Abstract
Cotranslational protein transport into dog pancreas microsomes involves the Sec61p complex plus a luminal heat shock protein 70. Posttranslational protein transport into the yeast endoplasmic reticulum (ER) involves the so-called Sec complex in the membrane, comprising a similar Sec61p subcomplex, the putative signal peptide receptor subcomplex, and the heat shock protein 40-type subunit, Sec63p, plus a luminal heat shock protein 70. Recently, human homologs of yeast proteins Sec62p and Sec63p were discovered. Here we determined the concentrations of these two membrane proteins in dog pancreas microsomes and observed that the canine homologs of yeast proteins Sec62p and Sec63p are abundant proteins, present in almost equimolar concentrations as compared with Sec61αp monomers. Furthermore, we detected fractions of these two proteins in association with each other as well as with the Sec61p complex. The J domain of the human Sec63p was shown to interact with immunoglobulin heavy chain binding protein. Thus, the membrane of the mammalian ER contains components, known from the posttranslationally operating protein translocase in yeast. We suggest that these components are required for efficient cotranslational protein transport into the mammalian ER as well as for other transport processes.
The decisive initial step in the biogenesis of most extracellular and many organellar proteins of eukaryotic cells is their integration into the membrane or their transport into the lumen of the endoplasmic reticulum (ER). Typically, protein integration and transport into the ER requires signal peptides at the amino terminus of the respective precursor proteins and a transport machinery, comprising soluble and membrane proteins (1). Protein integration or transport into the ER can occur co- or posttranslationally in yeast as well as in mammalian cells (1, 2). Posttranslational protein transport into the yeast ER involves a protein translocase in the membrane (also known as translocon or the Sec complex), comprising the Sec61p subcomplex (Sec61p, Sbh1p, and Sss1p) (3–5), the putative signal peptide receptor subcomplex (Sec62p, Sec66p/Sec71p, and Sec67p/Sec72p) (6–8), and the DnaJ-domain-containing subunit, Sec63p (also termed Ptl1p and Npl1) (9–11), plus a luminal heat shock protein 70, i.e., Kar2p (12–16) or Lhs1p (also named Cer1p and Ssi1p) (17–19). Sec63p and Kar2p also were reported to be involved in cotranslational transport into the yeast ER (20). Cotranslational protein transport into dog pancreas microsomes involves a similar Sec61p complex (comprising Sec61αp, Sec61βp, and Sec61γp) (21–24). Furthermore, mounting evidence suggests that a luminal heat shock protein 70, i.e., immunoglobulin heavy chain binding protein (BiP)/glucose-regulated protein 78 (Grp78) or glucose-regulated protein 170 (Grp170), is involved in cotranslational protein transport into dog pancreas microsomes (25–28).
Recently, human homologs of yeast proteins Sec62p (termed HTP1) (29) and Sec63p (30) were discovered. Here we asked whether these two membrane proteins are present in dog pancreas microsomes to any significant extent and whether these proteins interact with the Sec61p complex and/or luminal heat shock protein 70. We observed that the canine homologs of yeast proteins Sec62p and Sec63p are abundant proteins in pancreas microsomes, present in almost equimolar concentrations as compared with Sec61αp monomers. Fractions of the two proteins were detected in association with each other as well as with the Sec61p complex. The J domain of the human Sec63p was shown to interact with BiP in a productive manner. Thus, the membrane of the mammalian ER contains components, known from the posttranslationally operating protein translocase in yeast.
Materials and Methods
Materials.
The so-called protein ladder (10–200 kDa) was from Life Technologies (Grand Island, NY). The peroxidase conjugate of anti-rabbit IgG-goat antibodies and carbonic anhydrase were purchased from Sigma; enhanced chemiluminescence was from Amersham Pharmacia. Coomassie brilliant blue and the electrophoresis reagents were from Serva; poly(vinylidene difluoride) (PVDF) membranes were from Millipore; and x-ray films (X-Omat, AR) were from Kodak. BSA was from New England Biolabs. [γ-32P]ATP was from ICN.
Antibodies.
Antibodies were made against peptides plus either an additional amino- or carboxyl-terminal Cys. These peptides were coupled to keyhole limpet hemocyanine (Sigma) which had been activated with N-succinimidyl-3-maleinimido-propionate (Fluka). To obtain antibodies for use in immunoaffinity chromatography, 2 mg of coupled peptide for each immunization was mixed with 200 μg of adjuvant peptide (Sigma) and incomplete Freund's adjuvant and injected into rabbit (22). Antibodies were affinity purified on immobilized peptides (Sulfolink; Pierce). The acid-eluted antibodies were coupled to a mixture of Protein A- and Protein G-Sepharose (fast flow; Amersham Pharmacia) with dimethyl pimelimidate (Pierce). The antibodies that were used for immunoaffinity purification of Sec62p, Sec63p, and the Sec61p complex were directed against the carboxyl-terminal peptide of Sec62p, and the amino-terminal peptides of Sec63p (Fig. 1) and Sec61βp (PGPTPSGTNC), respectively. Furthermore, antibodies were made successfully against the amino-terminal peptide of Sec62p, the carboxyl-terminal peptide of Sec63p (Fig. 1), as well as against the carboxyl-terminal peptides of Sec61αp (CKEQSEVGSMGALLF) and the SPase 22-kDa subunit (CSVPFPDTYEITKSY). All purified antibodies react only with the expected proteins in immunoblots.
Figure 1.
The sequences of the human homologs of yeast proteins Sec62p (A) and Sec63p (B) and partial sequences of the corresponding canine proteins (shown below the human sequences). The canine sequences were derived from amino-terminal sequencing (B) and sequence analysis of cyanogen bromide cleavage products (A and B), respectively. Note that (i) the two dots represent an identical amino acid residue present in the canine protein and the respective human protein; (ii) x represents an ambiguous result in the amino acid analysis; (iii) in the case of cyanogen bromide fragments, a methionine (M) was added to the amino terminus of the fragment, assuming that an internal fragment was subjected to sequencing; (iv) the underlined human sequences represent putative transmembrane domains; (v) italic print indicates the J domain within Sec63p; and (vi) peptide sequences shown above the human protein sequences represent peptides which were used successfully for immunization.
Purification of Sec62p and Sec63p.
Dog pancreas microsomes were prepared and treated with nuclease and EDTA as described (31). The absorbance at 280 nm of the final microsomal suspension was 50, as measured in 2% SDS. This corresponds to a protein concentration of about 15 mg/ml. The microsomes were stripped with respect to ribosomes according to published procedures (22). After reisolation of the stripped microsomes by centrifugation, the pellets were resuspended in extraction buffer (20 mM Hepes⋅KOH (pH 7.5)/400 mM KCl/1 mM EDTA/1.5 mM MgCl2/15% glycerol/0.65% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate) at a concentration of 0.66 equivalents per μl. The nonsolubilized material was pelleted by centrifugation for 30 min at 2°C and 68,000 rpm in a Beckman TLA 100.3 rotor. The detergent extracts were incubated with different affinity-purified and immobilized peptide antibodies (2 equivalents per μl antibody resin) for 3 h at 4°C. The suspension was subsequently transferred into a column (Mobi-Tec, Gottingen, Germany). After collecting the flow-through, the column was washed five times with 1.6 (vol/vol) of loading buffer to antibody resin, before eluting the protein that had bound to the peptide antibodies with 2 (vol/vol) of the corresponding peptide at 1 mg/ml in loading buffer to antibody resin.
Purification of Glutathione S-Transferase (GST)–Sec63J Hybrid.
A PCR product, coding for amino acid residues 91–189 of human Sec63p, was inserted into plasmid pGEX-4T, resulting in a plasmid, coding for GST and the J domain of human Sec63p. Escherichia coli JM101 cells were transformed with this plasmid. The cells were grown in LB medium plus ampicillin (final concentration, 50 μg/ml) at 37°C to an OD600 of 1.5. GST–Sec63J hybrid production was then induced with isopropyl β-d-thiogalactoside (final concentration, 0.3 mM). After 2.5 h of induction, cells were harvested by centrifugation for 10 min at 2°C and 5,000 rpm in a Beckman JA10 rotor. The bacterial pellet was resuspended in application buffer [1 mM MgCl2/3 mM KCl/150 mM NaCl/2 mM NaH2PO4⋅H2O/10 mM Na2HPO4⋅2H2O (pH 7.3)]. Subsequently, the cells were lysed by three cycles of sonication for 1 min at 0°C. The resulting crude extract was cleared by centrifugation (30 min at 2°C and 20,000 rpm in a Beckman JA20 rotor). The supernatant was applied to glutathione-Sepharose 4B (Amersham Pharmacia). The column was washed with application buffer plus Tween 20 (final concentration, 0.1%). Then, GST–Sec63J hybrid was eluted with 50 mM Tris⋅HCl (pH 8.0)/10 mM glutathione. The eluate was applied to a Mono Q column (Amersham Pharmacia), equilibrated with 25 mM Tris⋅HCl (pH 8.0)/50 mM NaCl/10% glycerol. The protein was eluted with a salt gradient, ranging from 50–400 mM NaCl. Purification of His-tagged Grp78 was described elsewhere (32).
Surface Plasmon Resonance Spectroscopy.
Surface plasmon resonance spectroscopy was performed in a BIAlite upgrade system. Monoclonal goat anti-GST-antibodies (BIACORE, Uppsala) were immobilized on a sensor chip CM5 research grade (BIACORE) by amine coupling according to the manufacturer's protocol. No interaction of BiP in the presence of ATP was detected when GST was bound to these immobilized antibodies (data not shown). Therefore, GST was bound to the immobilized antibodies in the reference cell in subsequent experiments. The chip was equilibrated with application buffer, described above, which was supplemented with ATP (final concentration, 2 mM) and Tween 20 (final concentration, 0.1%), termed running buffer (flow rate, 15 μl/min). The GST–Sec63J hybrid was bound to the immobilized antibodies in the measuring cell (600 response units). Subsequently, solutions containing increasing concentrations of purified BiP (2–20 μM) were passed over the chip in the absence or presence of ATP. Each BiP application was followed by application of running buffer. The analysis was carried out by using the bia evaluation software Version 2.2.4.
Analytical Procedures.
ATPase assays were performed as described (33). Before electrophoresis, proteins were concentrated by precipitation with trichloroacetic acid (final concentration, 10%). After centrifugation at 4°C, the pellets were washed with ice cold acetone and boiled in SDS sample buffer. SDS/PAGE analysis, electroblotting to PVDF membranes, cyanogen bromide cleavage of Coomassie-stained proteins in gel slices, and amino acid sequencing by Edman degradation in a Procise 491 Protein sequencer (Applied Biosystems) was performed as described (27).
Results
Canine Sec62p and Sec63p Are Abundant Proteins in Pancreatic Microsomes.
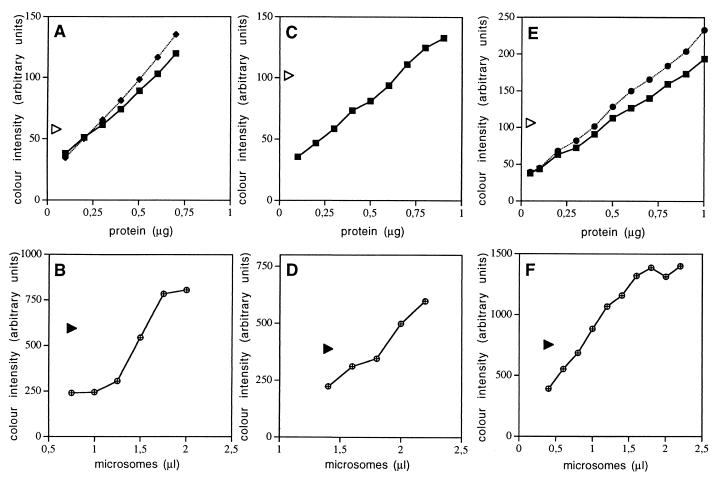
To determine to what extent Sec62p and Sec63p are present in dog pancreas microsomes, immunoaffinity purification of these proteins from microsomal detergent extracts with antipeptide antibodies (Fig. 1) was used. Subsequently, the purified proteins (Fig. 2) were subjected to amino acid sequence analysis (Fig. 1) and used as standards for the determination of their respective concentration in microsomal suspensions (Fig. 3). In the latter case, Sec61αp served as an internal control, because its concentration had been determined previously as being 1.67 μM (24). Dog pancreas microsomes were solubilized in detergent at high salt concentration and the extract was applied to immobilized antipeptide antibodies that were directed against Sec62p or Sec63p. The immunoaffinity resins were washed and the proteins were eluted with the respective peptides and subjected to SDS/PAGE analysis (Fig. 2). The dominant products of the Sec62p and Sec63p resins were identified as canine Sec62p and Sec63p, respectively, by immunoblot analysis (Fig. 2). In addition, the proteins of interest and/or cyanogen bromide cleavage products thereof were transferred to PVDF membranes and subjected to sequence analysis (Fig. 1). The sequence analyses confirmed the identity of the purified proteins and established that the respective protein bands correspond to single proteins, i.e., Sec62p and Sec63p. Based on this information, the amount of protein, present in the respective band of a gel of a certain protein preparation, was determined by comparison with protein standards, which were run on the same gel and stained simultaneously (Fig. 3 A and C). Subsequently, an aliquot of the same sample of purified protein was run on the same gel together with increasing amounts of microsomes, and the known amount of purified protein served as a standard for the Western blot signals, as determined by luminescence and densitometry of the x-ray films (Fig. 3 B and D). In parallel, the analyses were carried out for Sec61αp, present in purified Sec61p complex (34) (Fig. 3 E and F). We calculated concentrations of 1.96 μM for Sec62p, 1.98 μM for Sec63p, and 2.12 μM for Sec61αp in the microsomal suspensions. Thus, Sec62p and Sec63p are abundant proteins of dog pancreas microsomes, present in almost equimolar concentrations as compared with Sec61αp monomers.
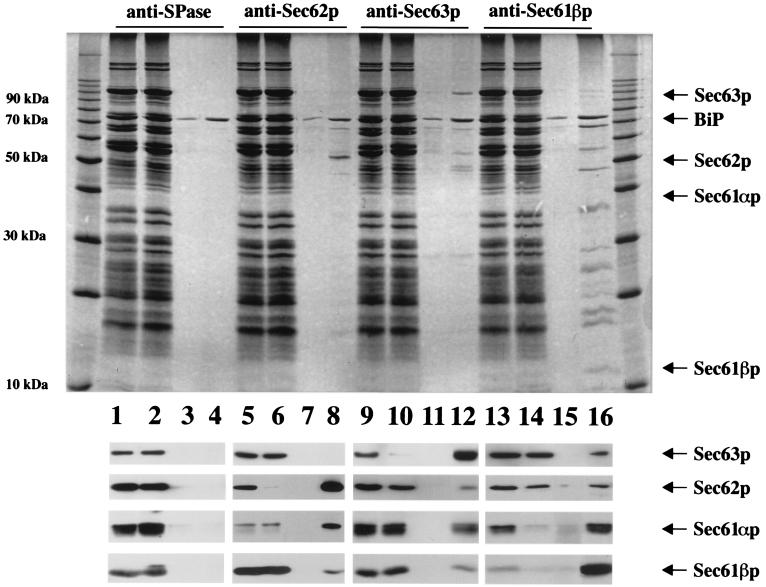
Figure 2.
Immunoaffinity purification of the canine homologs of yeast proteins Sec62p and Sec63p from pancreatic microsomes. A detergent extract of microsomal proteins was incubated with antibody resins which were directed against nonnative SPase 22-kDa subunit (lanes 1–4), Sec62p (lanes 5–8), Sec63p (lanes 9–12), or Sec61p complex (lanes 13–16) for 3 h at 4°C. After various washing steps, the specifically bound material was eluted with the respective peptide. Aliquots of the extract (lanes 1, 5, 9, and 13) and the pass-through (lanes 2, 6, 10, and 14) as well as 50× larger aliquots of the last washing step (lanes 3, 7, 11, and 15) and the eluate (lanes 4, 8, 12, and 16) were subjected to precipitation. The precipitates were analyzed by electrophoresis in 10 or 15% polyacrylamide gels and subsequent staining with Coomassie brilliant blue (Upper) or subsequent electroblotting to PVDF membranes, followed by incubation with specific antibodies and peroxidase conjugate of anti-rabbit IgG-goat antibodies (Lower). The antibodies were visualized by incubation of the blots in enhanced chemiluminescence and subsequent exposure to x-ray film. The protein ladder (10–200 kDa) was run on the same gel. Note that BiP was detected in all washing buffers and peptide eluates. Probably, this reflects the known ability of this protein to bind to denatured proteins and peptides. In addition, two unknown polypeptides with masses of 55 and 65 kDa, respectively, were routinely observed in peptide eluates, although to varying degrees (most prominent in the Sec61βp eluate).
Figure 3.
Quantitation of canine Sec62p and Sec63p in pancreatic microsomes. Serial dilutions of BSA (squares) and carbonic anhydrase (diamonds and circles, respectively) were run on SDS/PAGE gels in parallel to preparations, containing immunoaffinity-purified Sec62p (A), Sec63p (C), and purified Sec61 complex (E), respectively. The proteins were stained with Coomassie brilliant blue and the staining intensity was quantified by densitometry (Personal Densitometer, Applied Biosystems). The same preparations of these purified proteins were run on SDS/PAGE gels in parallel to serial dilutions of dog pancreas microsomes (B, D, and F). Subsequently, the proteins were transferred to PVDF membranes and incubated with specific rabbit antibodies and with a peroxidase conjugate of anti-rabbit IgG-goat antibodies. The bound antibodies were made visible by incubation with enhanced chemiluminescence and exposure to x-ray film. The intensity of silver precipitation was quantified by densitometry. The calculation of the molar concentrations of the various Sec proteins in microsomal suspensions was based on the predicted molecular masses of human Sec62p (45.9 kDa), human Sec63p (88 kDa), and canine Sec61αp (52.2 kDa), respectively, as calculated by the protean option of the dnastar sequence analysis software. The triangles represent the color intensities that were obtained for the purified proteins of interest. We note that the values observed for the purified proteins were within the linear ranges of the titrations.
Canine Sec62p and Sec63p Are Associated with Each Other as Well as with the Sec61p Complex.
The immunoaffinity purification, mentioned above, also allowed us to address the question of whether Sec62p and Sec63p are present in complexes with each other as well as with the Sec61p complex (Fig. 2). Here, immunoaffinity resins with antibodies that are directed against Sec61βp served as the positive control (22) and resins with antibodies that are directed against the 22-kDa subunit of the signal peptidase complex and do not recognize the native enzyme were used as the negative control (Fig. 2). From Western blotting analysis of the peptide eluates of the Sec62p and Sec63p affinity resins, we conclude that Sec61p complex is associated with Sec62p and Sec63p and that Sec62p is associated with Sec63p. For unknown reasons, we failed to reproducibly detect Sec63p in the eluate of the anti-Sec62p resin. Furthermore, Sec62p and Sec63p were detected in the peptide eluate of the anti-Sec61βp but not the anti-SPase 22-kDa subunit resins. However, on quantitative analysis, i.e., by asking to what extent the pass-through of the respective column is depleted with respect to a certain protein as compared with the applied material, one has to conclude that the observed coimmunoprecipitations represent only a small number of higher-order complexes. Thus, at least small fractions of Sec62p and Sec63p are associated with each other as well as with Sec61p complex after solubilization of dog pancreas microsomes in detergent. We obtained no indication for a specific interaction of canine Sec63p with BiP or Grp170 under these conditions.
The J Domain of Sec63p Functionally Interacts with BiP.
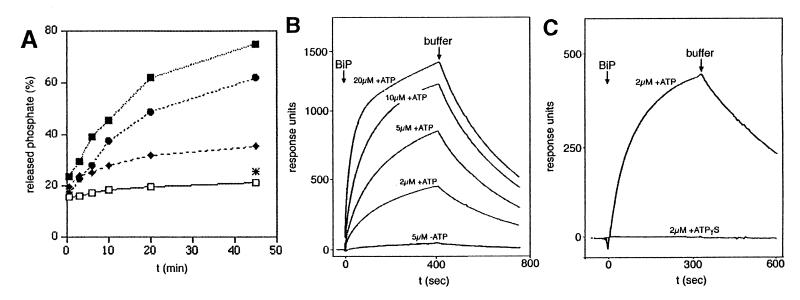
To determine whether the mammalian Sec63p contains a functional DnaJ domain, a hybrid protein, comprising GST and amino acid residues 91–189 of human Sec63p, was constructed (termed GST–Sec63J hybrid) and subjected to three activity assays. In the first set of experiments, we asked if the GST–Sec63J hybrid stimulates the ATPase activity of BiP. BiP was purified from dog pancreas microsomes and incubated with [γ-32P]ATP in the absence or presence of GST–Sec63J hybrid as described (33). After various times of incubation, the samples were analyzed by TLC and the amounts of ATP and free phosphate were determined by autoradiography and densitometry (Fig. 4A). According to the time-dependent release of 32Pi (versus the total label added) under the different conditions, the J domain of human Sec63p stimulated the ATPase activity of BiP in a concentration-dependent manner. Similar results were obtained with recombinant BiP (data not shown). A priori, the possibility cannot be excluded that the observed stimulation of ATPase activity of BiP by the GST–Sec63J hybrid was caused by a BiP/substrate rather than a BiP/cochaperone interaction. However, GST had no such stimulatory effect, even at much higher concentrations when compared with GST–Sec63J hybrid (Fig. 4A). A GST–Sec63J hybrid-based pulldown assay was used to further address this issue. Again, GST served as a negative control. GST or GST–Sec63J hybrid were immobilized on glutathione-Sepharose and incubated with detergent extracts of dog pancreas microsomes in the absence and presence, respectively, of ATP. The bound proteins were eluted and subjected to SDS/PAGE and subsequent staining with Coomassie brilliant blue (data not shown). The GST–Sec63J hybrid selectively pulled down BiP from the detergent-solubilized microsomal proteins in the presence of ATP and much less efficiently in the absence of ATP. We also observed that immobilized BiP binds full-length Sec63p, albeit not selectively, which is present in detergent extracts of microsomal proteins (data not shown). From our results, we conclude that BiP and the DnaJ domain of human Sec63p interact in a productive manner because (i) GST did not pull down BiP, and (ii) the other molecular chaperones, present in the detergent extract of dog pancreas microsomes (such as Grp94 and calreticulin), did not bind to the GST–Sec63J hybrid. In addition, we determined the apparent affinity of BiP for the GST–Sec63J hybrid by surface plasmon resonance spectroscopy (Fig. 4 B and C). Anti-GST antibodies were immobilized on a sensor chip. Then GST–Sec63J hybrid was bound to the immobilized antibodies (measuring cell). Again, GST, bound to anti-GST antibodies, served as a negative control (and was used in the reference cell). Subsequently, solutions containing increasing concentrations of BiP, purified as a recombinant protein from E. coli, were passed over the chip in the absence or presence of ATP and in the presence of ATPγS, respectively. We determined an apparent affinity of BiP for the GST–Sec63J hybrid in the presence of ATP (KD) of 5 × 10−6 M. However, we note that this apparent affinity has to be used with caution because the kinetics could not be fitted perfectly to a 1:1 binding model. There was no interaction seen in the absence of ATP (Fig. 4B) or in the presence of ATPγS (Fig. 4C). Similar results were obtained with BiP purified from microsomes (data not shown).
Figure 4.
The human homolog of yeast protein Sec63p functionally interacts with BiP (Grp78). (A) Stimulation of the ATPase activity of BiP by increasing concentrations of GST–Sec63J hybrid. BiP (250 μg/ml), purified from dog pancreas microsomes, was incubated in the absence (□) or presence of GST–Sec63J hybrid (2.5, ♦; 12.5, ●; and 62.5 μg/ml, ■) or in the presence of GST (212.5 μg/ml; [*]) as described (33). The thin-layer chromatograms were exposed to x-ray films. The x-ray films were analyzed by densitometry (Personal Densitometer, Applied Biosystems). (B and C) Apparent affinity of GST–Sec63J hybrid for BiP as measured by surface plasmon resonance spectroscopy. Monoclonal goat anti-GST antibodies were immobilized on a sensor chip CM5. GST–Sec63J hybrid was bound to the immobilized antibodies in the measuring cell; GST was bound to the immobilized antibodies in the reference cell. At t = 0, solutions containing defined concentrations of recombinant BiP were passed over the chip in the absence or presence of ATP (B) and in the presence of ATP or ATPγS (C), respectively. Each BiP application was followed by application of running buffer. The response units were recorded as the difference between measuring and the reference cell dependent on time. We note that the residuals, representing the difference between measured and fitted data, showed an amplitude of up to 15% of the maximum signal and a nonrandom distribution.
Discussion
The data reported in this paper provide insights into the molecular architecture of the protein transport apparatus of the mammalian ER. We showed that the canine homologs of yeast proteins Sec62p and Sec63p are abundant proteins in pancreatic microsomes, present in almost equimolar concentrations when compared with Sec61αp monomers. This distinguishes the dog pancreas microsomes from yeast microsomes where it was estimated that Sec61p is present in 3- to 5-fold excess when compared with Sec62p and Sec63p (5). Furthermore, fractions of the two mammalian Sec proteins were found in association with each other as well as with the Sec61p complex. Taken together, it is tempting to speculate that in the intact pancreas ER, all Sec61p complexes either transiently or permanently associate with Sec62p and Sec63p. However, when compared with the situation in yeast where almost all Sec62p and Sec63p were found in heptameric Sec complexes (5), only minor amounts of higher-order complexes were detected in the extracts of dog pancreas microsomes. Thus, the nature and/or stability of the putative mammalian higher-order Sec complexes are different when compared with the equivalent yeast complexes. Additionally, mammalian Sec63p was shown to interact with BiP in a chaperone/cochaperone manner as had been reported for Kar2p and the J domain of yeast Sec63p (35, 36). In summary, we conclude that the dog pancreas microsomes contain components known from the posttranslationally operating protein translocase in yeast. In contrast with the situation in yeast where at least two Sec complexes exist that differ in subunit composition and, apparently, in function (5), our data favor the presence of one type of higher-order complex in the mammalian ER. Therefore, we suggest that Sec62p and Sec63p are part of a multifunctional transport complex, prepared to function in posttranslational and cotranslational protein transport into as well as protein transport out of the mammalian ER. With respect to posttranslational transport, direct support for our suggestion was provided by a cross-linking approach (J. D. Oliver, B. C. Knight, J.T., R.Z., & S. High, unpublished data). Studying the membrane integration of tail-anchored proteins (i.e., Sec61βp, Sec61γp, and synaptobrevin 2), we observed transient interaction of these newly synthesized proteins with Sec61βp and Sec62p. On the basis of these results, we concluded that Sec61βp and Sec62p play a specific role during posttranslational membrane integration of tail-anchored proteins. Furthermore, because a role for luminal heat shock protein 70, i.e., BiP (28) and/or Grp170 (27), previously had been shown for cotranslational transport into dog pancreas microsomes, and because Sec63p was shown here to productively interact with BiP and associate with the Sec61p complex, the involvement of Sec63p in cotranslational transport would not be unexpected. The observations that protein export into the cytosol, delivering proteins to the proteasome for degradation, depends on Sec61αp and Kar2p/BiP in mammals and yeasts (37–40) and on Sec63p in yeast (39) further support our suggestion of Sec63p being part of a multifunctional transport complex.
Acknowledgments
The purified Sec61p complex and purified GST were kindly donated by our colleagues Martin Jung and Jens Solsbacher, respectively. C.B. was supported by a fellowship from the Graduiertenkolleg “Zelluläre Regulation und Wachstum.” This work was supported by the Deutsche Forschungsgemeinschaft SFB 530 (R.Z.) and SFB 352 (I.H.) and the Fonds der Chemischen Industrie.
Abbreviations
- ER
endoplasmic reticulum
- Hsp
heat shock protein
- BiP
immunoglobulin heavy chain binding protein
- Grp
glucose-regulated protein
- GST
glutathione S-transferase
- PVDF
poly(vinylidene difluoride)
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The protein sequence data reported in this paper has been deposited in the SWISS-PROT Protein Data Bank (accession nos. P82008 and P82009).
References
- 1.Zimmermann R. Biol Chem. 1998;379:275–282. [PubMed] [Google Scholar]
- 2.Zimmermann R, Meyer D I. Trends Biochem Sci. 1986;11:512–515. [Google Scholar]
- 3.Deshaies R J, Schekman R. J Cell Biol. 1987;105:633–645. doi: 10.1083/jcb.105.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esnault Y, Blondel M-O, Deshaies R J, Scheckman R, Kepes F. EMBO J. 1993;12:4083–4093. doi: 10.1002/j.1460-2075.1993.tb06092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panzner S, Dreier L, Hartmann E, Kostka S, Rapoport T A. Cell. 1995;81:561–570. doi: 10.1016/0092-8674(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 6.Deshaies R J, Schekman R. J Cell Biol. 1989;109:2653–2664. doi: 10.1083/jcb.109.6.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshaies R J, Sanders S L, Feldheim D A, Schekman R. Nature (London) 1991;349:806–808. doi: 10.1038/349806a0. [DOI] [PubMed] [Google Scholar]
- 8.Green N, Fang H, Walter P. J Cell Biol. 1992;116:597–604. doi: 10.1083/jcb.116.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothblatt J A, Deshaies R J, Sanders S L, Daum G, Schekman R. J Cell Biol. 1989;109:2641–2652. doi: 10.1083/jcb.109.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadler I, Chiang A, Kurihara T, Rothblatt J A, Way J, Silver P. J Cell Biol. 1989;109:2665–2675. doi: 10.1083/jcb.109.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toyn J, Hibbs A R, Sanz P, Crowe J, Meyer D I. EMBO J. 1988;7:4347–4353. doi: 10.1002/j.1460-2075.1988.tb03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogel J P, Misra L M, Rose M D. J Cell Biol. 1990;110:1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen T H, Law D T S, Williams D B. Proc Natl Acad Sci USA. 1991;88:1565–1569. doi: 10.1073/pnas.88.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders S L, Whitfield K M, Vogel J P, Rose M D, Schekman R. Cell. 1992;69:353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- 15.Lyman S K, Schekman R. Cell. 1997;88:85–96. doi: 10.1016/s0092-8674(00)81861-9. [DOI] [PubMed] [Google Scholar]
- 16.Lyman S K, Schekman R. J Cell Biol. 1995;131:1163–1171. doi: 10.1083/jcb.131.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craven R A, Egerton M, Stirling C J. EMBO J. 1996;15:2640–2650. [PMC free article] [PubMed] [Google Scholar]
- 18.Baxter B K, James P, Evans T, Craig E. Mol Cell Biol. 1996;16:6444–6456. doi: 10.1128/mcb.16.11.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton T G, Flynn G C. J Biol Chem. 1996;271:30610–30613. doi: 10.1074/jbc.271.48.30610. [DOI] [PubMed] [Google Scholar]
- 20.Brodsky J L, Goeckeler J, Schekman R. Proc Natl Acad Sci USA. 1995;92:9643–9646. doi: 10.1073/pnas.92.21.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Görlich D, Prehn S, Hartmann E, Kalies K-U, Rapoport T A. Cell. 1992;71:489–503. doi: 10.1016/0092-8674(92)90517-g. [DOI] [PubMed] [Google Scholar]
- 22.Görlich D, Rapoport T A. Cell. 1993;75:615–630. doi: 10.1016/0092-8674(93)90483-7. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann E, Sommer T, Prehn S, Görlich D, Jentsch S, Rapoport T A. Nature (London) 1994;367:654–657. doi: 10.1038/367654a0. [DOI] [PubMed] [Google Scholar]
- 24.Hanein D, Matlack K E S, Jungnickel B, Plath K, Kalies K-U, Miller K R, Rapoport T A, Akey C W. Cell. 1996;87:721–732. doi: 10.1016/s0092-8674(00)81391-4. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman D L, Walter P. Cell Regul. 1991;2:851–859. doi: 10.1091/mbc.2.10.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klappa P, Mayinger P, Pipkorn R, Zimmermann M, Zimmermann R. EMBO J. 1991;10:2795–2803. doi: 10.1002/j.1460-2075.1991.tb07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dierks T, Volkmer J, Schlenstedt G, Jung C, Sandholzer U, Zachmann K, Schlotterhose P, Neifer K, Schmidt B, Zimmermann R. EMBO J. 1996;15:6931–6942. [PMC free article] [PubMed] [Google Scholar]
- 28.Hamman B D, Hendershot L M, Johnson A E. Cell. 1998;92:747–758. doi: 10.1016/s0092-8674(00)81403-8. [DOI] [PubMed] [Google Scholar]
- 29.Daimon M, Susa S, Suzuki K, Kato T, Yamatani K, Sasaki H. Biochem Biophys Res Commun. 1997;230:100–104. doi: 10.1006/bbrc.1996.5892. [DOI] [PubMed] [Google Scholar]
- 30.Skowronek M H, Rotter M, Haas I G. Biol Chem. 1999;380:1133–1138. doi: 10.1515/BC.1999.142. [DOI] [PubMed] [Google Scholar]
- 31.Watts C, Wickner W, Zimmermann R. Proc Natl Acad Sci USA. 1983;80:2809–2813. doi: 10.1073/pnas.80.10.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bies C, Guth S, Janoschek K, Nastainczyk W, Volkmer J, Zimmermann R. Biol Chem. 1999;380:1175–1182. doi: 10.1515/BC.1999.149. [DOI] [PubMed] [Google Scholar]
- 33.Wiech H, Buchner J, Zimmermann M, Zimmermann R, Jakob U. J Biol Chem. 1993;268:7414–7421. [PubMed] [Google Scholar]
- 34.Möller I, Jung M, Beatrix B, Levy R, Kreibich G, Zimmermann R, Wiedmann M, Lauring B. Proc Natl Acad Sci USA. 1998;95:13425–13430. doi: 10.1073/pnas.95.23.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corsi A K, Schekman R. J Cell Biol. 1997;137:1483–1493. doi: 10.1083/jcb.137.7.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misselwitz B, Staeck O, Matlack K E S, Rapoport T A. J Biol Chem. 1999;274:20110–20115. doi: 10.1074/jbc.274.29.20110. [DOI] [PubMed] [Google Scholar]
- 37.Wiertz E J H, Tortoralla D, Bogyo M, Yu J, Mothes W, Jones T R, Rapoport T A, Ploegh H L. Nature (London) 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 38.Pilon M, Schekman R, Römisch K. EMBO J. 1997;16:4540–4548. doi: 10.1093/emboj/16.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plemper R K, Bömler S, Bordallo J, Sommer T, Wolf D H. Nature (London) 1997;388:891–895. doi: 10.1038/42276. [DOI] [PubMed] [Google Scholar]
- 40.Knittler M R, Dirks S, Haas I G. Proc Natl Acad Sci USA. 1995;92:1764–1768. doi: 10.1073/pnas.92.5.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]