role. Glycoconj. J. 17:669-676. [DOI] [PubMed] [Google Scholar]
- 12.Fukuta, K., T. Yokomatsu, R. Abe, M. Asanagi, and T. Makino. 2000. Genetic engineering of CHO cells producing human interferon-gamma by transfection of sialyltransferases. Glycoconj. J. 17:895-904. [DOI] [PubMed] [Google Scholar]
- 13.Gambaryan, A. S., A. B. Tuzikov, V. E. Piskarev, S. S. Yamnikova, D. K. Lvov, J. S. Robertson, N. V. Bovin, and M. N. Matrosovich. 1997. Specification of receptor-binding phenotypes of influenza virus isolates from different hosts using synthetic sialylglycopolymers: non-egg-adapted human H1 and H3 influenza A and influenza B viruses share a common high binding affinity for 6′-sialyl(N-acetyllactosamine). Virology 232:345-350. [DOI] [PubMed] [Google Scholar]
- 14.Gambaryan, A. S., V. P. Marinina, A. B. Tuzikov, N. V. Bovin, I. A. Rudneva, B. V. Sinitsyn, A. A. Shilov, and M. N. Matrosovich. 1998. Effects of host-dependent glycosylation of hemagglutinin on receptor-binding properties of H1N1 human influenza A virus grown in MDCK cells and in embryonated eggs. Virology 247:170-177. [DOI] [PubMed] [Google Scholar]
- 15.Gambaryan, A. S., J. S. Robertson, and M. N. Matrosovich. 1999. Effects of egg-adaptation on the receptor-binding properties of human influenza A and B viruses. Virology 258:232-239. [DOI] [PubMed] [Google Scholar]
- 16.Govorkova, E. A., G. Murti, B. Meignier, C. de Taisne, and R. G. Webster. 1996. African green monkey kidney (Vero) cells provide an alternative host cell system for influenza A and B viruses. J. Virol. 70:5519-5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govorkova, E. A., M. N. Matrosovich, A. B. Tuzikov, N. V. Bovin, C. Gerdil, B. Fanget, and R. G. Webster. 1999. Selection of receptor-binding variants of human influenza A and B viruses in baby hamster kidney cells. Virology 262:31-38. [DOI] [PubMed] [Google Scholar]
- 18.Gubareva, L. V., M. N. Matrosovich, M. K. Brenner, R. C. Bethell, and R. G. Webster. 1998. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J. Infect. Dis. 178:1257-1262. [DOI] [PubMed] [Google Scholar]
- 19.Gubareva, L. V., L. Kaiser, and F. G. Hayden. 2000. Influenza virus neuraminidase inhibitors. Lancet 355:827-835. [DOI] [PubMed] [Google Scholar]
- 20.Gubareva, L. V., L. Kaiser, M. N. Matrosovich, Y. Soo-Hoo, and F. G. Hayden. 2001. Selection of influenza virus mutants in experimentally infected volunteers treated with oseltamivir. J. Infect. Dis. 183:523-531. [DOI] [PubMed] [Google Scholar]
- 21.Ito, T., Y. Suzuki, A. Takada, A. Kawamoto, K. Otsuki, H. Masuda, M. Yamada, T. Suzuki, H. Kida, and Y. Kawaoka. 1997. Differences in sialic acid-galactose linkages in the chicken egg amnion and allantois influence human influenza virus receptor specificity and variant selection. J. Virol. 71:3357-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ives, J., J. Carr, N. A. Roberts, C. Y. Tai, D. B. Mendel, L. Kelly, R. Lambkin, and J. Oxford. 2000. An oseltamivir treatment-selected influenza A/Wuhan/359/95 virus with E119V mutation in the neuraminidase gene has reduced infectivity in vivo. J. Clin. Virol. 18:255-256. [Google Scholar]
- 23.Knibbs, R. N., I. J. Goldstein, R. M. Ratcliffe, and N. Shibuya. 1991. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. J. Biol. Chem. 266:83-88. [PubMed] [Google Scholar]
- 24.Lee, E. U., J. Roth, and J. C. Paulson. 1989. Alteration of terminal glycosylation sequences on N-linked oligosaccharides of Chinese hamster ovary cells by expression of beta-galactoside alpha 2,6-sialyltransferase. J. Biol. Chem. 264:13848-13855. [PubMed] [Google Scholar]
- 25.Lin, S., W. Kemmner, S. Grigull, and P. M. Schlag. 2002. Cell surface alpha 2,6 sialylation affects adhesion of breast carcinoma cells. Exp. Cell Res. 276:101-110. [DOI] [PubMed] [Google Scholar]
- 26.Macken, C., H. Lu, J. Goodman, and L. Boykin. 2001. The value of a database in surveillance and vaccine selection, p. 103-106. In A. D. Osterhaus, N. Cox, and A. W. Hampson (ed.), Options for the control of influenza IV. Elsevier Science, Amsterdam, The Netherlands.
- 27.Matrosovich, M., N. Zhou, Y. Kawaoka, and R. Webster. 1999. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J. Virol. 73:1146-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matrosovich, M., A. Tuzikov, N. Bovin, A. Gambaryan, A. Klimov, M. R. Castrucci, I. Donatelli, and Y. Kawaoka. 2000. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J. Virol. 74:8502-8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKimm-Breschkin, J. L. 2000. Resistance of influenza viruses to neuraminidase inhibitors—a review. Antivir. Res. 47:1-17. [DOI] [PubMed] [Google Scholar]
- 30.Mendel, D. B., and R. W. Sidwell. 1998. Influenza virus resistance to neuraminidase inhibitors. Drug Resist. Updates 1:184-189. [DOI] [PubMed] [Google Scholar]
- 31.Rees, S., J. Coote, J. Stables, S. Goodson, S. Harris, and M. G. Lee. 1996. Bicistronic vector for the creation of stable mammalian cell lines that predisposes all antibiotic-resistant cells to express recombinant protein. BioTechniques 20:102-110. [DOI] [PubMed] [Google Scholar]
- 32.Roberts, N. A. 2001. Treatment of influenza with neuraminidase inhibitors: virological implications. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:1895-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo, S. H., O. Goloubeva, R. Webby, and R. G. Webster. 2001. Characterization of a porcine lung epithelial cell line suitable for influenza virus studies. J. Virol. 75:9517-9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tisdale, M. 2000. Monitoring of viral susceptibility: new challenges with the development of influenza NA inhibitors. Rev. Med. Virol. 10:45-55. [DOI] [PubMed] [Google Scholar]
- 35.Tsuji, S. 1996. Molecular cloning and functional analysis of sialyltransferases. J. Biochem. 120:1-13. [DOI] [PubMed] [Google Scholar]
- 36.Wagner, R., M. Matrosovich, and H.-D. Klenk. 2002. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev. Med. Virol. 12:159-166. [DOI] [PubMed] [Google Scholar]
- 37.Woods, J. M., R. C. Bethell, J. A. V. Coates, N. Healy, S. A. Hiscox, B. A. Pearson, D. M. Ryan, J. Ticehurst, J. Tilling, S. M. Walcott, and C. R. Penn. 1993. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob. Agents Chemother. 37:1473-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zambon, M., and F. G. Hayden. 2001. Position statement: global neuraminidase inhibitor susceptibility network. Antivir. Res. 49:147-156. [DOI] [PubMed] [Google Scholar]