Abstract
Antibodies (Abs) contribute to the control of influenza virus infection in vivo by reducing progeny virus yield from infected cells (yield reduction [YR]) and by inhibiting progeny virus from spreading the infection to new host cells (virus neutralization [VN]). Previous studies showed that the infection could be resolved in severe combined immunodeficiency (SCID) mice by treatment with hemagglutinin (HA)-specific monoclonal antibodies (MAbs) that exhibit both VN and YR activities but not by MAbs that exhibited only YR activity. To determine whether virus clearance requires both activities, we measured the therapeutic activity of an HA-specific MAb (VN and YR) and its Fab fragment (VN) by intranasal (i.n.) administration to infected SCID mice. Immunoglobulin G (IgG) and Fab cleared the infection with i.n. 50% effective doses (ED50s) of 16 and 90 pmol, respectively. To resolve an established infection solely by VN activity, Fab must be present in the respiratory tract at an effective threshold concentration until all infected cells have died and production of virus has ceased. Because IgG and Fab had different half-lives in the respiratory tract (22 and 8 h, respectively) and assuming that both operated mainly or solely by VN, it could be estimated that clearance was achieved 24 h after Ab treatment when both reagents were present in the respiratory tract at ∼10 pmol. This dose was ∼200 times larger than the respiratory tract-associated Ab dose resulting from administration of the intraperitoneal ED50 (270 pmol) of IgG. This indicated that our procedure of i.n. administration of Ab did not make optimal use of the Ab's therapeutic activity.
Many innate and adaptive components of the host defense system have been shown to participate in the control of influenza virus infection. Among these, antibodies (Abs) play a central role, particularly in the immune host, where they may provide “sterilizing immunity” or greatly impair virus replication, depending on their specificity and titer (7, 8, 16, 30). We have been interested in identifying the mechanisms by which Abs contribute to the control of this infection. In principle, they can act at two distinct stages of the viral replication cycle: (i) reaction with viral proteins expressed on the surface of live infected cells may result, directly or indirectly, in reduced production or release of infectious progeny virus; and (ii) reaction with released virus may impair the ability of the virus to infect new host cells. We refer to the former activities as yield reduction (YR) and to the latter as virus neutralization (VN). YR activities may comprise the targeting of complement deposition and Fc receptor (FcR)-expressing effector cells to infected host cells, Ab-mediated catalysis of hydrogen peroxide and ozone formation (50), and possibly the mere cross-linking of viral antigens in the plasma membrane of infected cells (11, 15, 42, 48). Similarly, VN activity may comprise various mechanisms that reduce the ability of free virus to spread the infection to new host cells (8, 12, 33).
Previous studies have shown that treatment of infected SCID mice with Abs that exhibited YR but no measurable VN activity decreased virus titers in the respiratory tract but failed to resolve the infection (29). In contrast, treatment with hemagglutinin (HA)-specific Abs that exhibited VN and presumably YR activity was capable of resolving the infection (28, 32). Indeed, a mixture of HA-specific monoclonal antibodies (MAbs) with different epitope specificities (to prevent outgrowth of viral escape mutants) was therapeutically effective when given to SCID mice with massive pulmonary infections (32). These findings raised the question of whether HA-specific Abs were therapeutically so effective because they concomitantly expressed VN and YR activities or because of VN alone. Consistent with the former possibility was the finding that an Ab mixture that contained a saturating dose of YR-exhibiting MAb and a small dose of an HA-specific MAb exhibited greatly improved therapeutic activity compared to the individual components of the mixture (29).
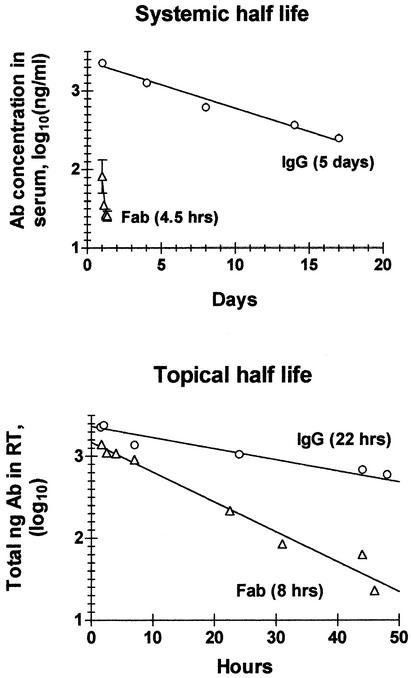
Here, we addressed the question presented above by measuring the therapeutic activities of an intact HA-specific MAb and its Fab fragment. Both the intact MAb and Fab exhibited high VN activities in vitro, but they presumably differed greatly in Fc- and bivalency-dependent YR activities (although the latter could not be measured in the presence of VN activity). Ab treatment was administered by the intranasal (i.n.) route, because the half-lives of Fab and IgG were less dissimilar after i.n. administration (22 and 8 h, respectively) than after intraperitoneal (i.p.) administration (IgG, 120 h; Fab, 4.5 h). The study shows that the infection could be resolved by a single i.n. administration of Fab, indicating that YR activity is not required for Ab-mediated resolution of influenza virus infection. The finding that Fab had a larger i.n. 50% effective dose (ED50) than IgG (5.6 times on a molar basis and 4.8 times on the basis of VN activity) is probably in large part due to the shorter half-life of Fab in the respiratory tract. However, we cannot exclude a minor contribution by Fc-dependent YR activities in virus clearance by the intact IgG.
MATERIALS AND METHODS
Virus.
Influenza type A virus PR8 (A/Puerto Rico/8/34[H1N1], Mt. Sinai strain) was propagated in the allantoic cavity of embryonated hen's eggs. The titer of infectious virus was determined as described (36) by limiting dilution in microcultures of Madin-Darby canine kidney (MDCK) cells and was expressed as the 50% tissue culture infectious dose (TCID50). Infectious stocks typically contained ∼109 TCID50s/ml. Aliquots were stored frozen (−70°C) and used once for infection of mice or determination of Ab-mediated VN activity. Purified virus was prepared from a large batch of allantoic fluid by differential centrifugation and banding in a sucrose gradient and quantified as described previously (28).
Antibodies.
H36-4-5.2 (H36-4) is a mouse hybridoma Ab specific for the Sb site (4, 44) of the HA of PR8. It is of the κ/G2a isotype. Its sequence (5, 26) and affinity (13) have been determined. The MAb used here was purified from hybridoma culture supernatant by adsorption to and elution from protein A-agarose (Pierce). The MAb was concentrated by ultrafiltration through a collodium bag with a 75,000-molecular-weight (MW) exclusion (Schleicher & Schuell, Keene, N.H.) and dialyzed against phosphate-buffered saline (PBS). Rat hybridoma Abs 187.1 (ATCC HB58) and G2a-3-40-6.8 are specific for mouse Cκ and CHγ2a. They were purified from hybridoma culture by adsorption and elution from protein G-agarose (Pierce), concentrated as described above, and biotinylated with EZ-Link NHS-biotin (Pierce) according to the manufacturer's protocol.
Preparation of Fab.
Papain digestion of Ab was done as described by Mage (24). Briefly, purified MAb H36-4 (10 mg/ml in PBS) was incubated, with constant resuspension, at 37°C with 5 U of agarose-bound papain (P-4406; Sigma) in the presence of 1 mM EDTA and 25 mM 2-mercaptoethanol (2-ME). Digestion was stopped by removal of the sample from the papain-agarose and inactivation of 2-ME by addition of iodoacetamide to a final concentration of 30 mM. Two to 22 h of digestion gave similar yields of Fab. Digestion samples were then passed through a column of protein A-agarose to remove Fc fragments and residual intact IgG molecules, and the effluent was concentrated and dialyzed against PBS by using collodium bags (Schleicher & Schuell) with a 10,000-MW exclusion. Samples were sterilized by passage through a 0.45-μm-pore filter (Corning). The protein concentration was determined by UV absorption and a Bio-Rad protein assay with bovine IgG as standard. The mean value from both determinations was used to estimate the sample's protein concentration.
PAGE and Western blotting.
One microgram of protein in sodium dodecyl sulfate (SDS) sample buffer was electrophoresed under nonreducing conditions in 10% Bis-Tris NuPAGE polyacrylamide gel electrophoresis (PAGE) gels with MOPS (morpholinepropanesulfonic acid) as the running buffer (Invitrogen). Protein bands were detected by staining with colloidal Coomassie blue. In Western blots, Ig light chains were detected with biotinylated rat anti-mouse Cκ MAb 187.1 and developed with streptavidin-alkaline phosphatase (AP) (ExtrAvidin; Sigma) and BCIP/NBT (5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium) (Sigma Fast; Sigma).
ELISA.
The enzyme-linked immunosorbent assay (ELISA) was performed as described previously (28) with polyvinyl plastic plates coated with 25 hemagglutination units of purified PR8 virus (∼175 ng) as an immunoadsorbent. Biotinylated MAb 187.1 or G2a-3-40-6.8 (rat anti-mouse G2a) was used for detection of bound mouse Ab and streptavidin-AP and pNPP (both Sigma) were used for development. All assays were standardized by titration of a series of dilutions of purified MAb H36-4. Optical density at 405 and 750 nm (OD405-750) was measured with an Emax ELISA reader (Molecular Devices, Sunnyvale, Calif.). Data were analyzed with Softmax Pro software (Molecular Devices) and expressed as concentration relative to the Ab standard.
VN activity.
VN activity was measured by MDCK assay as described previously (39) and was expressed as the Ab concentration at which the infectivity of 25 to 100 TCID50s of PR8 was inhibited by 96 to 99%. Virus-Ab mixtures, which had been incubated in vitro for 1 h at room temperature, were also tested for residual infectivity in vivo by inoculation into the respiratory tract of SCID mice. The virus dose used in the latter experiments was ∼300 TCID50s of PR8, which corresponds to ∼60 50% mouse infectious doses (MID50s). In this case, VN activity indicated the Ab concentration during preincubation of virus in vitro, at which 50% of the mice showed no evidence of infection 7 days after inoculation.
YR activity.
YR activity was tested as described previously (29) by incorporation of Ab into the medium of multicycle virus growth cultures. The activity is expressed as the Ab concentration at which the viral yield is reduced by 75% compared to that in Ab-free control cultures. However, this activity can only be assigned if the Ab lacks VN activity at the concentration that results in YR, and both intact H36-4 and its Fab fragment displayed VN activity at the YR concentration (data not shown).
Half-life of Ab in vivo after topical administration.
SCID mice were anesthetized by i.p. injection of ketamine-xylazine (70 mg of ketamine and 7 mg of xylazine per kg of body weight), and Ab was administered i.n. by placing 30 μl of Ab preparation onto the nares. After various intervals, mice were again anesthetized as described above and exsanguinated by heart puncture, and bronchoalveolar lavage (BAL) was performed as follows. A 20-gauge needle was inserted right below the larynx into the exposed trachea ∼1 cm deep, and three 0.5-ml batches of PBS containing EDTA (1 mM) and bovine serum albumin (BSA [0.1 mg/ml]) (PBS/EDTA/BSA) were each injected once, withdrawn, and pooled. The thorax was opened, the aorta was severed, and the lung was perfused in situ by slow injection of 5 ml of PBS containing EDTA (10 mM) into the right ventricle. Because of the low Ab concentration in serum, the perfusion was omitted from some experiments. The heart was removed, and the trachea with attached lung lobes was dissected out of the thorax. The tissue was then minced into small fragments with a tissue chopper (Brinkman), transferred into 3 ml of PBS/EDTA/BSA, and incubated for 30 min at room temperature with frequent resuspension. Tissue debris was pelleted, and the fluid (lung eluate) was collected. Contamination of BAL fluid (BALF) and lung eluate with blood was assessed in some experiments by counting erythrocytes, but this was omitted from later experiments because of low Ab concentration in serum. BALF, lung eluate, and serum were then tested by ELISA for the concentration of anti-viral Ab, and the total amount of recovered Ab was calculated.
Therapeutic activity in vivo.
Anesthetized SCID mice were inoculated i.n. with 300 TCID50s of PR8 in 20 μl. Three hours later, they were again anesthetized and inoculated i.n. with Ab in 30 μl. The intention behind using a larger volume of Ab sample than virus inoculum was to increase the likelihood that Ab became distributed over the entire region of the respiratory tract that had been exposed to the prior virus inoculum. After 7 days, mice were anesthetized and then exsanguinated by heart puncture, and lungs and tracheas were harvested and stored frozen for subsequent measurement of infectious virus content. The latter was done by mechanical disruption of the frozen tissue in 1 (trachea and extrapulmonary bronchi) or 2 (lung lobes) ml of PBS and injection of a sample of debris-free extract into the allantoic cavity of 10-day embryonated hen's eggs.
RESULTS
Characterization of the Fab preparation.
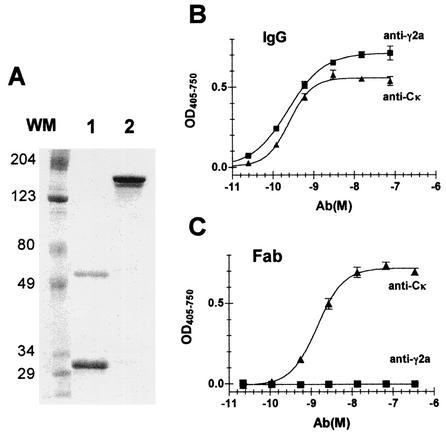
Intact IgG and Fab preparations were analyzed under nonreducing conditions by SDS-PAGE (Fig. 1A). The Fab preparation resolved into two bands: one with a molecular mass of ∼60 kDa and the other with a molecular mass of ∼30 kDa. Both bands reacted with the anti-Cκ MAb in a Western blot (data not shown). This is consistent with the 60-kDa bands being disulfide-linked heterodimeric (VL-Cκ/VH-CH1) Fab and the 30-kDa band being dissociated light (VL-Cκ) and heavy (VH-CH1, Fd) chains. The Cκ-CH1 interchain disulfide bond apparently had been reduced in some of the Fab fragments during digestion, which was performed in the presence of 25 mM 2-ME. Intact H36-4 migrated as a double band, probably because of differential glycosylation, with a molecular mass of ∼150 kDa. Neither sample appeared to be significantly contaminated by extraneous protein. The lack of residual intact IgG in the Fab preparation was verified by ELISA using purified virus as immunoadsorbent and biotinylated Cκ- or CH-specific reagents for detection of bound Ab (Fig. 1B and C). No contamination of the Fab preparation by intact IgG (<0.1%) was detectable (Fig. 1C).
FIG. 1.
Purity of IgG and Fab preparations. (A) Two micrograms of protein was heated in SDS sample buffer and electrophoresed under nonreducing conditions through a 10% Bis-Tris gel with MOPS running buffer. The gels were stained with colloidal Coomassie blue. Molecular mass markers (WM) are indicated in kilodaltons. (B) IgG and (C) Fab were titrated in ELISA against a solid-phase immunoadsorbent of PR8. In each titration, bound Ab was measured with either biotinylated anti-CK (triangles) or anti-CHγ2a, and the assay was developed with streptavidin-AP and pNPP. One of three assays is shown.
Comparison of binding and VN activities of IgG and Fab in vitro.
The molar concentration for 50% maximum binding in ELISA was only 1.7 times larger for Fab than for IgG (Table 1). This is consistent with studies by Dimmock and collaborators (13, 18), which showed that H36-4 bound monovalently to virus and that intact IgG and the Fab fragment displayed similar affinities. Apparently, H36-4 reacted mainly monovalently as well with viral antigen in the ELISA.
TABLE 1.
Binding and VN activity of intact IgG and Faba
| Assay | EC50 (log10 M)
|
Fab/IgG ratio | |
|---|---|---|---|
| IgG | Fab | ||
| ELISA | −9.52 | −9.28 | 1.7 |
| VN activity | |||
| MDCK | −10.1 | −9.92 | 1.5 |
| Respiratory tract | −9.51 | −9.22 | 1.9 |
Dilutions of H36-4 IgG and Fab were tested in ELISA for binding to purified PR8 adsorbed to wells of polyvinyl plastic plates. Biotinylated MAb 187 (anti-Cκ) was used for detection of bound IgG and Fab. The mean EC50 values of two independent assays are shown. VN activity was measured in two types of assays. In both, virus-Ab mixtures were first incubated in vitro for 1 h and then inoculated either into MDCK cell microcultures (12 cultures per Ab dilution) or into the respiratory tracts of SCID mice under ketamine-xylazine anesthesia (three to four mice per Ab dilution). Three days later, MDCK cultures and SCID mice were tested for evidence of infection. In each case, the mean of two independent assays is shown.
Fab and IgG exhibited similar activities in the VN assay in vitro, again indicating that the intact IgG bound mainly monovalently to HA molecules on intact infectious virus. In contrast, Edwards and Dimmock (13) reported that Fab had a substantially lower VN activity than intact MAb H36-4. However, they measured VN activity by ELISA and noted that the difference in activity decreased with increasing fraction of neutralized virus from 100- to 15-fold at 50 and 90% neutralization of input virus, respectively. The VN assay used here measured neutralization of 96 to 99% of input virus. VN activity was also tested by i.n. inoculation of in vitro-incubated virus-Ab mixtures into SCID mice. Seven days later, the mice were killed and tested for the presence of virus in lung and trachea. Here too, intact IgG and Fab showed similar activities.
We also tested IgG and Fab for YR activity in multicycle virus replication cultures. However, no values could be assigned to this activity because it occurred at a concentration at which both Ab preparations mediated VN (data not shown).
In conclusion, Fab was only slightly less effective on a molar basis than intact IgG in all in vitro activities tested.
Half-lives of IgG and Fab in the respiratory tract after i.n. administration.
Therapeutic activity is greatly affected by the half-life of the therapeutic reagent. After i.p. administration, the half-life of intact MAb H36-4 in serum (5 days) was ∼25 times longer than that of Fab (4.5 h) (Fig. 2A). This large difference in half-life would have made a comparison of the therapeutic activities of IgG and Fab by systemic application difficult to conduct and interpret. We next measured the half-lives of IgG and Fab in the respiratory tract after i.n. administration. The premise was that neonatal FcR, which is abundantly expressed by vascular endothelial cells and is known to prolong the half-life of IgG in serum (17), may not play an equally dominant role in the respiratory tract. If so, IgG and Fab might display less-divergent half-lives in this compartment. This was indeed the case (Fig. 2B), because IgG had a half-life only three times longer (22 h) than that of Fab (8 h). In addition, the data (Fig. 2B) indicated that only approximate one-third of the i.n.-administered inocula ended up in the respiratory tract. This was suggested by the finding that the decay curves intersected the y axis at values that were smaller than the initially applied doses: i.e., at 2,330 (39%) instead of 6,000 ng in the case of IgG and at 1,480 (36%) instead of 4,100 ng in the case of Fab. Thus, assuming that the entire amount of Ab in respiratory tract was recoverable by BAL and elution from lung fragments, only approximately one-third of the i.n.-administered Ab sample may have entered the respiratory tract, while the rest may have ended up in the gastrointestinal tract.
FIG. 2.
Half-lives of IgG and Fab in serum and respiratory tract (RT). (A) Fab (130 μg) or IgG (7.5 μg) was injected i.p. into 2- to 3-month-old SCID mice. At various intervals, mice were exsanguinated by heart puncture under ketamine-xylazine anesthesia. The concentration of Fab or IgG in serum was determined by ELISA against PR8 immunoadsorbent, using biotinylated anti-Cκ for Ab detection and purified MAb H36-4 for standardization. Each point shows the log10 of the Ab concentration (nanograms per milliliter) in serum of an individual mouse. Linear regression analysis indicated systemic half-lives of 120 h for IgG and 4.5 h for Fab. (B) IgG (6 μg) and Fab (5 μg) were administered in a volume of 30 μl by the i.n. route to anesthetized SCID mice. At various intervals, mice were anesthetized and exsanguinated by heart puncture. BAL was performed and residual Ab eluted from finely minced lung tissue. The Ab concentration was determined as indicated above by ELISA of BALF and lung eluate. The total amount (concentration × sample volume) of lung-associated Ab was determined and is plotted on a log10 basis against the interval (hours) between administration and recovery. Linear regression analysis indicated topical half-lives of 8 h for Fab and 22 h for IgG.
The relatively small difference in half-lives made i.n. administration the route of choice for comparing the therapeutic activities of IgG and Fab.
Therapeutic activities of IgG and Fab after i.n. administration.
IgG and Fab were administered 3 h after the SCID mice had been exposed to an inoculum of ∼300 TCID50s of PR8 (∼60 MID50s). The interval of 3 h between exposure to virus and treatment was chosen to provide the virus with sufficient time to initiate an infection but not enough time to progress through a complete cycle of replication. The latter would have increased the likelihood of occurrence of viral escape mutants. In addition, it was possible that the infection would spread to sites of the respiratory tract that were less effectively treatable by i.n. Ab administration. Mice were killed seven days later, and tracheal and pulmonary tissues were tested for the presence of infectious virus. Given that the threshold of detection was 10 to 20 infectious virus particles per tissue, and since passive IgG and Fab had decreased in concentration by ≥100-fold by day 5, it is almost certain that a failure to detect infectious virus by day 7 indicated a complete clearance of the infection.
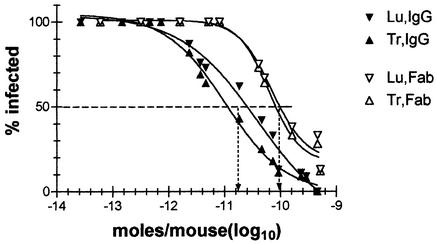
Seven experiments were performed. Each included several Ab doses with one to three mice receiving each dose and one to two untreated control mice. All of the untreated control mice scored positive for infection (data not shown). The infection could be cleared by treatment with both IgG and Fab (Fig. 3). On a molar basis, IgG was therapeutically 5.6 times more effective than Fab: the 50% effective concentrations (EC50s) of IgG and Fab were 10−10.8 and 10−10.1 mol, or 2.4 and 4 μg per mouse, respectively.
FIG. 3.
Therapeutic activity of i.n.-administered IgG and Fab. SCID mice were inoculated i.n. with ∼60 MID50s of PR8 in 20 μl of PBS and 3 h later with various doses of IgG (solid symbols) or Fab (open symbols) in 30 μl of PBS. Seven days later, trachea (Tr) and lung (Lu) were tested for presence of infectious virus. Seven experiments were performed. Together, they comprised 41 and 29 mice treated with IgG and Fab, respectively. The percentage of infected tissues was calculated from the cumulative number of infected and noninfected tissues at the given dosage. Trachea and lungs are indicated by triangles pointing up and down, respectively. Sigmoidal curves were fitted to the each set of data with Prism software (GraphPad Software, Inc., San Diego, Calif.). The stipulated horizontal line shows the 50% clearance level, and the vertical arrows point to the average dose (in moles per mouse) of IgG and Fab at which 50% clearance was achieved.
The therapeutic effectiveness of Ab in the respiratory tract is lower after i.n. administration than after i.p. administration.
We were surprised to find that resolution of the infection required such large Ab doses. For example, 2.4 μg of intact H36-4 per 100 μl of medium exhibited in the MDCK assay in vitro a VN titer of 2,000 (Table 1). We wondered, therefore, whether the large i.n. ED50 may indicate a generally low therapeutic effectiveness of the i.n. route of Ab administration. We thought this question could be answered by comparing the ED50s of intact Ab in the respiratory tract after i.n and i.p. administration. We had shown previously (28) that the i.p. ED50 of MAb H36-4 was 40 μg (i.e., ∼20 times larger than the i.n. ED50) and confirmed this value with the current Ab preparation in two groups of mice (dat not shown). Similar observations have been made by other investigators, who reported that i.p. administration required 100 to 160 times more Ab than i.n. administration (36, 38). However, these comparisons did not take into account the fact that only a fraction of i.p.-administered Ab ended up in the respiratory tract. To estimate this fraction, we determined the relationship between (i) Ab dose given i.p. and Ab concentration in serum and (ii) Ab concentration in serum and BALF. One day after i.p. administration, the Ab concentration per milliliter of serum was approximately one-fourth (27% ± 2.5% [mean ± standard error; n = 4) of the administered dose in 2- to 3-month-old (body weight, ∼25 g) SCID mice. The ratio of Ab concentration in BALF versus that in serum was ∼1/1,500 (0.00066 ± 0.00015; n = 4) on day 1 but was ∼1/400 (0.00236 ± 0.00033; n = 5) on days 4 to 17. We verified the latter ratio by measuring the concentrations of total IgG2a in serum and BALF of normal BALB/c mice and obtained a similar value (0.00281 ± 0.00149; n = 4). The increase in the ratio between day 1 and later time points is probably a reflection of the time it takes until an equilibrium is reached between influx of passive Ab into the respiratory tract by transudation from serum and its topical half-life. In addition, because, the relationships presented above were measured in noninfected mice and infection is known to increase transudation of serum components into the respiratory tract (14, 31, 34), we measured total IgG2a concentration in serum and BALF of BALB/c mice after infection with PR8. Compared to the ratio measured in normal mice, the IgG2a concentration increased 4-fold by day 5 (0.0112 ± 0.0022; n = 9) and 27-fold by day 8 (0.0753 ± 0.0181; n = 8). We assume that most of the increase on day 5 was due to increased transudation of serum IgG2a, because local virus-specific IgG Ab production has been shown not to commence before day 5 after infection (22, 46). The additional increase between day 5 and day 8 was likely due to a combination of transudation and local Ab synthesis.
The total amount of passive Ab in the respiratory tract after i.n. administration of 2.4 μg was estimated to be 800 ng (one-third of the inoculum [Fig. 2B]) right after administration, and given a topical half-life of 22 h, the amounts were 400 ng on day 1 and 20 ng on day 5 (Table 2).
TABLE 2.
Estimated amounts of MAb H36-4 in respiratory tract after i.n. or i.p. administration of a 50% therapeutic dose
| Route | Total amt inoculated (μg) | Total Ab concn in serum on day 1 (μg/ml) | Total amt of Ab (ng) in respiratory tract after:
|
|
|---|---|---|---|---|
| 24 h | 5 days | |||
| i.n. | 2.4 | <0.01 | 400a | 21b |
| i.p | 40 | 10 | 2c | 16d |
(1/3 of inoculum)/2 (one half-life).
400 ng/20 (4.4 half-lives).
10 μg × 0.00066 (BALF/serum on day 1) × 0.3 (ml of BALF). A single BAl was performed with 0.3 ml.
5.8 μg (concn in serum on day 5) × 0.0023 (day 4 to 17 BALF/serum) × 0.3 (ml of BALF) × 4 (increased transudation relative to day 1).
Based on the measurements provided above, we estimated the total amount of MAb in the respiratory tract 1 day after i.p. administration of 40 μg of H36-4 to be ∼200 times smaller than after i.n. administration of 2.4 μg. However, similar amounts would be present 5 days after Ab administration. Because we believe (see Discussion) that the passive MAb most likely resolved the infection within 24 h after administration, the data indicate that i.n. administration, as performed here, did not make optimal use of the MAb's therapeutic activity.
DISCUSSION
The main goal of this study was to determine whether the ability of HA-specific Abs to clear an influenza virus infection depended on the concomitant expression of VN and YR activities or could be achieved solely by VN activity. We addressed this question by testing the Fab fragment of an HA-specific MAb for its therapeutic activity in infected SCID mice. The VN activity of the Fab fragment was almost equal to that of the intact MAb, but Fab obviously lacked YR activity that was Fc and/or bivalency dependent. The only presently known mechanism by which Fab could have mediated residual YR activity may be through catalytic generation of reactive oxygen intermediates (ROIs) (50, 51). However, this recently described activity requires singlet molecular oxygen (1O2*), and an adequate source for it, such as activated neutrophils that participate in an acute inflammatory response (51), is probably lacking during the first day of Fab-mediated virus clearance. Thus, the finding that the infection in SCID mice could be resolved by treatment with Fab provides strong evidence that this infection can be resolved by Ab-mediated VN activity alone and does not require concomitant YR activity.
To our knowledge, Fab or other types of monovalent Ab fragments have not previously been tested for either prophylactic or therapeutic activity in vivo against influenza virus. However, the protective activity of monovalent Ab fragments has been tested against other viruses, including Venezuelan equine encephalitis virus (25), respiratory syncytial virus (RSV) (6, 9, 10), murine hepatitis virus (20), hantavirus (23), herpes simplex virus (52), and vesicular stomatitis virus (19). These studies showed that Fab fragments from VN-negative Abs were ineffective in vivo (6, 9), which is consistent with the notion that Fab contributes to the control of infection mainly, if not exclusively, by VN activity. In addition, they showed that Fab or single-chain Fv (scFv) fragments with VN activity were usually ineffective when administered systemically. This was attributed either to their short half-lives (13) or to a requirement for accessory Fc-dependent activities (23, 25). In most other studies, in which Fab or scFv fragments displayed some protective activity in vivo, treatment with Fab was initiated prior to infection (20), or virus was incubated in vitro with Ab fragments prior to injection into mice (19), or Fab and virus were administered topically at the same time and site (52). The only studies in which Fab was tested in a purely therapeutic mode were those of Crowe and collaborators (9, 10). Crowe et al. showed that i.n. administration of Fab to immunocompetent mice 3 days after infection with RSV resulted in a temporary reduction in lung virus titer but not in clearance, because the virus titer rebounded 2 days after treatment with Fab. Since previous studies indicated that Fab tended to be more protective when given prophylactically or simultaneously with virus, we verified immunohistologically (presence of NP-containing nuclei in epithelial cells) that the infection had indeed been initiated in the respiratory tract by the time Fab was administered (data not shown). Thus, this study provides the first example of an established virus infection that can be resolved by treatment with Fab.
A VN-based resolution of influenza virus infection is entirely conceivable, because infected epithelial cells release progeny virus only for a limited period of time and then die (1, 43, 45) and because virus is released from the apical cell pole (47) into respiratory tract-LF, which it must traverse in order to infect other cells. Thus, if Ab were present at an effective threshold level in this compartment until all infected cells have died, one would expect the infection to be resolved. Infections that do not meet these conditions, such as by RSV, which can spread from cell to cell, or by human immunodeficiency virus, which can establish a chronic infection, would not be expected to be curable by Ab-mediated VN activity alone (9, 10, 35).
From the present study, we can estimate the time required for clearing the infection by computing the time point at which both Fab and IgG were present in the respiratory tract at the same therapeutic dose. Given that VN activity accounted for the entire therapeutic activity of Fab and assuming that the same applied to IgG, we can estimate the time of equal Fab- and IgG-mediated therapeutic activity as follows. The i.n. ED50s of Fab and IgG were 90 and 16 pmol, respectively (Fig. 3). Since Fab had a VN activity 1.5 times lower than that of IgG, the i.n. ED50 values given above would correspond to (arbitrary) VN units of 60 (90/1.5) and 16, respectively. With half-lives of 8 and 22 h, respectively, Fab and IgG would display the same VN activity ∼24 h after administration, when Fab had decreased in concentration eightfold and IgG had decrease approximately twofold, respectively. However, if the therapeutic activity of IgG was larger than its VN activity—for instance because of contributing Fc- and bivalency-dependent activities—Fab- and IgG-mediated therapeutic activities would intersect earlier. Thus, the data indicate that the infection was resolved (in 50% of mice) in 24 h at most and possibly even quicker. This implies that all infected cells had died by this time point in 50% of the mice. This is consistent with results of studies showing that cells in vitro already showed evidence of apoptosis 5 to 8 h after infection (43, 45) and that cultures of primary respiratory tract epithelial cells were massively necrotic 24 h after infection with PR8 (1).
The amount of Ab in the respiratory tract at 24 h, the time at which the infection was resolved in 50% of the mice, was estimated to be ∼10 pmol. This was larger than expected, considering that an amount of Ab ∼2,000 times smaller in 100 μl protected 50% of MDCK cultures from infection in vitro. The large i.n. ED50 appeared to reflect a generally low therapeutic effectiveness of Ab in the respiratory tract when administered i.n. This conclusion was supported by the finding that the dose of passive Ab present in the respiratory tract 1 day after i.p. injection of 40 μg, the i.p. ED50 of MAb H36-4, was ∼200 times smaller. Apparently, transudation of Ab from serum into respiratory tract resulted in a more even and thus therapeutically more effective distribution of Ab throughout the respiratory tract than application i.n. as performed here. Topical administration of aerosolized Ab may be therapeutically more effective.
The role of Fc-dependent activities in Ab-mediated prophylaxis and therapy has been investigated in many virus systems. VN-negative Abs were consistently found to require an intact Fc region for protective activity in vivo (3, 6, 21, 40). This is consistent with the notion that YR activities are dominated by Ab-mediated targeting of FcR-expressing cells and the complement system to infected host cells. Divergent findings were made, however, with VN-positive Abs. The majority of studies (19-21, 37, 49, 52), including one dealing with influenza virus (38), concluded that Fc-dependent activities were not required for Ab-mediated protection in vivo. In other cases, however, Fc-dependent activities appeared to play a substantial role (2, 23, 25, 27, 41). Although the short half-life in vivo of Fc-deficient Ab fragments may have contributed to the reported loss of Ab-mediated protection by Ab fragments in some of these studies, this possibility was excluded in two of them (23, 41). Thus, Fc-dependent activities clearly are important for the protective activity in vivo of some but not other VN-positive Abs. The MAb studied here fits better into the latter group, although we cannot exclude, for the reasons outlined above, that Fc-dependent activities made some contribution to its therapeutic activity in vivo. Further studies are needed to clarify the relation between Fc-dependent activities, VN activity, and protection in vivo.
Acknowledgments
This study was supported by grant 13989 from NIAID and the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health.
We thank Bill Wunner and Jacek Mozdzanowski for reviewing the manuscript.
REFERENCES
- 1.Arndt, U., G. Wennemuth, P. Barth, M. Nain, Y. Al-Abed, A. Meinhardt, D. Gemsa, and M. Bacher. 2002. Release of macrophage migration inhibitory factor and CXCL8/interleukin-8 from lung epithelial cells rendered necrotic by influenza A virus infection. J. Virol. 76:9298-9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldridge, J. R., and M. J. Buchmeier. 1992. Mechanisms of antibody-mediated protection against lymphocytic choriomeningitis virus infection: mother-to-baby transfer of humoral protection. J. Virol. 66:4252-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boere, W. A. M., B. J. Benaissa-Trouw, T. Harmsen, T. Erich, C. A. Kraaijeveld, and H. Snippe. 1985. Mechanisms of monoclonal antibody-mediated protection against virulent Semliki Forest virus. J. Virol. 54:546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caton, A. J., G. G. Brownlee, J. W. Yewdell, and W. Gerhard. 1982. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 31:417-427. [DOI] [PubMed] [Google Scholar]
- 5.Clarke, S. H., K. Huppi, D. Ruezinsky, L. Staudt, W. Gerhard, and M. Weigert. 1985. Inter- and intraclonal diversity in the antibody response to influenza virus hemagglutinin. J. Exp. Med. 161:687-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbeil, S., C. Seguin, and M. Trudel. 1996. Involvement of the complement system in the protection of mice from challenge with respiratory syncytial virus long strain following passive immunization with monoclonal antibody 18A2B2. Vaccine 14:5621-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couch, R. B., and J. A. Kasel. 1983. Immunity to influenza in man. Annu. Rev. Microbiol. 37:529-549. [DOI] [PubMed] [Google Scholar]
- 8.Crowe, J. E. 1999. Host responses to respiratory virus infection and immunization. Curr. Top. Microbiol. Immunol. 236:191-214. [DOI] [PubMed] [Google Scholar]
- 9.Crowe, J. E., B. R. Murphy, R. M. Chanock, R. A. Q. Williamson, C. F. Barbas, and D. R. Burton. 1994. Recombinant human respiratory syncytial virus (RSV) monoclonal antibody Fab is effective therapeutically when introduced directly into the lungs of RSV-infected mice. Proc. Natl. Acad. Sci. USA 91:1386-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowe, J. E., P. S. Gilmore, B. R. Murphy, R. M. Chanock, L. Duan, R. J. Pomerantz, and G. R. Pilkington. 1998. Isolation of a second recombinant human respiratory syncytial virus monoclonal antibody fragment (Fab RSVF2-5) that exhibits therapeutic efficacy in vivo. J. Infect. Dis. 177:1073-1076. [DOI] [PubMed] [Google Scholar]
- 11.Dietzschold, B., M. Kao, Y. M. Zheng, Z. Y. Chen, G. Maul, Z. F. Fu, C. E. Rupprecht, and H. Koprowski. 1992. Delineation of putative mechanisms involved in antibody-mediated clearance of rabies virus from the central nervous system. Proc. Natl. Acad. Sci. USA 89:7252-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimmock, N. J. 1993. Neutralization of animal viruses. Curr. Top. Microbiol. Immunol. 183:1-149. [DOI] [PubMed] [Google Scholar]
- 13.Edwards, M. J., and N. J. Dimmock. 2000. Two influenza A virus-specific Fabs neutralize by inhibiting virus attachment to target cells, while neutralization by their IgGs is complex and occurs simultaneously through fusion inhibition and attachment inhibition. Virology 278:423-435. [DOI] [PubMed] [Google Scholar]
- 14.Fazekas de St. Groth, S. 1951. Studies in experimental immunology of influenza. IX. The mode of action of pathotopic adjuvants. Aus. J. Exp. Biol. Med. Sci. 29:339-352. [DOI] [PubMed] [Google Scholar]
- 15.Fujinami, R. S., and M. B. A. Oldstone. 1979. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature 279:529-530. [DOI] [PubMed] [Google Scholar]
- 16.Gerhard, W. 2001. The role of the antibody response in influenza virus infection. Curr. Top. Microbiol. Immunol. 260:171-190. [DOI] [PubMed] [Google Scholar]
- 17.Ghetie, V., and E. S. Ward. 2000. Multiple roles for the major histocompatibility complex class I-related receptor FcRn. Annu. Rev. Immunol. 18:739-766. [DOI] [PubMed] [Google Scholar]
- 18.Hardy, S. A., and N. J. Dimmock. 2003. Valency of antibody binding to enveloped virus particles as determined by surface plasmon resonance. J. Virol. 77:1649-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalinke, U., A. Krebber, C. Krebber, E. Bucher, A. Pluckthun, R. M. Zinkernagel, and H. Hengartner. 1996. Monovalent single-chain Fv fragments and bivalent miniantibodies bound to vesicular stomatitis virus protect against lethal infection. Eur. J. 26:2801-2806. [DOI] [PubMed] [Google Scholar]
- 20.Lamarre, A., and P. J. Talbot. 1995. Protection from lethal coronavirus infection by immunoglobulin fragments. J. Immunol. 154:3975-3984. [PubMed] [Google Scholar]
- 21.Lefrancois, L. 1984. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J. Virol. 51:208-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang, B., L. Hyland, and S. Hou. 2001. Nasal-associated lymphoid tissue is a site of long-term virus-specific antibody production following respiratory virus infection of mice. J. Virol. 75:5416-5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang, M., Y.-K. Chu, and C. Schmaljohn. 1996. Bacterial expression of neutralizing mouse monoclonal antibody Fab fragments to Hantaan virus. Virology 217:262-271.8599211 [Google Scholar]
- 24.Mage, M. G. 1980. Preparation of Fab fragments from IgGs of different animal species. Methods Enzymol. 70:142-150. [DOI] [PubMed] [Google Scholar]
- 25.Mathews, J. H., J. T. Roehrig, and D. W. Trent. 1985. Role of complement and the Fc portion of immunoglobulin G in immunity to Venezuelan equine encephalomyelitis virus infection with glycoprotein-specific monoclonal antibodies. J. Virol. 55:594-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKean, D., K. Huppi, M. Bell, L. Staudt, W. Gerhard, and M. Weigert. 1984. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc. Natl. Acad. Sci. USA 81:3180-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKendall, R. R. 1985. IgG-mediated viral clearance in experimental infection with herpes simplex virus type 1: role for neutralization and Fc-dependent functions by not C′ cytolysis and C5 chemotaxis. J. Infect. Dis. 151:464-470. [DOI] [PubMed] [Google Scholar]
- 28.Mozdzanowska, K., M. Furchner, G. Washko, J. Mozdzanowski, and W. Gerhard. 1997. A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. J. Virol. 71:4347-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mozdzanowska, K., K. Maiese, M. Furchner, and W. Gerhard. 1999. Treatment of influenza virus-infected SCID mice with non-neutralizing antibodies specific for the transmembrane proteins matrix 2 and neuraminidase reduces the pulmonary virus titer but fails to clear the infection. Virology 254:138-146. [DOI] [PubMed] [Google Scholar]
- 30.Murphy, B. R., and M. L. Clements. 1989. The systemic and mucosal immune response of humans to influenza A virus. Curr. Top. Microbiol. Immunol. 146:109-115. [DOI] [PubMed] [Google Scholar]
- 31.Owens, S. L., J. W. Osebold, and Y. C. Zee. 1981. Dynamics of B-lymphocytes in the lungs of mice exposed to aerosolized influenza virus. Infect. Immun. 33:231-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palladino, G., K. Mozdzanowska, G. Washko, and W. Gerhard. 1995. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J. Virol. 69:2075-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parren, P. W. H. I., and D. R. Burton. 2001. The antiviral activity of antibodies in vitro and in vivo. Adv. Immunol. 77:195-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Persson, C. G. A., J. S. Erjefalt, L. Greiff, I. Erjefalt, M. Korsgren, M. Linden, F. Sundler, M. Andersson, and C. Svensson. 1998. Contribution of plasma-derived molecules to mucosal immune defence, disease and repair in the airways. Scand. J. Immunol. 47:302-313. [DOI] [PubMed] [Google Scholar]
- 35.Poignard, P., R. Sabbe, G. R. Picchio, M. Wang, R. J. Gulizia, H. Katinger, P. W. H. I. Parren, D. E. Mosier, and D. R. Burton. 1999. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity 10:431-438. [DOI] [PubMed] [Google Scholar]
- 36.Prince, G. A., V. G. Hemming, R. L. Horswood, P. A. Baron, and R. M. Chanock. 1987. Effectiveness of topically administered neutralizing antibodies in experimental immunotherapy of respiratory syncytial virus infection in cotton rats. J. Virol. 61:1851-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prince, G. A., V. G. Hemming, R. L. Horswood, P. A. Baron, B. R. Murphy, and R. M. Chanock. 1990. Mechanism of antibody-mediated viral clearance in immunotherapy of respiratory syncytial virus infection of cotton rats. J. Virol. 64:3091-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramisse, F., F. X. Deramoudt, M. Szatanik, A. Bianchi, P. Binder, C. Hannoun, and J.-M. Alonso. 1998. Effective prophylaxis of influenza A virus pneumonia in mice by topical passive immunotherapy with polyvalent human immunoglobulins of F(ab′)2 fragments. Clin. Exp. Immunol. 111:583-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scherle, P. A., G. Palladino, and W. Gerhard. 1992. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted T cells. J. Immunol. 148:212-217. [PubMed] [Google Scholar]
- 40.Schlesinger, J. J., M. Foltzer, and S. Chapman. 1993. The Rc portion of antibody to Yellow Fever virus NS1 is a determinant of protection against YF encephalitis in mice. Virology 192:132-141. [DOI] [PubMed] [Google Scholar]
- 41.Schlesinger, J. J., and S. Chapman. 1995. Neutralizing F(ab′)2 fragments of protective monoclonal antibodies to Yellow Fever virus (YF) envelope protein fail to protect mice against lethal YF encephalitis. J. Gen. Virol. 76:217-220. [DOI] [PubMed] [Google Scholar]
- 42.Schneider-Schaulies, S., U. G. Liebert, Y. Segev, B. Rager-Zisman, M. Wolfson, and V. Ter Meulen. 1992. Antibody-dependent transcriptional regulation of measles virus in persistently infected neural cells. J. Virol. 66:5534-5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schultz-Cherry, S., N. Dybdahl-Sissoko, G. Neumann, Y. Kawaoka, and V. S. Hinshaw. 2001. Influenza virus NS1 protein induces apoptosis in cultured cells. J. Virol. 75:7875-7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staudt, L. M., and W. Gerhard. 1983. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. I. Significant variation in repertoire expression between individual mice. J. Exp. Med. 157:687-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takizawa, T., S. Matsukawa, Y. Higuchi, S. Nakamura, Y. Nakanishi, and R. Fukuda. 1993. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J. Gen. Virol. 74:2347-2355. [DOI] [PubMed] [Google Scholar]
- 46.Tamura, S., T. Iwasaki, A. H. Thompson, H. Asanuma, Z. Chen, Y. Suzuki, C. Aizawa, and T. Kurata. 1998. Antibody-forming cells in the nasal-associated lymphoid tissue during primary influenza virus infection. J. Gen. Virol. 79:291-299. [DOI] [PubMed] [Google Scholar]
- 47.Tucker, S. P., and R. W. Compans. 1993. Virus infection of polarized epithelial cells. Adv. Virus Res. 42:187-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ubol, S., B. Levine, S.-H. Lee, N. S. Greenspan, and D. E. Griffin. 1995. Roles of immunoglobulin valency and the heavy-chain constant domain in antibody-mediated downregulation of Sindbis virus replication in persistently infected neurons. J. Virol. 69:1990-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virgin, H. W., IV, R. Bassel-Duby, B. B. N. Fields, and K. L. Tyler. 1988. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J. Virol. 62:4594-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wentworth, A. D., L. H. Jones, P. Wentworth, Jr., K. D. Janda, and R. A. Lerner. 2000. Antibodies have the intrinsic capacity to destroy antigens. Proc. Natl. Acad. Sci. USA 97:10930-10935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wentworth, P., Jr., J. E. McDunn, A. D. Wentworth, C. Takeuchi, J. Nieva, T. Jones, C. Bautista, J. M. Ruedi, A. Gutierrez, K. D. Janda, B. M. Babior, A. Eschenmoser, and R. A. Lerner. 2002. Evidence for antibody-catalyzed ozone formation in bacterial killing and inflammation. Science 298:2195-2199. [DOI] [PubMed] [Google Scholar]
- 52.Zeitlin, L., K. J. Whaley, P. P. Sanna, T. R. Moench, R. Bastidas, A. De Logu, R. A. Williamson, D. R. Burton, and R. A. Cone. 1996. Topically applied human recombinant monoclonal IgGa antibody and its Fab and F(ab′)2 fragments protect mice from vaginal transmission of HSV-2. Virology 225:213-215. [DOI] [PubMed] [Google Scholar]