Abstract
Susceptibility to ecotropic murine leukemia viruses (MLV) is restricted to mice and rats at the level of virus binding to the host cell receptor. Asparagine 232, valine 233, tyrosine 235, and glutamic acid 237 in the third extracellular domain (EL3) of the receptor are critical determinants of the host range difference between mice and humans. However, placing these residues in the human homolog confers only partial binding, indicating that other divergent sequences are involved. We sought to determine if the other sequences lie within or outside EL3. Here we report the identification of lysine 234 as another critical residue that influences virus binding and infection, as well as evidence that the unidentified sequences lie outside EL3. Each of the four basic residues in the third extracellular domain were changed to an acidic residue and initially examined in combination with a change at position 235 or position 237. Substitution of lysine 211, 215, or 222 combined with substitution of the critical tyrosine 235 or glutamic acid 237 did not affect virus infection. However, combined substitution of lysine 234, a conserved residue between mice and humans, and tyrosine 235 resulted in a marked decrease in virus infection and binding. A lysine 234 change alone reduced virus binding, contrary to previous observations that at least two of the other four residues must be changed before binding is reduced. Interestingly, there was no decrease in infection when lysine 234 was replaced in combination with glutamic acid 237. This result suggests that residue 234 may act by influencing the local structure of residues 233 to 235, whereas the presence of a glycine at position 236 may prevent this influence from extending to residue 237. With this report, the involvement of all the residues divergent between mice and humans in the third extracellular domain has been ruled out, suggesting that as yet unidentified determinants lie in other extracellular domains.
The host range of the ecotropic murine leukemia virus (MLV) is restricted to mice and rats by the availability of their cell surface receptor ATRC1, an integral membrane protein with 14 membrane-spanning domains (3). In the host cell, ATRC1 functions as the principal transporter of cationic amino acids (9, 18). Other mammals, including humans, have homologous proteins that function as cationic amino acid transporters but lack ecotropic MLV receptor capability (1, 2).
Homology scanning mutagenesis of the receptor and its human homolog led to the identification of residues 211 to 239 as the putative virus binding domain (2, 21) (Fig. 1A). This domain resides on the extracellular side of the plasma membrane since the two N-linked glycosylation sites (asparagines 223 and 229) within it are glycosylated in vivo (10). Replacing two or more of asparagine 232, valine 233, tyrosine 235, and glutamate 237 results in loss of virus surface protein (SU) binding and virus infection, although single substitution of any of these residues did not reduce binding or infection (2), indicating that each of these residues was critical but that none alone was essential to virus binding and infection.
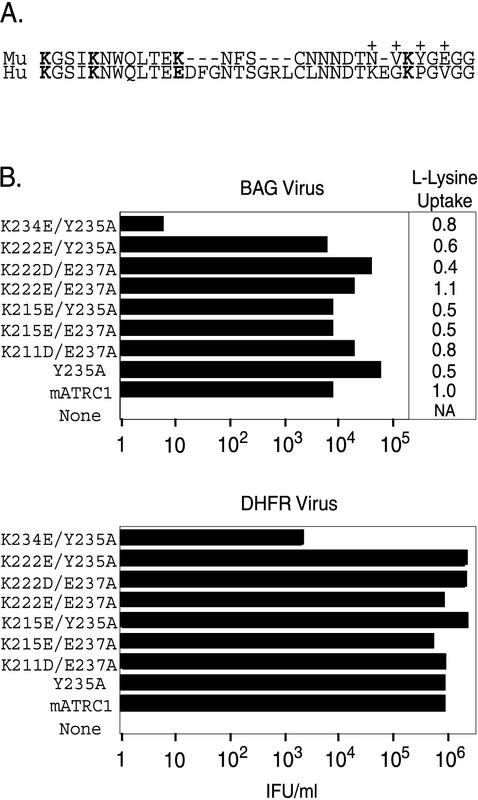
FIG. 1.
Combined replacement of lysine 234 with glutamate and tyrosine 235 with alanine in the third extracellular domain of mATRC1 reduces virus infection. (A) Amino acid sequence (single-letter code) of the third extracellular domain of ATRC1. Mu, murine sequence (residues 211 to 239); Hu, human sequence (residues 211 to 246). Lysine residues at positions 211, 215, 222, and 234 of mATRC1 are in boldface. The corresponding positions of the human sequence are also in boldface. +, previously identified residues that influence infection and binding. (B) HEK 293 cells stably expressing receptors with substitutions were exposed to serial 10-fold dilutions of ecotropic MLV-pseudotyped BAG or DHFR virus, and infectious titers were determined from the end point dilution (n = 4). The substitutions present on the mutant receptors are designated by the single-letter amino acid codes for the amino acids in the wild-type receptor followed by the residue numbers and then by the single-letter codes for the substitute amino acids. For receptors containing two changes, the designations are separated by a slash. IFU, infection-forming units. To determine l-lysine uptake, the relative levels of surface expression of wild-type and mutant receptors were estimated by measuring l-lysine uptake. Relative l-lysine uptake was calculated by normalizing l-lysine uptake in human cells expressing mutant receptors to that in the human cell line expressing the wild-type receptor.
Placing the critical tyrosine 235 and glutamate 237 in the corresponding divergent positions of the human homolog gave a chimera that mediates infection at levels as high as those for the wild-type mouse receptor. But this chimera does not bind detectable levels of SU (2). Addition of asparagine 232 and valine 233 rendered the chimera capable of a low level of binding activity, 30% of the binding activity of the wild-type receptor (2). This finding suggested that additional residues divergent between mice and humans were involved in the interactions with the envelope protein. However, it is not known if the other influential divergent residues lie in the third extracellular loop or in other extracellular domains. In particular, it has proven difficult to determine if other receptor domains are involved in infection and virus binding.
The possible involvement in binding of all the divergent residues in the third extracellular domain except lysine 222 has been assessed previously (2, 13). This residue is a glutamate in human ATRC1. Here we report the characterization of a new set of altered receptors and examine the possible involvement of lysine 222 in virus attachment. In addition, three conserved lysine residues were examined. The results indicate that lysine 222 is not involved in receptor function but that virus binding and infection are influenced by lysine 234. We constructed cDNAs encoding replacement of each of the basic residues (lysines 211, 215, 222, and 234) with an acidic amino acid. These changes were chosen because the divergent lysine 222 is a glutamate in human ATRC1 (Fig. 1A) and because a similar charge substitution strategy had proven effective in the characterization of the critical glutamate 237 (13).
The first set of mutant receptors bore double substitutions of one of the lysines and of a residue previously established as critical. The initial strategy of analyzing double-mutant receptors was used because in the previous studies single-mutant receptors displayed wild-type receptor levels of infection and binding, whereas mutant receptors with substitution of at least two of the critical residues exhibited loss of function that revealed their involvement (2, 13). The effect of single substitution of a lysine residue was then determined for any double-mutant receptor showing reduced function. The relative capability of each altered receptor was assessed by comparing the susceptibility and virus binding capacity of control cells expressing wild-type murine ATRC1 (mATRC1) to those of a human cell-derived population stably expressing the mutant receptor. To control for differences in receptor expression, the relative amounts of cell surface expression of mutant receptors were quantified and compared to that of the wild-type receptor on control cells.
Plasmid pcDNA mATRC1 encodes the wild-type murine Atrc-1 cDNA (accession number M26687) and plasmid pcDNA ATRC1-HA encodes a fusion of the HA1 epitope tag from the influenza virus hemagglutinin (HA) protein (20) immediately after the carboxy-terminal lysine 622; the latter plasmid was made by replacing nucleotides (nt) 2064 through 2420, including the translation termination codon (nt 2064 to 2066), with the sequence encoding three copies of the HA1 epitope tag followed by a new termination codon. Both plasmids are in the eukaryotic expression vector pcDNA3 (Invitrogen). Oligonucleotide-directed mutagenesis (11) was used to generate nucleotide substitutions. The sequence of each plasmid was verified by dideoxynucleotide sequencing (U.S. Biochemicals, Cleveland, Ohio) prior to their introduction into cells. Plasmids were transfected into nonsusceptible human embryonic kidney 293 (HEK 293) cells by calcium phosphate coprecipitation, and selection for the neomycin resistance gene carried by pcDNA3 was achieved by growth in medium containing 750 μg of G418 (GIBCO)/ml for 5 weeks. Stable transfectant lines were then maintained in medium containing 250 μg of G418/ml. Cell lines stably producing a replication-defective ecotropic Moloney MLV-pseudotyped virus which transduces Escherichia coli β-galactosidase (BAG virus) (16) or a mutant dihydrofolate reductase that confers methotrexate resistance (DHFR virus) (19) were cultured in Dulbecco's modified Eagle's medium (GIBCO, Grand Island, N.Y.) supplemented with 8% fetal bovine serum (GIBCO). In addition, high-titer-replication-defective ecotropic Moloney MLV-pseudotyped BAG virus was produced by transient transfection of H1BAG packaging cells with plasmid pcDNA MoMLV as previously described (22).
Replacement of lysine 234 by negatively charged glutamate in combination with substitution of tyrosine 235 markedly reduced virus infection, while double substitutions that included lysines 211, 215, and 222 had no effect.
The HEK 293-derived cells stably expressing altered receptors were exposed to serial dilutions of virus to compare their susceptibilities to that of HEK 293 cells expressing wild-type mATRC1. Virus titration by end point dilution of ecotropic MLV-pseudotyped DHFR virus was performed exactly as previously described (13). Briefly, cells exposed to serial 10-fold virus dilutions were selected for growth in medium containing 150 μM methotrexate (Sigma) and 8% dialyzed donor calf serum (GIBCO), indicative of transduction of the mutant dhfr gene. After 3 weeks, methotrexate-resistant colonies were fixed and stained with 2% crystal violet in 90% ethanol. End point dilution titration of ecotropic MLV-pseudotyped BAG virus was performed similarly except that exposed cells were fixed 48 h later and stained for β-galactosidase activity transduced by the virus.
Substitution of lysine 211, 215, or 222 combined with a tyrosine 235-to-alanine change (Y235A), or in some cases combined with a glutamate 237-to-alanine change (E237A), did not reduce virus entry, indicating that these lysine residues are not critical for receptor function (Fig. 1B). However, a receptor bearing K234E and Y235A substitutions was 2,000-fold less efficient at mediating virus entry than was mATRC1 (Fig. 1B). Similar results were obtained in independent titrations of the ecotropic MLV-pseudotyped DHFR and BAG viruses.
To control for variations attributable to differences in receptor levels, the level of cell surface expression of mutant receptors on the stable cell lines was quantified by two methods. Since expression of an exogenously encoded receptor on the cell surface increases cationic amino acid uptake above that mediated by the endogenous human transporter in the parental HEK 293 cells (13, 17), the increase in l-lysine transport in the human cell lines expressing receptors with substitutions or wild-type receptors was determined as a measure of relative surface expression. Cationic amino acid uptake assays were performed as previously described (13, 17). Briefly, cationic amino acid uptake was initiated by incubating monolayers of cells with l-[14C]lysine for 2 min at 37°C and then washing cells with ice-cold phosphate-buffered solution and lysing them. The protein concentration of each lysate was determined by a Bradford protein assay (Bio-Rad) of the trichloroacetic acid (TCA)-precipitable pellet. Cytoscint (ICN) was added to the TCA-soluble component of the cell lysate, and the sample was counted in a liquid scintillation counter. The total amino acid uptake by cells in an individual well was normalized to its protein concentration, and the relative transport was calculated as the mean increase in amino acid uptake over the mean uptake in parental HEK 293 cells measured in the same assay.
HEK 293 cells expressing mutant receptors at 50 to 170% the levels of the wild-type receptor have been shown to exhibit comparable levels of susceptibility to ecotropic MLV infection (13). The relative amino acid transport increase of all the double lysine and tyrosine mutant receptors fell within this previously established range (Fig. 1), including the double-mutant receptor with mutations K234E plus Y235A (80%). These results indicate that the reduction in infection was not due to lack of cell surface expression of this mutant receptor.
Single replacement of lysine 234 with glutamate or aspartate does not reduce virus infection.
A cDNA encoding a chimera with a single change of lysine 234 to glutamate (K234E) was subsequently constructed. In addition, a chimera containing a single lysine 234-to-aspartate (K234D) change was constructed. To facilitate specific detection on immunoblots, the mutations resulting in the double and single substitutions were transferred into plasmid pcDNA mATRC1-HA. HEK 293-derived populations stably expressing the cDNAs were generated. Cells expressing the HA-tagged double mutant receptor were less susceptible by 3 log units, but the single replacements for residue 234 conferred susceptibility comparable to that of wild-type mATRC1-HA (Fig. 2A).
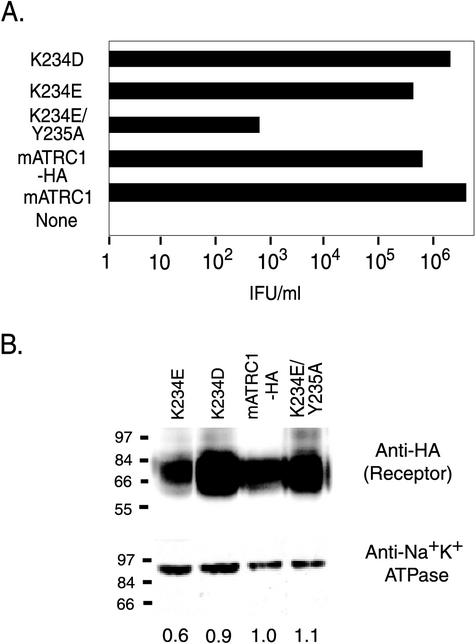
FIG. 2.
Lysine 234 is critical to ecotropic MLV infection. (A) HEK 293 cells stably expressing HA1 epitope-tagged receptors were exposed to 10-fold serial dilutions of high-titer ecotropic MLV-pseudotyped BAG virus stock, and infectious titers were determined from the end point dilution (n = 4). None, parental HEK 293 cells lacking ecotropic MLV receptor; mATRC1, untagged wild-type receptor; mATRC1-HA, HA-tagged wild-type receptor; K234E/Y235A, HA-tagged mutant receptor with combined replacement of lysine 234 with glutamate and tyrosine 235 with alanine; K234E and K234D, HA-tagged mutant receptors with single replacements of lysine 234 with glutamate and aspartate, respectively. IFU, infection-forming units. (B) Immunoblot analysis of the surface expression of mutant receptors. Surface proteins on live HEK 293 cells stably expressing HA-tagged mATRC1 or mutant receptors were labeled with biotin by using a membrane-impermeant biotinylation reagent, and then cells were lysed and equal masses of each lysate were incubated with avidin-agarose beads to affinity purify plasma membrane proteins as described in the text. (Top) Half of the total purified surface membrane proteins were applied to each lane and immunoblotted to mouse anti-HA1 monoclonal antibody HA11. Detection was performed with goat anti-mouse immunoglobulin G conjugated to HRP and a chemiluminescent substrate for HRP. (Bottom) The other half of total purified surface membrane proteins were immunoblotted to anti-Na+K+ATPase as a control to normalize for efficiency of recovery of total surface proteins during affinity purification. The values shown underneath the lanes represent the average relative surface expression levels of mutant receptors compared to that of mATRC1 from two independent experiments calculated from the scanned images with ImageQuant software.
The relative surface expression of each receptor was evaluated by biotinylating surface proteins so that they could be affinity purified and then determining the relative amounts of mutant and wild-type receptors present in the purified surface proteins by immunoblot analysis with a receptor-specific antibody. Briefly, plasma membrane proteins on live, adherent cells were specifically labeled on surface amine groups by incubation with 1 mg of sulfosuccinimidyl-6-(biotinamido)hexanoate (N-hydroxysuccimide-LC-biotin; Pierce), a membrane-impermeant biotinylation reagent, at 4°C for 2 h as described by Kahne and Ansorge and Sargiacomo et al. (8, 14). After 2 h, biotinylation was stopped by addition of 20 mM l-glycine, labeled cells were lysed in NP-40 buffer (150 mM NaCl, 1% Nonidet P-40, 50 mM Tris-HCl [pH 8.0]), and nuclei were removed by brief centrifugation, after which sodium dodecyl sulfate (SDS) was added to a final concentration of 1% (vol/vol). Total protein in each cell lysate was determined with a Bradford protein assay kit (Bio-Rad), and then equal masses were incubated with 50 μl of avidin-agarose beads (Sigma) overnight at 4°C to allow binding of biotinylated proteins for their purification. The next day, samples were centrifuged for 4 min at 14,000 rpm in an Eppendorf microcentrifuge to pellet the avidin-agarose and bound protein. The pellet was washed three times in NP-40 buffer, resuspended in Laemmli sample buffer containing 8 M urea, and heated at 80°C to release bound protein, and equal-volume samples were subjected to SDS-polyacrylamide gel electrophoresis. The size-fractionated proteins were then immunoblotted to anti-HA1 monoclonal antibody HA11 (Covance-Berkeley Antibody Co.). A duplicate immunoblot was probed with a mouse monoclonal anti-Na+K+ATPase antibody (anti-Na+K+ATPase α clone M7-PB-E9; Affinity Bioreagents, Inc.) as a control to ascertain if equal amounts of membrane proteins were recovered from each lysate during avidin-agarose affinity purification. Goat anti-mouse conjugated to horseradish peroxidase (HRP; Jackson ImmunoResearch) was used as the secondary antibody, and detection of the antibody sandwich was performed by chemiluminescence (SuperSignal; Pierce).
HEK 293 cells transfected with the mATRC1-HA cDNA expressed an anti-HA1-reactive protein of the expected size (a broad doublet of approximately 70 kDa) on their surfaces (Fig. 2B, left). A broad protein band is characteristic of ATRC1, for which it has been shown that variations in the glycosylation at asparagines 223 and 229 result in proteins with a range of sizes (5). Negative control HEK 293 cell membrane proteins did not contain an anti-HA-reactive species (data not shown). Relative anti-HA-reactive band intensities were estimated with ImageQuant on scans of the immunoblots and normalized to the relative band intensities of the control anti-Na+K+ATPase reactive species (Fig. 2B, right). Based on this quantification, the K234E mutant receptor was expressed at a slightly lower level than mATRC1-HA, while the K234D and the doubly substituted K234E-plus-Y235A mutant receptors were expressed at almost the same level as mATRC1-HA (Fig. 2B, left). These results indicate that the loss of infection in the double-mutant receptor was not a result of lower levels of surface expression, in agreement with the results for the untagged mutant receptors quantified by cationic amino acid uptake.
The reduction in infection correlates with a marked reduction of virus binding.
The ability of the lysine 234 mutant receptors to confer virus binding on the human cells was quantified to determine if the reduction in susceptibility correlated with reduced ability to bind. Virus binding assays were performed as previously described by Kadan et al. (7) with the modifications described in Zavorotinskaya and Albritton (22). Briefly, shed SU was removed from Moloney MLV particles by size fractionation using low-speed centrifugation through a Centricon 100 device (100-kDa molecular mass cutoff). This method also concentrated the virus so that saturating amounts of particles could be incubated with HEK 293 cells expressing the HA-tagged mATRC1, HA-tagged mutant receptors, or no receptor, then with goat anti-SU antiserum (Quality Biotech, Inc.; serum no. 80S000018), and last with a fluorescein isothiocyanate-conjugated mouse anti-goat antibody and propidium iodide. In addition, incubations were carried out at 4°C because HEK 293 cells showed appreciable cell death when binding was performed at 37°C but maintained viability at 4°C (less than 5% cell death, as judged by exclusion of propidium iodide). Virus binding was quantified as the increase in green fluorescence of live cells (negative for propidium iodide). In some cases, virus binding to HEK 293 cells stably expressing untagged wild-type mATRC1 was performed as an additional positive control.
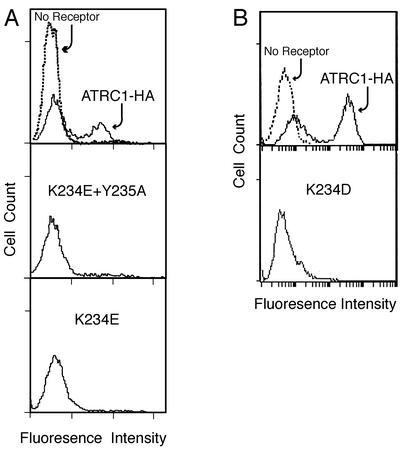
Typically, about half of the positive-control cell population showed greater than a 20-fold increase in fluorescence compared to negative-control HEK 293 cells lacking receptor, indicating that high levels of receptor were on their surfaces (Fig. 3, top). A cell population expressing untagged mATRC1 showed a similar factor of increase (data not shown). The other half of the mATRC1-HA cell population showed very little increase, suggesting that these cells expressed few or no receptors. The double K234E-plus-Y235A change profoundly affected the ability of receptor to bind virus envelope protein. The cell population expressing this mutant receptor showed less than a twofold increase in fluorescence (Fig. 3A). Surprisingly, the susceptible cell populations with receptors expressing the single K234E replacement also showed a marked loss of binding (Fig. 3A). A similar decrease in binding was observed in a separate experiment for the cells expressing receptors with the single K234D mutation (Fig. 3B).
FIG. 3.
A change in lysine 234 correlates with a reduction in virus binding. The markedly reduced binding of the double K234E-plus-Y235A mutant receptor correlates with the decrease in its ability to mediate infection. Single K234E and K234D mutant receptors also produced greatly reduced binding, even though they mediated infection comparably to the wild-type receptor. HEK 293 cells stably expressing the HA-tagged wild-type receptor (mATRC1-HA) or HA-tagged mutant receptors with either combined replacement of lysine 234 by glutamate and tyrosine 235 by alanine (K234E/Y235A) or a single replacement of lysine 234 by glutamate (K234E) or by aspartate (K234D) were incubated with ecotropic Moloney MLV particles at 4°C for 1 h, then with the goat anti-envelope surface protein (SU) at 4°C for 1 h, and finally with mouse anti-goat antiserum conjugated to fluorescein isothiocyanate at 4°C for 1 h. Propidium iodide was added during the final 5 min of incubation with the secondary antibody. Parental HEK 293 cells lacking the ecotropic MLV receptor were treated in the same manner as a negative control (no receptor, dashed line). Relative virus binding to live cells (propidium iodide negative) was quantified by flow cytometry.
Since cells were incubated with saturating amounts of virus and virus-cell complexes were not fixed, only those interactions that were maintained for the duration of the protocol (about 4 h) were detected by flow cytometry. The interactions between wild-type virus and wild-type receptor are strong enough to last this long, but weaker interactions may not be measured. This appears to be the case for mutant receptors with a single replacement of lysine 234. The single-mutation receptors conferred susceptibility on the human cells comparable to that conferred by the wild-type receptor, suggesting that their interaction must be strong enough to allow transient virus attachment and that the interaction must be sufficient to secure virus entry at physiologic temperature. Others have shown that binding to endogenous mATRC1 on murine NIH 3T3 cells is greater at 37°C than at 4°C (7), so we also quantified binding to mATRC1- and K234D-expressing cells at the higher temperature. However, virus binding was not increased on either cell line (data not shown). Perhaps internalization of virus-receptor complexes and insertion of the envelope protein fusion peptide into the host cell membrane at 37°C influence the outcome of weak virus binding in favor of infection for mutant receptors with single K234E and K234D mutations.
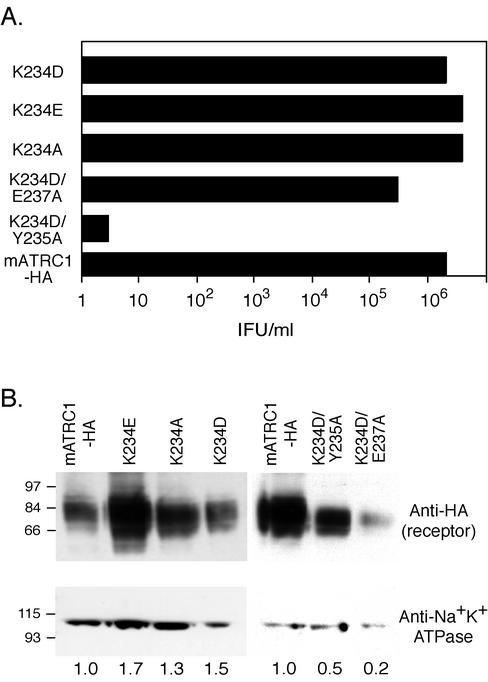
New HEK 293 populations expressing K234D and K234E single-mutant receptors were generated to obtain cell lines with more closely comparable receptor levels to determine if the slightly lower infection observed on the first K234E line (Fig. 2) was due to its lower expression. Indeed, levels of infection of the new K234E- and K234D-expressing cell lines were comparable and correlated with surface expression (Fig. 4). That is, infection became comparable when expression levels of the mutants were comparable. Substitution of alanine for lysine 234 (K234A) gave infection comparable to that for mATRC1-, K234D-, and K234E-expressing cells (Fig. 4). This result confirms that the side chain of lysine 234 is not essential for infection.
FIG. 4.
Infection via K234D- and K234E-expressing receptors is comparable when their cell surface expression levels are similar. Replacing lysine 234 with alanine (K234A) does not reduce infection. There is synergism between a lysine 234-to-aspartic acid change (K234D) and a tyrosine 235-to-alanine change (Y235A) but not between K234D and a glutamic acid 237-to-alanine change (E237A). New HEK 293 cells stably expressing HA-tagged single K234D and K234E mutant receptors at comparable levels were generated, as well as cell lines stably expressing receptors with a new single mutation, K234A, and two new double mutations, K234D plus Y235A and K234D plus E237A. (A) Infectious titers were determined from the end point dilution (n = 4) of cells exposed to serial 10-fold dilutions of ecotropic MLV-pseudotyped BAG virus stock. All receptors contained the HA1 epitope tag. IFU, infection-forming units. (B) Immunoblot analysis of the surface expression of mutant receptors was performed as described in the legend to Fig. 2.
Discussion.
Given that the double-mutant and K234D mutant receptors were expressed at the same levels as the wild-type receptor, the effect of changes in lysine 234 on the stability of the virus-receptor interactions appears to be profound. These results contrast with those observed for two other critical residues. It was previously shown that replacing both tyrosine 235 and glutamate 237 markedly reduced binding but that their single substitution gave no reduction (2). The reduction in binding observed for a single change of lysine 234 suggests that this residue has a more profound influence on the binding interaction than either residue 235 or 237.
Given the reduction in binding and the close proximity of lysine 234 and tyrosine 235, the question arose as to the phenotype of a lysine 234 change in the context of a change at the critical glutamic acid 237. Consequently, we compared infection via a K234D-plus-E237A mutant receptor to that via a K234D-plus-Y235A mutant receptor. Interestingly, the double K234D-plus-E237A mutant receptor gave infection comparable to that for mATRC1, a sharp contrast to the substantial reduction in infection via the combination with Y235A (Fig. 4). Considering that the defective mutant receptor was expressed at more than twice the cell surface levels of the K234D-plus-E237A mutant receptors, low expression does not appear to be the primary cause of the reduced infection. Further, this finding indicates that the influence of lysine 234 is somehow related to its proximity to the critical tyrosine. One possibility is that replacing residue 234 changes the local structure, resulting in a synergism between changes at positions 234 and 235, whereas a lysine 234 substitution may not be synergistic with respect to glutamic acid 237 because the intervening glycine 236 prevents the structural changes from extending further.
The first 200 amino acids of the virus envelope protein have been shown to be sufficient to specify host range (4, 6). Although the receptor binding site on the envelope protein has not been precisely defined, several charged residues in this amino-terminal region of SU have been identified (12, 15). In particular, a negatively charged residue (aspartate 84) was shown to be critical for virus binding and infection (12). Perhaps an interaction between this critical acidic residue on SU and the positively charged side chain of lysine 234 on the virus receptor is a part of the virus-receptor contact in attachment.
The previous finding that placing the critical tyrosine 235, glutamate 237, asparagine 232, and valine 233 in the third extracellular domain of the human ATRC1 resulted in a gain of only 30% of envelope protein binding suggested that additional residues were involved in virus attachment (2). The results reported here indicate that the putative virus binding site includes lysine 234, but because this residue is conserved it is not one of the unidentified components lacking in human ATRC1. Because the involvement of all the residues divergent between mouse and humans in the third extracellular domain has now been ruled out by mutagenesis (2, 13, 21; this report), the unidentified determinants must be in other extracellular domains.
Acknowledgments
We thank Amy G. Scott for technical assistance and Krishnakumar Kizhatil, Tatiana Zavarotinskaya, and David Armbruster for critical reading of the manuscript. Some of the DNA sequence analysis and the oligonucleotides synthesis used in this work were performed in the Molecular Resource Center of the University of Tennessee Health Sciences Center.
This work was supported by NIH grant AI33410 (L.M.A.).
REFERENCES
- 1.Albritton, L. M., A. M. Bowcock, R. L. Eddy, C. Morton, L. A. Farrerra, L. Cavalli-Sforza, T. Shows, and J. M. Cunningham. 1992. The human cationic amino acid transporter: physical and genetic mapping to 13q12-q14. Genomics 12:430-434. [DOI] [PubMed] [Google Scholar]
- 2.Albritton, L. M., J. W. Kim, L. Tseng, and J. M. Cunningham. 1993. Envelope-binding domain in the cationic amino acid transporter determines the host range of ecotropic murine retroviruses. J. Virol. 67:2091-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albritton, L. M., L. Tseng, D. Scadden, and J. M. Cunningham. 1989. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell 57:659-666. [DOI] [PubMed] [Google Scholar]
- 4.Battini, J.-L., O. Danos, and J. M. Heard. 1995. Receptor-binding domain of murine leukemia virus envelope glycoproteins. J. Virol. 69:713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Closs, E. I., L. M. Albritton, J. W. Kim, and J. M. Cunningham. 1993. Identification of a low affinity, high capacity transporter of cationic amino acids in mouse liver. J. Biol. Chem. 268:7538-7544. [PubMed] [Google Scholar]
- 6.Heard, J., and O. Danos. 1991. An amino-terminal fragment of the Friend murine leukemia virus binds the ecotropic receptor. J. Virol. 65:4026-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadan, M. J., S. Sturm, W. F. Anderson, and M. A. Eglitis. 1992. Detection of receptor-specific murine leukemia virus binding to cells by immunofluorescence analysis. J. Virol. 66:2281-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahne, T., and S. Ansorge. 1994. Non-radioactive labelling and immunoprecipitation analysis of leukocyte surface proteins using different methods of protein biotinylation. J. Immunol. Methods 168:209-218. [DOI] [PubMed] [Google Scholar]
- 9.Kim, J. W., E. I. Closs, L. M. Albritton, and J. M. Cunningham. 1991. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature 352:725-728. [DOI] [PubMed] [Google Scholar]
- 10.Kim, J. W., and J. M. Cunningham. 1993. N-linked glycosylation of the receptor for murine ecotropic retroviruses is altered in virus-infected cells. J. Biol. Chem. 268:16316-16320. [PubMed] [Google Scholar]
- 11.Kunkel, T. A. 1985. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc. Natl. Acad. Sci. USA 82:477-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacKrell, A. J., N. W. Soong, C. M. Curtis, and W. F. Anderson. 1996. Identification of a subdomain in the Moloney murine leukemia virus envelope protein involved in receptor binding. J. Virol. 70:1768-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malhotra, S., A. G. Scott, T. Zavorotinskaya, and L. M. Albritton. 1996. Analysis of the murine ecotropic leukemia virus receptor reveals a common biochemical determinant on diverse cell surface receptors that is essential to retrovirus entry. J. Virol. 70:321-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sargiacomo, M., M. Lisanti, L. Graeve, A. LeBivic, and E. Rodriquez-Boulan. 1989. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J. Membr. Biol. 107:277-286. [DOI] [PubMed] [Google Scholar]
- 15.Skov, H., and K. B. Andersen. 1993. Mutational analysis of Moloney murine leukemia virus surface protein gp70. J. Gen. Virol. 74:707-714. [DOI] [PubMed] [Google Scholar]
- 16.Turner, D. L., and C. L. Cepko. 1987. A common progenitor for neuron and glia persists in rat retina late in development. Nature 328:131-136. [DOI] [PubMed] [Google Scholar]
- 17.Wang, H., E. Dechant, M. Kavanaugh, R. A. North, and D. Kabat. 1992. Effects of ecotropic murine retroviruses on the dual-function cell surface receptor/basic amino acid transporter. J. Biol. Chem. 267:23617-23624. [PubMed] [Google Scholar]
- 18.Wang, H., M. P. Kavanaugh, R. A. North, and D. Kabat. 1991. Cell-surface receptor for ecotropic murine retroviruses is a basic amino acid transporter. Nature 352:719-731. [DOI] [PubMed] [Google Scholar]
- 19.Williams, D. A., K. Hsieh, A. DeSilva, and R. C. Mulligan. 1984. Introduction of new genetic material into pluripotent haematopoietic stem cells of mouse. Nature 310:476-479. [DOI] [PubMed] [Google Scholar]
- 20.Wilson, I. A., H. L. Niman, R. A. Houghten, A. R. Cherenson, M. L. Connolly, and R. A. Lerner. 1984. The structure of an antigenic determinant in a protein. Cell 37:767-778. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimoto, T., E. Yoshimoto, and D. Meruelo. 1993. Identification of amino acid residues critical for infection with ecotropic murine leukemia retrovirus. J. Virol. 67:1310-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zavorotinskaya, T., and L. M. Albritton. 1999. Suppression of a fusion defect by second site mutations in the ecotropic murine leukemia virus surface protein. J. Virol. 73:5034-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]