Abstract
Human papillomavirus type 16 (HPV16) is an oncogenic virus that causes persistent infections in cervical epithelium. The chronic nature of HPV16 infections suggests that this virus actively evades the host immune response. Intraepithelial Langerhans cells (LC) are antigen-presenting cells that are critical in T-cell priming in response to viral infections of the skin. Here we show that HPV16 infection is directly associated with a reduction in the numbers of LC in infected epidermis. Adhesion between keratinocytes (KC) and LC, mediated by E-cadherin, is important in the retention of LC in the skin. Cell surface E-cadherin is reduced on HPV16-infected basal KC, and this is directly associated with the reduction in numbers of LC in infected epidermis. Expression of a single viral early protein, HPV16 E6, in KC reduces levels of cell surface E-cadherin thereby interfering with E-cadherin-mediated adhesion. Through this pathway, E6 expression in HPV16-infected KC may limit presentation of viral antigens by LC to the immune system, thus preventing the initiation of a cell-mediated immune response and promoting survival of the virus.
Human papillomaviruses (HPV) cause persistent disease, despite producing immunogenic proteins throughout the replicative cycle. These viruses are therefore likely to possess an army of mechanisms to avoid the host immune system. Some types of HPV, in particular type 16, are strongly associated with the development of cancer after infections of the cervical epithelium (54). Consequently, the chronic nature of the infection, in association with high-risk oncogenic types of HPV, results in an increased risk of cellular transformation and malignancy.
HPV is a nonlytic virus that is only permissive for viral replication in epidermal keratinocytes (KC). The ability of the virus to influence the immune system is therefore limited to the localized environment of the infected epidermis. Furthermore, activation of the adaptive immune response to HPV is dependent on cross-presentation of viral antigens to antigen-presenting cells (APC) resident in the skin (43). In the present study we explore the influence of HPV on the host's capacity to initiate the immune response by reducing numbers of APC resident at the site of infection.
Langerhans cells (LC) are the epidermal contingent of the potent antigen-presenting dendritic cells (34) and constitute the primary APC in the skin. Immature LC form a contiguous network throughout the epithelium. Under steady-state conditions, CD14+, E-cadherin-negative LC precursors migrate from the dermis into the epidermis in response to macrophage inflammatory protein 3α (MIP-3α) (9) and differentiate into CD14+, E-cadherin-positive immature LC when exposed to transforming growth factor β1 (TGF-β1) (25). Both MIP-3α and TGF-β1 are constitutively expressed by KC. Immature LC initiate migration from the epidermis in response to proinflammatory stimuli such as tumor necrosis factor alpha and interleukin-1β (12, 49). These cells become responsive to MIP-3β, which directs their migration to the T-cell-dependent regions of local lymph nodes via the afferent lymphatics. There they present antigen in association with major histocompatibility complex and provide costimulatory signals for T-cell activation (1, 26).
Detachment of LC from surrounding KC is an essential step in the initiation of their migration from the epidermis. Immature LC adhere to KC via the homophilic, calcium-dependent cell adhesion molecule E-cadherin, which is constitutively expressed by KC in the basal and suprabasal layers of the epidermis. E-cadherin is not expressed on the surface of dermal LC but is upregulated on immature LC when they are resident in the epidermis. The interaction between E-cadherin on the surface of immature LC and KC is important for both LC localization and retention into the basal layers of the epidermis (22, 40). Cell surface expression of E-cadherin on LC is reduced upon the initiation of their migration to permit LC detachment from surrounding KC (6, 35), and this process is partly under the control of proinflammatory cytokines (11). In concordance with this, dendritic cells that have migrated from the skin express low levels of E-cadherin in the lymph nodes (35).
LC are essential for the initiation of an adaptive immune response against viral antigens encountered within the epidermis, and this has been substantiated by several observations (39). There is an inverse correlation between LC density at the site of infection and the severity of herpes simplex virus type 1 infections (36, 37). A similar relationship was observed with vaccinia virus, where infection is more severe when the virus is inoculated into LC-depleted skin (3). A number of studies have shown that LC are depleted in dysplastic cervical tissue (31, 41). Dysplastic cervical tissue is frequently infected with HPV, and we postulate that the LC depletion observed is not simply a consequence of cellular transformation but that it is an active mechanism of immune evasion by the virus. By reducing the density of APC at the site of infection, the virus could restrict the initiation of the adaptive immune response and thereby sustain a persistent infection. Because there is no evidence to support the competence of HPV to infect LC, LC depletion could only be achieved through virally mediated effects on infected KC. We hypothesize that HPV16 decreases LC deposition within the infected epidermis by reducing surface expression of E-cadherin on infected KC.
The HPV genome is comprised of an early region, a late region and a regulatory region. The virus life cycle is tightly linked to the differentiation of KC with the early proteins being expressed in the lower layers and the late proteins being expressed in the upper, terminally differentiating layers of the epithelium. The early region encodes six open reading frames (E1, E2, E4, E5, E6, and E7) which are involved in viral replication and transformation of host cells (reviewed in reference 33). The expression of these early proteins in the lower layers of the epithelium, where LC are normally abundant, may influence levels of cell surface E-cadherin on infected KC, thereby affecting the numbers of LC resident in the infected tissue.
We provide here in vivo evidence that active HPV16 infection in cervical epithelium causes a depletion of LC, which closely correlates with reduced and atypical E-cadherin expression. With further in vitro studies we show that the reduction in cell surface E-cadherin is brought about by the HPV type 16 (HPV16) E6 protein. The decrease in levels of surface E-cadherin by E6 is functionally significant, resulting in reduced E-cadherin-positive aggregates in a cell adhesion assay. These data provide evidence of a novel mechanism of viral immune evasion, whereby HPV16 infection decreases E-cadherin expression on the surface of KC, consequently depleting LC at the site of infection. Decreased LC density in the infected epidermis provides a virally mediated mechanism that may contribute to the inability of the host immune system to mount an effective response to HPV16.
MATERIALS AND METHODS
Patients.
Formalin-fixed, paraffin-embedded tissue sections of cervical epithelium were obtained locally from punch biopsies of low-grade or atypical lesions from 96 patients. A further three HPV16 E4+ samples were obtained from low grade or atypical lesions from British patients. Normal cervical epithelium was obtained locally from individuals with no evidence of cervical dysplasia.
Immunostaining of tissue sections.
Paraffin sections of human cervical epithelium were immunostained with antibodies to E4 (TVG405) (14), E-cadherin (HECD-1), or LC (S100; Sigma Chemical Co.). Antigen retrieval of deparaffinized and rehydrated tissue was carried out by boiling sections in a pressure cooker for 2 min in 0.08 M sodium citrate buffer (pH 6.0) for E4 staining and by microwaving sections in Tris-HCl (pH 9.5) containing 5% urea for 20 min for S100 positive LC and E-cadherin staining. The E4 protein was detected by incubating sections with TVG405 for 1.5 h at room temperature (RT), followed by incubation with the 9E10 antibody (14) for 40 min at RT. The staining was detected after subsequent incubations with a biotinylated sheep anti-mouse immunoglobulin for 30 min at RT, a streptavidin-labeled horseradish peroxidase (HRP; Amersham Life Science) for 30 min at RT and diaminobenzidine (Sigma Chemical Co.). For the detection of E-cadherin and LC, sections were incubated at RT overnight with the antibody to E-cadherin and for 1 h with the antibody to S100, and this staining was detected by using the Histostain-DS Doublestaining kit (Zymed), according to the manufacturer's instructions. E-cadherin staining within the epidermis was evaluated by using a semiquantitative scale measuring the intensity and distribution of the staining in the following manner: 3, strong and normal distribution; 2, heterogeneous distribution; 1, weak and fragmented distribution; and 0, loss of staining (46).
Cell culture.
The HaCaT cell line used in the present study was originally obtained from the American Type Culture Collection (Rockville, Md.). HaCaT cells are derived from spontaneously transformed epithelial cells and are mutant p53 (H179R, R282W) (7). The K1neo cell line is a derivative of the human cancer cell line K1, cloned from a differentiated thyroid carcinoma, and was a gift from D. Wynford-Thomas (51). K1neo cells express wild-type p53 and are HPV negative.
Cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS; Invitrogen), 2 mM l-glutamine (Sigma Chemical Co.), and 0.1 mg of penicillin-streptomycin (Invitrogen)/ml. Prior to transfections, HaCaT cells were adapted to minimum essential medium, Joklik-modified (SMEM) supplemented as for DMEM but with FCS that had been extensively dialyzed against phosphate-buffered saline. When it was necessary to preserve cell surface E-cadherin, cells were dissociated with nonenzymatic dissociation solution (Sigma Chemical Co.). Otherwise, cells were enzymatically dissociated with trypsin.
Plasmids.
HPV E4 and E5 genes were amplified by PCR from pBR16 (15). The primers used for E4 were 5′-CGGGATCCAGATCTATGGCTGATCCTGCAGCAGCAACGAAGTATCCTCTCCTGAAATTATTAGGCAGCACT-3′ and 5′-TCGAATTCGGATCCCTATGGGTGTAGTGTTAC-3′ and for E5 were 5′-CGGGATCCATGACAAATCTTGATACT-3′ and 5′-CGGGATCCTTATGTAATTAAAAAGCGTG-3′. The E2, E6, and E7 genes were cloned from pGEX16 E2 (20), pGEX16 E6 (24), and pGEX16 E7 (10), respectively. The E1 gene was cloned from DNA originally derived from W12 cells (38). Genes were inserted in frame with the C terminus of the enhanced green fluorescent protein (EGFP) gene in pEGFP-C1 (Clontech). In all cases the sequences of the inserted genes were confirmed by DNA sequencing. The pET21d (Invitrogen) was used as a nonspecific carrier plasmid to maintain a constant total amount of input DNA in cotransfection experiments.
Expression of HPV genes in HaCaT cells.
HaCaT cells were grown in supplemented SMEM prior to transfection. Transfections of HaCaT cells were carried out in antibiotic and serum-free SMEM with FuGENE 6 transfection reagent (Roche) according to the manufacturer's instructions, by using 1 μg of DNA and 11 μl of FuGENE 6 for each transfection. HaCaT cells were incubated in serum-free SMEM for the first 5 h after transfection, after which time the media was supplemented with a final concentration of 10% FCS and cells were incubated for a further 43 h. Transfections in K1neo cells were carried out similarly but in serum free DMEM.
Flow cytometric analysis.
Vector control (pEGFP), HPV16 early gene transfectants and cervical carcinoma-derived cell lines were stained and analyzed for the expression of E-cadherin. Briefly, cells were dissociated with nonenzymatic dissociation solution and washed twice in staining buffer (phosphate-buffered saline containing 1% FCS and 0.1% sodium azide). Cells were incubated with an anti-E-cadherin antibody (HECD-1; R&D Systems) in the dark for 1 h on ice. Cells were washed twice between subsequent 30-min incubations on ice with biotinylated sheep anti-mouse immunoglobulin (Amersham Pharmacia Biotech) and Cy5.5-labeled streptavidin (Rockland). Cells were washed and incubated with propidium iodide, 5 min prior to acquisition, to permit the identification of dead cells during analysis. For transfection experiments 2,000 live, GFP-positive cells were acquired and analyzed by using a FACScalibur (Becton Dickinson) and CellQuest software. For all other experiments 10,000 events were acquired.
Homotypic adhesion assay.
HaCaT cells that had been transfected 48 h earlier were treated with 0.25% trypsin and 0.015% EDTA in phosphate-buffered saline to dissociate cells and cleave cell surface E-cadherin. Cells were transferred into rehydrated low attachment plates (Corning Costar Corp.) and incubated for 3.5 h in supplemented DMEM. At that time, cells were removed from the plate and stained for E-cadherin and with propidium iodide. Cell aggregation was measured by an increase in the forward scatter of live, GFP-positive cells with equivalent levels of GFP fluorescence and E-cadherin-mediated aggregation was distinguished by E-cadherin staining.
Localization of EGFP and EGFP-E6 in HaCaT cells.
EGFP and EGFP-E6-transfected HaCaT cells were grown on coverslips and, at 48 h after transfection, cells were fixed for 20 min at RT in 3% paraformaldehyde. The localization of the EGFP or EGFP-E6 was detected by using immunofluorescence microscopy.
Immunoblots.
K1neo cells were transfected with pEGFP or pEGFP-E6 and harvested 48 h later. Lysates prepared from equivalent numbers of cells were resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and transferred to Hybond-C extra nitrocellulose (Amersham Biosciences). p53 was detected by using mouse monoclonal antibody Ab-6 (Oncogene Research) and an HRP-labeled anti-mouse immunoglobulin (Dako), and actin was detected by using C-11 goat polyclonal antibody (Santa Cruz) and HRP-labeled anti-goat immunoglobulin (Sigma). Bands were visualized by using Supersignal West Pico chemiluminescent substrate (Pierce, Ill.).
Statistical analysis.
Statistical analysis of the data was performed by using the two-tailed unpaired Mann-Whitney U test or the Spearman rank correlation.
RESULTS
LC are depleted in HPV16-infected skin.
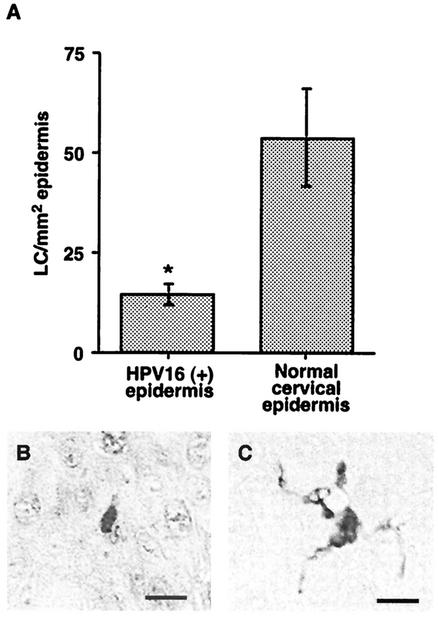
To investigate the effect of HPV16 infection on the density of epidermal LC, we first identified samples in which late events in the viral life cycle were supported by staining histologically atypical and low-grade lesions for the HPV16 E4 protein. Approximately 5% of the locally obtained low-grade and atypical tissue samples were positive for HPV16 E4. There was a significant reduction in LC density (P = 0.015) in HPV16-positive regions of epidermis from infected patients (n = 8) compared to histologically normal cervical tissue from uninfected (n = 8) individuals (Fig. 1A). Overall, there was a 2.5-fold reduction in the number of LC in HPV16-infected regions of epidermis compared to adjacent, uninfected regions of the same tissue samples, clearly indicating that the reduction in LC density in the skin was a direct result of the active viral infection. Interestingly, the LC in HPV-infected tissue were generally morphologically less dendritic (Fig. 1B) than LC in normal tissue (Fig. 1C). This has previously been observed in several studies of LC density in cervical intraepithelial neoplasia (30, 31).
FIG. 1.
HPV16 infection reduces the number of LC resident in the epidermis and the remaining LC have a less dendritic morphology. (A) S100 positive LC cells were enumerated in cervical epidermis that was identified as HPV16+ by the presence of E4 staining (n = 8) and in cervical epidermis from normal individuals (n = 8). The numbers of LC/square millimeter are presented, and the mean ± the standard error of the mean is shown. The asterisk indicates a statistically significant difference (Mann Whitney test) between the two groups (P = 0.015). (B and C) LC in HPV16 epidermis are generally morphologically less dendritic (B) than LC in normal skin (C). Bar, 10 μm.
E-cadherin expression is reduced and atypical in HPV16-infected skin.
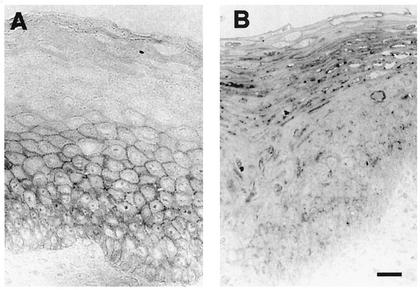
Because E-cadherin is required for the retention of LC in the epidermis, we sought to determine the effect of HPV on E-cadherin expression in infected skin. HPV16-infected and normal tissue sections were stained with an E-cadherin-specific antibody to evaluate the effect of HPV16 infection on E-cadherin. E-cadherin expression was strong in normal cervical epithelium and was localized to the cell surface of the basal and parabasal KC in 7/8 samples (Fig. 2A). In contrast, in all HPV16-infected samples (8 of 8) the E-cadherin staining was weak and fragmented or completely lost. The predominant E-cadherin staining pattern in HPV16-infected tissue, when it was detected, was in the KC of the intermediate and superficial layers of the epidermis (Fig. 2B). E-cadherin localization in these cells was intracellular and punctate, suggesting that there may be a defect in the translocation of the protein to the surface of virus-infected cells.
FIG. 2.
E-cadherin expression is reduced and cellular localization is altered in HPV16-infected tissue. (A) Normal epidermis showed a typical staining pattern after E-cadherin labeling, with a positive signal apparent on the cell surface of basal and suprabasal KC. (B) HPV16-infected epidermis showed punctate intracellular staining in the superficial layers of the epidermis. Bar, 10 μm.
There is a direct correlation between E-cadherin expression and LC number in the epidermis.
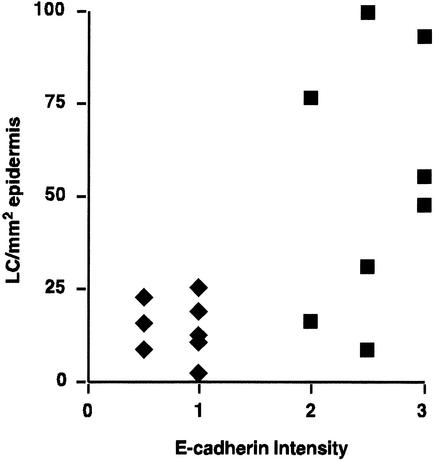
The relationship between E-cadherin intensity and distribution and LC numbers in infected epidermis was examined. In order to carry out the comparison, E-cadherin intensity and distribution was assessed on a semiquantitative scale, in which a high score (i.e., “3”) was given to the typical normal pattern of E-cadherin intensity and distribution and a low score (i.e., “0”) was given when E-cadherin was not detected. There was a significant difference (P = 0.0002) in the mean intensity and distribution of E-cadherin staining in HPV16-infected epidermis (0.8 ± 0.1) compared to normal tissue (2.6 ± 0.2). The relationship between E-cadherin intensity and distribution and LC number was examined (Fig. 3). There was a direct correlation between E-cadherin intensity and distribution and LC number in both normal and infected tissues. In HPV16-infected tissue samples, weak or absent E-cadherin staining was invariably associated with a low density of LC. The majority of normal samples (5 of 8) had high LC numbers and strong and normally distributed E-cadherin staining. However, a greater heterogeneity in LC numbers was observed in normal tissue and three of the normal samples had a lower density of LC despite having a normal distribution in E-cadherin staining. A Spearman rank correlation showed that there was a significant direct correlation between E-cadherin intensity and distribution and LC number in the epidermis (r = 0.6196; P = 0.01).
FIG. 3.
There is a direct correlation between E-cadherin expression and LC density in the skin. E-cadherin staining patterns in HPV-infected (♦) and normal tissues (▪) were graded on a semiquantitative scale (loss of staining = 0; weak and fragmented distribution = 1; heterogeneous distribution = 2; strong and normal distribution = 3) and were plotted against LC number. E-cadherin staining was low or absent in HPV16-infected tissues, and there was a low density of LC. E-cadherin staining was stronger and LC density was generally higher in normal tissues. There was a direct correlation between the level of E-cadherin and LC density in both normal and infected tissue (P = 0.01).
HPV16 E6 expression in cells reduces cell surface E-cadherin.
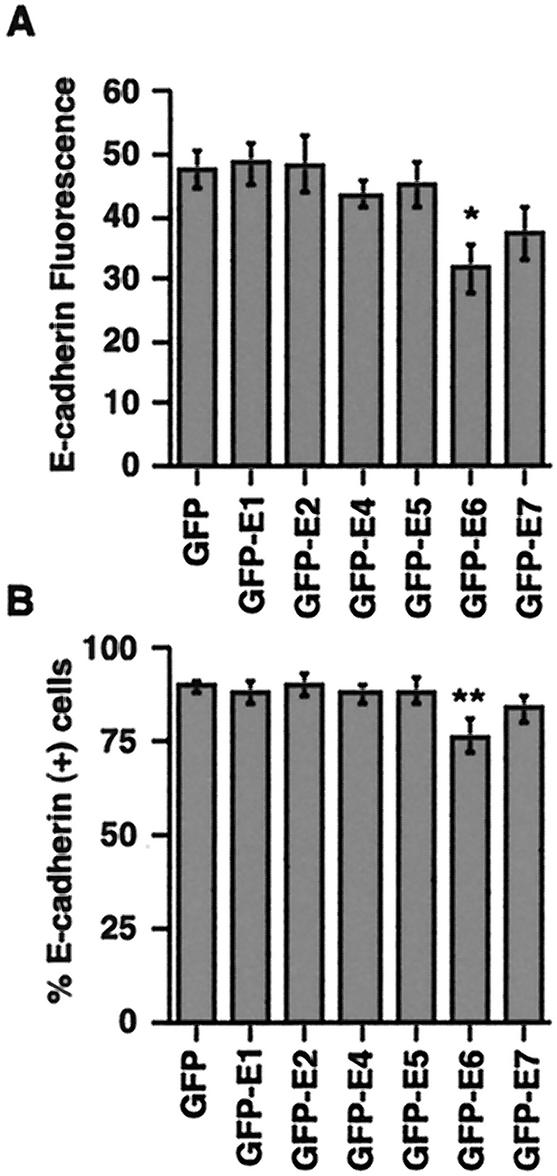
The reduction or loss of E-cadherin staining in the basal and parabasal layers of infected epidermis suggested that an early viral protein expressed in these KC was involved in E-cadherin down regulation. To identify which of the early proteins disrupted E-cadherin expression, we transfected a KC cell line (HaCaT cells) with individual HPV16 early proteins fused to GFP and measured the levels of cell surface E-cadherin. HPV16 E6 was the only early protein that significantly (P = 0.026) reduced, by ca. 30%, the cell surface E-cadherin expression on HaCaT cells (Fig. 4A). There was also a small but insignificant reduction in the level of surface E-cadherin on E7-transfected cells. HPV16 E6 not only decreased the level of cell surface E-cadherin but also rendered some cells E-cadherin negative. There was a 10% decrease in the percentage of E-cadherin-positive cells in EGFP-E6-transfected samples (P = 0.015) compared to samples transfected with EGFP alone (Fig. 4B).
FIG. 4.
Expression of HPV16 E6 reduces the level of cell surface E-cadherin on KC. HaCaT cells were transfected with individual HPV early proteins, and the levels of cell surface E-cadherin fluorescence (A) and E-cadherin-positive cells (B) on GFP+ cells were analyzed by flow cytometry after immunostaining with HECD-1. There was a statistically significant decrease in both the E-cadherin mean fluorescence intensity (∗, P = 0.026) and the percentage of E-cadherin-positive cells (∗∗, P = 0.015) in the GFP-E6-transfected HaCaT cells compared to HaCaT cells transfected with GFP alone.
EGFP-E6 fusion protein mediates p53 degradation.
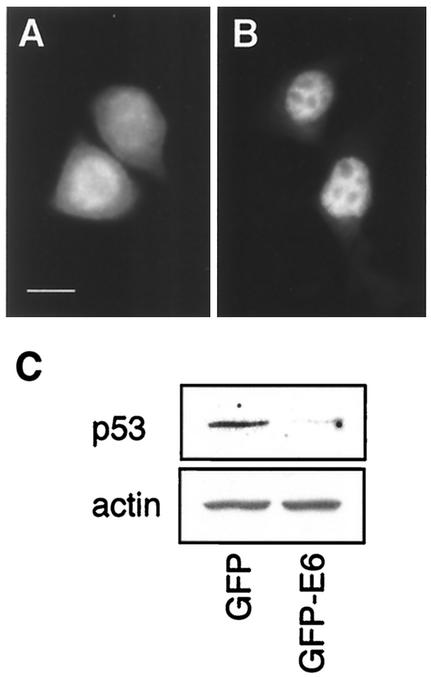
Others have shown that a GFP-E6 fusion similar to that expressed in the present study was localized to the nucleus and was able to sensitize cells to the induction of apoptosis in the presence of mitomycin C (28). We confirmed, by using immunofluorescence microscopy, that EGFP-E6 was localized to the nucleus of HaCaT cells, whereas EGFP was found in the cytoplasm (Fig. 5A and B). We sought to confirm that EGFP-E6 retained its ability to mediate p53 degradation, as another indicator of its functional activity. The wild-type p53 cell line K1neo was transfected with pEGFP or pEGFP-E6, and the amount of p53 in cells was determined by Western blotting at 48 h posttransfection. The efficiency of the uptake of DNA, as measured by the percentage of green fluorescent cells, was similar in both of the transfected samples (EGFP, 34.5%; EGFP-E6, 29.6%). There was a noticeable reduction in the amount of p53 detected in lysates prepared from the EGFP-E6-transfected cells compared to the cells expressing EGFP alone (Fig. 5C). This result showed that the biological function of E6-mediated degradation of p53 was retained when E6 was fused to the C terminus of EGFP.
FIG. 5.
The EGFP-E6 fusion protein is nuclear localized and degrades wild-type p53. HaCaT cells were transfected with pEGFP (A) or pEGFP-E6 (B), and the localization of the protein was visualized by fluorescence microscopy. Whereas EGFP was present in the cytoplasm, EGFP-E6 (B) was exclusively localized to the nucleus. Bar, 10 μm. The functional activity of EGFP-E6 was established by determining its ability to mediate p53 degradation in a wild-type p53 cell line. (C) p53 expression, measured by Western blotting, was reduced in K1neo cells transfected with pEGFP-E6 compared to cells transfected with pEGFP. Actin expression was shown to confirm equivalent loading of protein in both samples.
Expression of HPV16 E6 reduces E-cadherin-mediated adhesion.
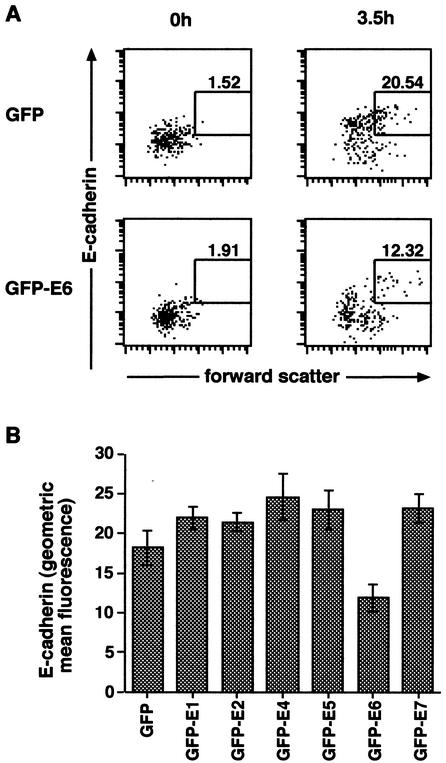
Although expression of HPV16 E6 in KC was sufficient to reduce levels of cell surface E-cadherin, we sought to ascertain the functional significance of this decrease on cellular adhesion. An in vitro cell adhesion assay with HaCaT cells was used to measure the homotypic binding of E-cadherin. Cell surface adhesion molecules were enzymatically cleaved from HaCaT cells expressing HPV early proteins, and the ability of HPV proteins to inhibit cellular adhesion after cleavage was measured by a decrease in forward scatter of E-cadherin-positive cells by flow cytometry. In the experiment shown in Fig. 6A, there was a 40% decrease in the percentage of E-cadherin-positive cell aggregates in EGFP-E6-expressing cells compared to cells expressing EGFP only. A reduction in the percentage of E-cadherin-positive cell aggregates was consistently observed after expression of E6 but not other HPV early proteins (Fig. 6B). The E6-mediated reduction of cell surface E-cadherin expression on HaCaT cells was associated with a reduction in E-cadherin-mediated adhesion and was therefore of functional significance.
FIG. 6.
E6 expression in HaCaT cells reduces E-cadherin-mediated adhesion. HaCaT cells transfected either with HPV early proteins fused to GFP or with GFP alone were stained with an anti-E-cadherin antibody prior to analysis by flow cytometry to distinguish E-cadherin-positive aggregates (gated region). (A) The plots on the left side of the figure show GFP-positive cells (upper left) and GFP-E6-positive cells (lower left) directly after cleavage of E-cadherin, and the plots on the right side of the figure show the same cells 3.5 h after incubation in low-adhesion plates. There was a 40% drop in E-cadherin-positive aggregates in the GFP-E6-positive cells (lower right plot) compared to the GFP-positive cells (upper right plot). (B) Effects of each of the individual early proteins. Only E6 reduced E-cadherin-positive aggregates, whereas the other early proteins had no effect. The mean percentage (± the standard error of the mean) of E-cadherin-positive aggregates over three experiments is shown.
DISCUSSION
LC are the primary APC of the epidermis. Under steady-state conditions LC numbers are homeostatically maintained, being retained in the epidermis with a half-life of about 9 days (17). LC are required for the initiation of the cellular immune response to skin pathogens, and there is an increase in susceptibility to disease when these cells are reduced in number or absent from the skin. In this report, we investigated the effects of HPV16 on LC retention in infected epidermis. We show that HPV16-infected epidermis is depleted of LC as a direct result of the viral infection. The depletion of LC is likely to contribute to the persistence of HPV16 infection by limiting presentation of viral antigens to the immune system.
The disruption of LC homeostasis after epidermal viral infections has been most extensively studied after vaccinia virus infection of murine skin. In the initial response to an epidermal viral infection, LC are mobilized and migrate to the draining lymph nodes (23). As early as 5 days after infection, LC numbers recover to near-normal levels (19) and the density of LC at the infection site is increased above normal levels for some weeks later (3). The net increase in LC in infected epidermis may reflect the disruption of homeostasis as a result of increased immigration of LC into the skin in response to proinflammatory cytokines, counterbalancing the increase in emigration of antigen loaded LC from the skin. These data are supported by increased LC numbers observed in infected skin after immunohistochemical analysis in patients infected with measles, rubella, herpes zoster, varicella, and herpes simplex viruses (44).
In the present study, we observed a net decrease in LC in HPV16-infected epidermis, resulting directly from the virus infection. A virus-mediated reduction in LC numbers in the skin has been similarly observed in human immunodeficiency virus (HIV)-infected individuals. However, HIV can infect LC and therefore may directly influence their numbers and function in the epidermis (13, 53). There is no evidence to support the replication competence of LC for HPV infection as gene expression in HPV is closely linked to cell differentiation in the epidermis and virus particles can only be produced in epidermal cells that have undergone this process (4).
In HPV16 infection, the virus has the ability to create a unique microenvironment at the infection site by influencing cellular functions of the infected KC. The virus may disrupt LC homeostasis by altering the expression of cellular adhesion molecules, such as E-cadherin, or the secretion of cytokines and chemokines that are essential for LC retention and migration. E-cadherin is reported to play an important role in the retention of LC in the epidermis through a homophilic interaction between E-cadherin on LC and KC (21, 40). We found that E-cadherin expression on KC directly correlated with LC number in both normal and infected epidermis, supporting a role for the homotypic E-cadherin interactions between KC and LC in LC retention in the epidermis. Furthermore, the expression of E-cadherin was reduced or lost on the surface of HPV16-infected KC in the basal layer of virus-infected epidermis, where LC normally reside. The reduction in LC numbers may therefore be a consequence of the loss of ability of LC to maintain E-cadherin adherens junctions with KC, an event that normally precedes the emigration of LC from the epidermis. This result suggested to us that one or more viral proteins could influence LC retention in the skin by altering the expression of E-cadherin on the surface of infected KC.
We assessed which of the viral proteins were involved in down regulation of E-cadherin on KC. HPV proteins are differentially expressed with the late proteins, L1 and L2, only being expressed in the superficial epidermis. We therefore disregarded these proteins as having a role in E-cadherin down regulation as reduced expression occurred in the lower, basal layers of the epidermis where early viral protein expression predominates. The only HPV16 early protein that consistently and significantly reduced the level of cell surface E-cadherin on HaCaT cells and reduced the total number of E-cadherin-positive cells after transfection was E6. We therefore propose a new function for E6 in the control of cell surface E-cadherin expression and in the regulation of the cutaneous immune response in virus-infected skin.
We do not discount the possibility that one or more further signals are required to direct LC away from HPV16-infected skin. One such signal may result from HPV16 regulation of cytokine expression by infected KC. There is increasing evidence supporting a role for HPV in the regulation of a number of cytokines, some of which are implicated in the maturation and migration of LC. These include TGF-β1, which is constitutively expressed by epidermal KC and is required for the infiltration and differentiation of LC precursors in the epidermis. TGF-β1−/− SCID mice are deficient of epidermal LC but LC infiltration can be induced at localized sites in these mice after injection with TGF-β1-expressing cells (42). HPV16+ cervical precancerous lesions display reduced expression of TGF-β1, and this is may impact on the infiltration and maturation state of LC at the site of the infection (16). These data suggest that in addition to the loss of E-cadherin in HPV16-infected tissue and its effect on LC retention, signals for LC immigration and maturation may also be impaired. Multiple factors are likely to contribute to the reduction of LC in the epidermis, thereby aiding viral evasion of host immunity.
The HPV E6 protein has been shown to interact with several cellular proteins but is best known for its ability to bind p53 and mediate its degradation by the ubiquitin system (50). There is conflicting evidence regarding the role of p53 in the regulation of E-cadherin. Mutations of p53 have been reported to be associated with E-cadherin down regulation in breast carcinoma (8) but an association between p53 and E-cadherin expression was discounted in soft tissue sarcoma and respiratory papillomatosis (18, 52). Furthermore, p53 transcriptionally regulates p21WAF1 expression, which was found to be required for E-cadherin induction in a p53 wild-type colon carcinoma cell line (32). We showed that E6 expression reduced cell surface E-cadherin in HaCaT cells, which are insensitive to E6-targeted degradation of p53 (27). Although we cannot discount a role for E6-mediated degradation of p53 in the down regulation of E-cadherin in wild-type p53 cells, the data presented here demonstrate that E6 was able to downregulate E-cadherin expression in the absence of p53 degradation in mutant p53 HaCaT cells.
A homotypic cell adhesion assay was carried out to evaluate whether the E6-mediated loss of E-cadherin was associated with a defect in cell to cell adhesion. We showed that expression of E6 in HaCaT cells reduced surface E-cadherin, thereby functionally impairing cell aggregation. Others have reported that a reduction, rather than a total loss of cell surface E-cadherin expression is sufficient to allow LC migration from the skin (35). This is further confirmed by the continued expression of E-cadherin on LC that have recently migrated from the skin to the draining lymph nodes (23). The reduction or loss of E-cadherin expression that we observed in HPV16-infected skin directly correlated with a reduction in LC density, further supporting the role of E6 down regulation of E-cadherin in LC depletion in the epidermis.
The implications of reduced E-cadherin expression on HPV16-infected KC on immune function was not directly assessed here. However, there is increasing evidence to support a role for pathogens in the disruption of LC function and consequently the host immune response. Pathogen-mediated effects on LC migration have recently been reported in schistosomiasis infection, where LC are retained in the epidermis as a result of the secretion of prostaglandin D2 by the infecting Schistosoma (2). The authors of that study speculated that the organism may influence immune reactions in the skin via this mechanism. An association between LC depletion and increased severity of virus infection has been shown with other viruses. The virus-mediated reduction in LC in HPV16-infected skin reported here may account for the persistence of this virus in individuals who are otherwise immunocompetent.
The down regulation of E-cadherin on the surface of tumor cells is an important step in metastasis (5). The E-cadherin-catenin complex plays a dominant role in the suppression of invasion and there is increasing evidence to support a causal role of E-cadherin-catenin complex disruption in tumor invasion (45). The E6 and E7 genes of HPV encode the major transforming activity of the virus, and both of these genes are retained in cervical carcinomas (47) and reduced E-cadherin expression is associated with cervical intraepithelial neoplasia (46). The reduction in E-cadherin in cervical neoplasia may be a consequenc of E6 expression, since HPV has been demonstrated in 99.7% of tumor biopsy specimens from cervical cancer patients (48). The E6-mediated down regulation of cell surface E-cadherin described in this report therefore may also have a role in HPV16-derived cervical carcinomas, not only in metastasis but also in immune evasion by the cancer.
Our findings have important consequences in the improvement of therapeutic treatments for the control of HPV. In contrast to cervical HPV16 infections, LC infiltration is frequently observed in HPV-associated lung adenocarcinomas and squamous cell carcinomas, contributing to a favorable prognosis for these cancers (29). We propose that in HPV16 infection the E6 protein interferes with E-cadherin expression on the surface of KC, reducing the retention of LC at the site of infection and thereby lessening the opportunity for these cells to acquire and process viral antigen. KC infected with HPV16 therefore might not be readily eliminated by the host immune system. Similarly, HPV16-transformed cells, which also express E6, may evade the host immune system by this same mechanism. Therapeutic agents that increase the density of LC in the epidermis may lead to a more favorable prognosis for patients with HPV16-associated disease.
Acknowledgments
K.M. and C.M.L. contributed equally to this study.
This work was supported by the Cancer Society of New Zealand and the Health Research Council of New Zealand. B.T.B. is a Wellcome Trust Senior Research Fellow.
We thank K. Raj, N. Keen, and F. Carlotti for pBabe E1, pGEX16 E6, and pGEX16 E7, respectively, and D. Wynford-Thomas for the K1neo cells. We thank Kirsty Nicolson and Nicky Real for technical assistance.
REFERENCES
- 1.Aiba, S., and S. I. Katz. 1990. Phenotypic and functional characteristics of in vivo-activated Langerhans cells. J. Immunol. 145:2791-2796. [PubMed] [Google Scholar]
- 2.Angeli, V., C. Faveeuw, O. Roye, J. Fontaine, E. Teissier, A. Capron, I. Wolowczuk, M. Capron, and F. Trottein. 2001. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J. Exp. Med. 193:1135-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, Y., and E. Sprecher. 1989. Langerhans cells in vaccinia virus infection in mouse skin. Arch. Virol. 107:307-313. [DOI] [PubMed] [Google Scholar]
- 4.Bedell, M. A., J. B. Hudson, T. R. Golub, M. E. Turyk, M. Hosken, G. D. Wilbanks, and L. A. Laimins. 1991. Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. J. Virol. 65:2254-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birchmeier, W., and J. Behrens. 1994. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim. Biophys. Acta 1198:11-26. [DOI] [PubMed] [Google Scholar]
- 6.Borkowski, T. A., B. J. Van Dyke, K. Schwarzenberger, V. W. McFarland, A. G. Farr, and M. C. Udey. 1994. Expression of E-cadherin by murine dendritic cells: E-cadherin as a dendritic cell differentiation antigen characteristic of epidermal Langerhans cells and related cells. Eur. J. Immunol. 24:2767-2774. [DOI] [PubMed] [Google Scholar]
- 7.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukholm, I. K., J. M. Nesland, R. Karesen, U. Jacobsen, and A. L. Borresen-Dale. 1997. Expression of E-cadherin and its relation to the p53 protein status in human breast carcinomas. Virchows Arch. 431:317-321. [DOI] [PubMed] [Google Scholar]
- 9.Charbonnier, A. S., N. Kohrgruber, E. Kriehuber, G. Stingl, A. Rot, and D. Maurer. 1999. Macrophage inflammatory protein 3α is involved in the constitutive trafficking of epidermal Langerhans cells. J. Exp. Med. 190:1755-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciccolini, F., G. Di Pasquale, F. Carlotti, L. Crawford, and M. Tommasino. 1994. Functional studies of E7 proteins from different HPV types. Oncogene 9:2633-2638. [PubMed] [Google Scholar]
- 11.Cumberbatch, M., R. J. Dearman, and I. Kimber. 1996. Adhesion molecule expression by epidermal Langerhans cells and lymph node dendritic cells: a comparison. Arch. Dermatol. Res. 288:739-744. [DOI] [PubMed] [Google Scholar]
- 12.Cumberbatch, M., R. J. Dearman, and I. Kimber. 1997. Langerhans cells require signals from both tumour necrosis factor-alpha and interleukin-1 beta for migration. Immunology 92:388-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittmar, M. T., G. Simmons, S. Hibbitts, M. O'Hare, S. Louisirirotchanakul, S. Beddows, J. Weber, P. R. Clapham, and R. A. Weiss. 1997. Langerhans cell tropism of human immunodeficiency virus type 1 subtype A through F isolates derived from different transmission groups. J. Virol. 71:8008-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doorbar, J., C. Foo, N. Coleman, L. Medcalf, O. Hartley, T. Prospero, S. Napthine, J. Sterling, G. Winter, and H. Griffin. 1997. Characterization of events during the late stages of HPV16 infection in vivo using high-affinity synthetic Fabs to E4. Virology 238:40-52. [DOI] [PubMed] [Google Scholar]
- 15.Durst, M., L. Gissmann, H. Ikenberg, and H. zur Hausen. 1983. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc. Natl. Acad. Sci. USA 80:3812-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Sherif, A. M., R. Seth, P. J. Tighe, and D. Jenkins. 2000. Decreased synthesis and expression of TGF-β1, β2, and β3 in epithelium of HPV 16-positive cervical precancer: a study by microdissection, quantitative RT-PCR, and immunocytochemistry. J. Pathol. 192:494-501. [DOI] [PubMed] [Google Scholar]
- 17.Ghaznawie, M., J. M. Papadimitriou, and P. J. Heenan. 1999. The steady-state turnover of murine epidermal Langerhans cells. Br. J. Dermatol. 141:57-61. [DOI] [PubMed] [Google Scholar]
- 18.Gupta, D., J. Holden, and L. Layfield. 2001. Topoisomerase alpha II, retinoblastoma gene product, and p53: potential relationships with aggressive behavior and malignant transformation in recurrent respiratory papillomatosis. Appl. Immunohistochem. Mol. Morphol. 9:86-91. [PubMed] [Google Scholar]
- 19.Hernando, R. A., J. C. Ruby, and G. M. Halliday. 1994. Changes in epidermal Langerhans cells, gamma delta T cells and CD4 T cells after intradermal infection with recombinant vaccinia virus expressing cytokine genes. Immunol. Cell Biol. 72:383-389. [DOI] [PubMed] [Google Scholar]
- 20.Hibma, M. H., K. Raj, S. J. Ely, M. Stanley, and L. Crawford. 1995. The interaction between human papillomavirus type 16 E1 and E2 proteins is blocked by an antibody to the N-terminal region of E2. Eur. J. Biochem. 229:517-525. [DOI] [PubMed] [Google Scholar]
- 21.Jakob, T., M. J. Brown, and M. C. Udey. 1999. Characterization of E-cadherin-containing junctions involving skin-derived dendritic cells. J. Investig. Dermatol. 112:102-108. [DOI] [PubMed] [Google Scholar]
- 22.Jakob, T., and M. C. Udey. 1998. Regulation of E-cadherin-mediated adhesion in Langerhans cell-like dendritic cells by inflammatory mediators that mobilize Langerhans cells in vivo. J. Immunol. 160:4067-4073. [PubMed] [Google Scholar]
- 23.Johnston, L. J., G. M. Halliday, and N. J. King. 2000. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J. Investig. Dermatol. 114:560-568. [DOI] [PubMed] [Google Scholar]
- 24.Keen, N., R. Elston, and L. Crawford. 1994. Interaction of the E6 protein of human papillomavirus with cellular proteins. Oncogene 9:1493-1499. [PubMed] [Google Scholar]
- 25.Larregina, A. T., A. E. Morelli, L. A. Spencer, A. J. Logar, S. C. Watkins, A. W. Thomson, and L. D. Falo, Jr. 2001. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat. Immunol. 2:1151-1158. [DOI] [PubMed] [Google Scholar]
- 26.Larsen, C. P., S. C. Ritchie, T. C. Pearson, P. S. Linsley, and R. P. Lowry. 1992. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J. Exp. Med. 176:1215-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magal, S. S., A. Jackman, X. F. Pei, R. Schlegel, and L. Sherman. 1998. Induction of apoptosis in human keratinocytes containing mutated p53 alleles and its inhibition by both the E6 and E7 oncoproteins. Int. J. Cancer 75:96-104. [DOI] [PubMed] [Google Scholar]
- 28.Mahajan, N. P., K. Linder, G. Berry, G. W. Gordon, R. Heim, and B. Herman. 1998. Bcl-2 and Bax interactions in mitochondria probed with green fluorescent protein and fluorescence resonance energy transfer. Nat. Biotechnol. 16:547-552. [DOI] [PubMed] [Google Scholar]
- 29.Miyagi, J., T. Kinjo, K. Tsuhako, M. Higa, T. Iwamasa, Y. Kamada, and T. Hirayasu. 2001. Extremely high Langerhans cell infiltration contributes to the favorable prognosis of HPV-infected squamous cell carcinoma and adenocarcinoma of the lung. Histopathology 38:355-367. [DOI] [PubMed] [Google Scholar]
- 30.Morelli, A. E., C. Sananes, G. Di Paola, A. Paredes, and L. Fainboim. 1993. Relationship between types of human papillomavirus and Langerhans' cells in cervical condyloma and intraepithelial neoplasia. Am. J. Clin. Pathol. 99:200-206. [DOI] [PubMed] [Google Scholar]
- 31.Morris, H. H., K. C. Gatter, G. Sykes, V. Casemore, and D. Y. Mason. 1983. Langerhans' cells in human cervical epithelium: effects of wart virus infection and intraepithelial neoplasia. Br. J. Obstet. Gynaecol. 90:412-420. [DOI] [PubMed] [Google Scholar]
- 32.Mueller, S., E. Cadenas, and A. H. Schonthal. 2000. p21WAF1 regulates anchorage-independent growth of HCT116 colon carcinoma cells via E-cadherin expression. Cancer Res. 60:156-163. [PubMed] [Google Scholar]
- 33.Park, T. W., H. Fujiwara, and T. C. Wright. 1995. Molecular biology of cervical cancer and its precursors. Cancer 76:1902-1913. [DOI] [PubMed] [Google Scholar]
- 34.Romani, N., S. Koide, M. Crowley, M. Witmer-Pack, A. M. Livingstone, C. G. Fathman, K. Inaba, and R. M. Steinman. 1989. Presentation of exogenous protein antigens by dendritic cells to T-cell clones: intact protein is presented best by immature, epidermal Langerhans cells. J. Exp. Med. 169:1169-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarzenberger, K., and M. C. Udey. 1996. Contact allergens and epidermal proinflammatory cytokines modulate Langerhans cell E-cadherin expression in situ. J. Investig. Dermatol. 106:553-558. [DOI] [PubMed] [Google Scholar]
- 36.Sprecher, E., and Y. Becker. 1987. Herpes simplex virus type 1 pathogenicity in footpad and ear skin of mice depends on Langerhans cell density, mouse genetics, and virus strain. J. Virol. 61:2515-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sprecher, E., and Y. Becker. 1989. Langerhans cell density and activity in mouse skin and lymph nodes affect herpes simplex type 1 (HSV-1) pathogenicity. Arch. Virol. 107:191-205. [DOI] [PubMed] [Google Scholar]
- 38.Stanley, M. A., H. M. Browne, M. Appleby, and A. C. Minson. 1989. Properties of a non-tumorigenic human cervical keratinocyte cell line. Int. J. Cancer 43:672-676. [DOI] [PubMed] [Google Scholar]
- 39.Stingl, G., S. I. Katz, L. Clement, I. Green, and E. M. Shevach. 1978. Immunologic functions of Ia-bearing epidermal Langerhans cells. J. Immunol. 121:2005-2013. [PubMed] [Google Scholar]
- 40.Tang, A., M. Amagai, L. G. Granger, J. R. Stanley, and M. C. Udey. 1993. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature 361:82-85. [DOI] [PubMed] [Google Scholar]
- 41.Tay, S. K., D. Jenkins, P. Maddox, M. Campion, and A. Singer. 1987. Subpopulations of Langerhans' cells in cervical neoplasia. Br. J. Obstet. Gynaecol. 94:10-15. [DOI] [PubMed] [Google Scholar]
- 42.Thomas, R. M., D. V. Belsito, C. Huang, L. Z. Chen Lz, I. Ormsby, W. J. Simmons, P. Cowin, J. Shaw, T. Doetschman, and G. J. Thorbecke. 2001. Appearance of Langerhans cells in the epidermis of Tgfb1(−/−) SCID mice: paracrine and autocrine effects of transforming growth factor-β1 and -β2(1). J. Investig. Dermatol. 117:1574-1580. [DOI] [PubMed] [Google Scholar]
- 43.Tindle, R. W. 2002. Immune evasion in human papillomavirus-associated cervical cancer. Nat. Rev. Cancer 2:59-65. [DOI] [PubMed] [Google Scholar]
- 44.Tsukahara, T., and Y. Horiuchi. 1996. Immunohistochemical study of cellular events in lesional skin during common virus infections. J. Dermatol. 23:22-32. [PubMed] [Google Scholar]
- 45.Vermeulen, S., V. Van Marck, L. Van Hoorde, F. Van Roy, M. Bracke, and M. Mareel. 1996. Regulation of the invasion suppressor function of the cadherin/catenin complex. Pathol. Res. Pract. 192:694-707. [DOI] [PubMed] [Google Scholar]
- 46.Vessey, C. J., J. Wilding, N. Folarin, S. Hirano, M. Takeichi, P. Soutter, G. W. Stamp, and M. Pignatelli. 1995. Altered expression and function of E-cadherin in cervical intraepithelial neoplasia and invasive squamous cell carcinoma. J. Pathol. 176:151-159. [DOI] [PubMed] [Google Scholar]
- 47.Vousden, K. H. 1994. Interactions between papillomavirus proteins and tumor suppressor gene products. Adv. Cancer Res. 64:1-24. [DOI] [PubMed] [Google Scholar]
- 48.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12-19. [DOI] [PubMed] [Google Scholar]
- 49.Wang, B., S. Kondo, G. M. Shivji, H. Fujisawa, T. W. Mak, and D. N. Sauder. 1996. Tumour necrosis factor receptor II (p75) signalling is required for the migration of Langerhans' cells. Immunology 88:284-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werness, B. A., A. J. Levine, and P. M. Howley. 1990. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248:76-79. [DOI] [PubMed] [Google Scholar]
- 51.Wyllie, F. S., M. F. Haughton, J. M. Rowson, and D. Wynford-Thomas. 1999. Human thyroid cancer cells as a source of iso-genic, iso-phenotypic cell lines with or without functional p53. Br. J. Cancer 79:1111-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoo, J., S. Park, C. S. Kang, S. J. Kang, and B. K. Kim. 2002. Expression of E-cadherin and p53 proteins in human soft tissue sarcomas. Arch. Pathol. Lab. Med. 126:33-38. [DOI] [PubMed] [Google Scholar]
- 53.Zimmer, M. I., A. T. Larregina, C. M. Castillo, S. Capuano III, L. D. Falo, Jr., M. Murphey-Corb, T. A. Reinhart, and S. M. Barratt-Boyes. 2002. Disrupted homeostasis of Langerhans cells and interdigitating dendritic cells in monkeys with AIDS. Blood 99:2859-2868. [DOI] [PubMed] [Google Scholar]
- 54.zur Hausen, H. 1991. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology 184:9-13. [DOI] [PubMed] [Google Scholar]