Abstract
Based on in vitro observations in scrapie-infected neuroblastoma cells, quinacrine has recently been proposed as a treatment for Creutzfeldt-Jakob disease (CJD), including a new variant CJD which is linked to contamination of food by the bovine spongiform encephalopathy (BSE) agent. The present study investigated possible mechanisms of action of quinacrine on prions. The ability of quinacrine to interact with and to reduce the protease resistance of PrP peptide aggregates and PrPres of human and animal origin were analyzed, together with its ability to inhibit the in vitro conversion of the normal prion protein (PrPc) to the abnormal form (PrPres). Furthermore, the efficiencies of quinacrine and chlorpromazine, another tricyclic compound, were examined in different in vitro models and in an experimental murine model of BSE. Quinacrine efficiently hampered de novo generation of fibrillogenic prion protein and PrPres accumulation in ScN2a cells. However, it was unable to affect the protease resistance of preexisting PrP fibrils and PrPres from brain homogenates, and a “curing” effect was obtained in ScGT1 cells only after lengthy treatment. In vivo, no detectable effect was observed in the animal model used, consistent with other recent studies and preliminary observations in humans. Despite its ability to cross the blood-brain barrier, the use of quinacrine for the treatment of CJD is questionable, at least as a monotherapy. The multistep experimental approach employed here could be used to test new therapeutic regimes before their use in human trials.
Transmissible spongiform encephalopathies are a group of neurodegenerative disorders including sporadic, genetic, and acquired forms of Creutzfeldt-Jakob disease (CJD) in humans, scrapie in sheep, and spongiform encephalopathy in cattle (bovine spongiform encephalopathy [BSE]). These diseases are characterized by the accumulation of a pathological form of the cellular prion protein (PrPc), called scrapie prion protein (PrPres), in the central nervous system and, in many instances, in the lymphoreticular system. PrPres shows several differences from PrPc: a high percentage of β-sheet secondary structure, resistance to proteolysis, insolubility in detergents, and a propensity to polymerize into amyloid-like fibrils (4, 27, 28). The disease-related form of PrPc, PrPres, is the only specific molecular marker of the infection, and the inhibition of its accumulation is often used to evaluate the efficacy of therapeutic drugs.
To date, several compounds have been described which decrease the PrPres concentration in different scrapie-infected cell lines or prolong the incubation period in animal models. These drugs belong to different classes, including sulfated polyanions (13, 17, 19; C. Farquhar, A. Dickinson, and M. Bruce, Letter, Lancet 353:117, 1999; C. Farquhar, I. McConnell, J. Graham, S. Cumming, R. Prescott, A. Boyle, G. R. Barclay, D. S. Pepper, M. L. Turner, and M. E. Bruce, presented at the International Conference on Transmissible Spongiform Encephalopathies, Edinburgh, United Kingdom, 2002), amphotericin B derivatives (1, 25), Congo red (5, 9), tetracyclic compounds (15, 35), tetrapyrroles (7, 26), branched polyamines (32, 33), and β-sheet breakers derived from PrP peptides (31). Nevertheless, none of them is effective when given around the time of the clinical phase, thereby restricting an evidence-based rationale for their use in the treatment of human disease. Recently, quinacrine, chlorpromazine, and some tricyclic derivatives with an aliphatic side chain were described as efficient inhibitors of PrPres formation in murine neuroblastoma cells chronically infected with the Chandler scrapie isolate (12, 18). Since these two compounds have been used in human medicine for many years (quinacrine as an antimalarial and chlorpromazine as an antipsychotic drug) and are able to cross the blood-brain barrier, they appeared to be interesting candidates for prion disease therapy. Consequently, quinacrine has been proposed for compassionate treatment of CJD, in particular for the new variant CJD causally linked to BSE, even though no data were available in experimental models to support its efficacy in the treatment of transmissible spongiform encephalopathies.
In this study, we used several complementary methods to extend our knowledge of the effect of quinacrine on prion replication in order to document the relevance of this therapeutic approach. Using biochemical and biophysical techniques, we examined the ability of the compound to interact with and to affect the protease resistance of synthetic PrP peptide aggregates and of PrPres from different brain homogenates. In addition, we investigated the ability of quinacrine to hinder the in vitro conversion of PrPc to PrPres. We also analyzed the effects of quinacrine and chlorpomazine on PrPres accumulation in three different models of chronically infected cells. Finally, we investigated their therapeutical effects in BSE-infected mice. In agreement with previous reports, the results obtained showed that quinacrine was effective against de novo generation of fibrillogenic prion protein and against PrPres accumulation in ScN2a cells. However, the compound was less efficient with ScGT1 cells, another chronically scrapie-infected murine neuronal cell line, which could be cured only after lengthy treatment. It was also less efficient in disrupting preexisting aggregated PrP fibrils and PrPres from different brain homogenates. In vivo, not only did quinacrine and chlorpromazine not efficiently inhibit the accumulation of PrPres, but a paradoxical increase in PrPres was observed. Moreover, preliminary results in treating mice that developed clinical signs have not shown evidence of clinical efficiency. We therefore propose that quinacrine can interact with PrP to inhibit PrPres formation but that it is unable to significantly disrupt preformed aggregates and therefore has a limited role in therapeutic interventions during the late stages of prion diseases.
MATERIALS AND METHODS
Chemicals.
Quinacrine, chlorpromazine, tetracycline hydrochloride, Congo red, and thioflavin T were purchased from Sigma Aldrich, and dextran sulfate 500 was purchased from Pharmacia. Amphotericin B derivatives, such as MS-8209 and MS-1191, were generous gifts from Mayoly-Spindler Laboratories. The drugs were diluted in a sterile 5% (wt/vol) glucose solution.
Peptide synthesis and purification.
The peptides PrP106-126 (KTNMKHMAGAAAAGAVVGGLG) and PrP82-146 (GQPHGGGWGQGGGTHSQWNKPSKPKTNMKAGAAAAGAAVVGGLGGYMLGSAMSRPIIHFGSDYE), based on the human PrP amino acid sequence, were synthesized by solid-phase chemistry on an Applied Biosystems 430A synthesizer as described previously (14, 36). The peptides were cleaved from the resin with phenol-thioanisole-trifluoroacetic acid, precipitated, washed several times with cold diethylether, and purified by preparative reverse-phase high-performance liquid chromatography (HPLC) (model 243; Beckman Instruments). The purities and identities of peptides were determined by analytical reverse-phase HPLC, capillary electrophoresis (Quanta 4000; Millipore), amino acid sequencing (6600 Prosequencer; Milligen), and mass spectrometry using a single quadrupole mass spectrometer equipped with an electrospray interface (Hewlett-Packard). The purities of the peptides were >95%.
Fluorescence microscopy.
To generate amyloid fibrils, the peptides PrP106-126 and PrP82-146 were dissolved in deionized water at a concentration of 2 mM, and the same volume of 200 mM Tris-HCl buffer, pH 7.4, was added. The samples were incubated for 1 week at 37°C. An aqueous solution of quinacrine, tetracycline hydrochloride, or the amyloid-binding fluorochrome thioflavin T was added to suspensions of peptide aggregates to a final concentration of 1 mM. After 2 h of incubation at room temperature, the samples were centrifuged at 16,000 × g for 5 min, and the pellet was washed several times with Tris-HCl buffer to remove any free dye in solution and then applied to gelatin-coated slides. Appropriate controls (solutions of gentamicin as a negative control, thioflavin T plus scrambled peptide, peptides in the absence of thioflavin T, and a solution of thioflavin T only) were used to avoid artifacts. The samples were then examined by fluorescence microscopy using selective filters (Zeiss).
Proteinase K digestion of synthetic peptides.
The synthetic peptides PrP106-126 and PrP82-146 were dissolved at a concentration of 1 mM in water-acetonitrile (1:1), and 30-μl aliquots were lyophilized. The samples were dissolved in 15 μl of deionized water alone or with either quinacrine or tetracycline, and the same volume of 200 mM Tris-HCl buffer, pH 7.4, containing 1 mM CaCl2 was added. The final PrP106-126- to- compound ratios were 1:1, 1:4, and 1:8, and the ratio of PrP82-146 to compound was equimolar. The samples of PrP106-126 and PrP82-146 were incubated at 37°C for 48 h and then digested with proteinase K (37°C for 15 min) at 20 (PrP106-126) or 16 (PrP82-146) μg/ml (the different concentrations of proteinase K used reflected the relative sensitivities of the two peptides to proteolysis). Proteolysis was blocked by the addition of EGTA (5 mM final concentration). After centrifugation at 16,000 × g for 5 min, the supernatant was removed, the pellet was dissolved in 30 μl of 10% formic acid containing 0.1% trifluoroacetic acid, and 20 μl was analyzed by reverse-phase HPLC. Parallel samples were run in the absence of quinacrine or tetracycline and analyzed as specified above with or without proteinase K digestion. The extent of proteolysis was calculated as the percentage of peptide present in the pellet compared to the total amount originally present.
Effects of quinacrine on protease resistance of PrPres.
PrPres was partially purified from brain tissue of patients with sporadic and variant CJD, 263K-infected hamsters, and 139A-infected mice following a procedure described previously (6). Sample aliquots containing ∼2 ng of PrPres were incubated at 37°C for 48 h either in the absence or in the presence of quinacrine or tetracycline (0.02, 0.2, and 2 mM concentrations) and then treated with proteinase K (50 μg/ml; 37°C; 1 h). The amount of PrP remaining after proteolysis was assessed by Western blot analysis using the monoclonal antibody (MAb) 3F4 (1:50,000) for CJD- and 263K-infected hamsters and the rabbit antiserum PrP95-108 (1:15,000) for 139A-infected mice (24).
Effects of quinacrine on cyclic amplification of PrPres.
Brains from healthy hamsters and 263K scrapie-infected hamsters at the terminal stage of disease were homogenized in 9 volumes of conversion buffer (phosphate-buffered saline [PBS], pH 7.4, containing 0.5% Triton X-100, 0.05% sodium dodecyl sulfate [SDS], and a complete cocktail of protease inhibitors from Boehringer Mannhein). The homogenates were clarified by centrifugation at 300 × g for 30 s and diluted 1:1 in conversion buffer. The scrapie brain homogenate was then diluted 1:300 in healthy brain homogenate; 20 μl of serial dilutions of quinacrine, melatonin, or tetracycline in TBSB (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% SB3-14) was then added to 40-μl sample aliquots to obtain final drug concentrations of 2, 5, and 10 mM. The samples were incubated at 37°C with agitation for 5 h and subjected to a cycle of sonication every hour as described previously (30). The samples were then digested with proteinase K (100 μg/ml; 37°C; 1 h) and analyzed by Western blotting using the antibody 3F4 (1:50,000).
Cell cultures.
Three cell lines were used in the present studies.
ScGT1 is an immortalized cell line from murine hypothalamus neurons kindly provided by S. Lehmann (Montpellier, France). These cells have been infected with the scrapie Chandler isolate and persistently express PrPres. The cells were grown at 37°C in Opti-Modified Eagle's medium supplemented with 5% fetal calf serum (FCS), 5% fetal horse serum, 1% penicillin-streptomycin, and 1% sodium pyruvate.
N2a58/22L cells are derived from the mouse neuroblastoma cell line N2a transfected with wild-type mouse Prnp cDNA (clone 58) infected with the mouse-adapted scrapie strain 22L (kindly provided by S. Lehmann) (23). The cells were grown at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated FCS, penicillin-streptomycin, 2 mM l-glutamine, and 250 μg of geneticin (G418)/ml.
In addition, ScN2a cells, similar to those used in previous PrPres-quinacrine studies (18), were grown in Ham's F-12 medium supplemented with penicillin, streptomycin, glutamine, and 2% FCS. The cells were plated at 2 × 104 per well in microtiter plates. In some studies, microglia obtained from newborn mice were added to quinacrine-treated and untreated ScN2a cells as previously described (2). Uninfected N2a cells acted as controls.
PrPres assay inhibition in the three cultured cellular models. (i) ScGT1 cells.
Three different treatment protocols were tested. First, 8 × 105 ScGT1 cells were grown in a 75-cm2 flask over 2 days. On the third day, when the cells were sufficiently adherent, different concentrations of drugs were added to the medium. The cells were incubated for 3 days in the presence of the drug. In the second protocol, 8 × 105 ScGT1 cells grown in a 75-cm2 flask were treated with the drugs for 6 days, with the medium and the drug changed every second day. In the last protocol, 3 × 105 ScGT1 cells grown in a 75-cm2 flask were treated with quinacrine daily for 3 weeks. PrPres accumulation was evaluated at the end of each type of treatment. The cytotoxic effects of quinacrine on ScGT1 cells were monitored using an MTT assay (2).
(ii) N2a58/22L cells.
N2a58/22L cells were grown in 12-well plates (105 cells/well) for 48 h. On the third day, quinacrine (0.2 to 0.4 μM) and Congo red (7.5 μM) were freshly dissolved in vehicle (PBS) and added to the culture every 24 h for 72 h. The cytotoxic effects of quinacrine on N2a58/22L cells were determined by microscopic examination and by an MTT assay (2).
(iii) ScN2a cells.
Quinacrine was added to ScN2a cells for 6 days, with the medium and the drug changed every second day, after which the amount of PrPres was determined. An MTT assay (2) was used to monitor the cytotoxic effects of quinacrine during this protocol. In further studies, the effects of quinacrine treatment on the ability of microglia to recognize and kill scrapie-infected cells was examined by adding 5 × 103 cell microglia per well containing ScN2a cells (i.e., to give a neurone/microglia ratio of ∼10:1) that were either untreated or had been preincubated with 0.1 mg of quinacrine/ml for 24 h. Cell survival was then assessed 24 h after the addition of the microglia using a standard MTT assay (2).
Analysis of PrPres in treated cells. (i) ScGT1 cells.
At the end of the treatment, the ScGT1 cells were washed in PBS and lysed in 1 ml of lysis buffer (50 mM Tris-HCl, pH 7.4, 0.5% Triton X-100, and 0.5% sodium deoxycholate) for 10 min at 4°C. The samples were submitted to a 10-min cycle of sonication at 4°C. After removal of insoluble debris by centrifugation at 20,000 × g for 1 min, the total protein concentration was measured by the bicinchoninic acid protein assay (Pierce). The samples were diluted in lysis buffer to normalize the protein concentration among them and then digested with proteinase K (20 μg/mg of protein) for 30 min at 37°C. Digestion was blocked by 4 mM Pefabloc, and the samples were centrifuged at 20,000 × g for 90 min at 4°C. The supernatant was removed, and the pellet was dissolved in 40 μl of Laemmli buffer (Bio-Rad). The samples were boiled for 5 min at 100°C. Fifteen microliters of each sample was subjected to 12% polyacrylamide gel electrophoresis. The proteins were transferred onto a nitrocellulose membrane in a buffer containing 0.15% Tris- 0.6% glycine-10% isopropanol overnight at 22 V and 160 mA. The membranes were blocked with 5% nonfat dry milk in PBS- 0.1% Tween 20 for 1 h at room temperature. PrPres was detected by incubation with the antibody SAF83 (a generous gift of J. Grassi, CEA, Saclay, France) recognizing the 126-164 PrP epitope for 1 h at room temperature. After three washes of 5 min each, the first antibody was revealed with a secondary antibody conjugated with peroxidase for 30 min at room temperature. The immunodetection was carried out by enhanced chemiluminescence (Amersham). The effect of the drug was determined by quantitating the PrPres signals of the treated cells and by comparison with a dilution scale of ScGT1 cells using Quantity One software (Bio-Rad).
(ii) N2a58/22L cells.
After 72 h of incubation, the N2a58/22L cells were washed twice with PBS and lysed in 0.5 ml of lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% Na-deoxycholate, 0.5% Triton X-100, 2 mM EDTA) containing protease inhibitors (leupeptin and pepstatin [1 μg/ml]) for 30 min at 4°C. After centrifugation (2 min at 10,000 × g), the supernatant was collected and the total protein concentration was measured. Protein (176 μg) was digested with 2.5 μg of proteinase K for 60 min at 37°C. The digestion was stopped by phenylmethylsulfonyl fluoride (10 mg/ml) at 4°C, and the proteins were then methanol precipitated. The samples were centrifuged (20 min at 10,000 × g), and the pellet was resuspended in SDS loading buffer and subjected to SDS- 12% polyacrylamide gel electrophoresis just after being boiled (2 h at 120 V in 25 mM Tris- 192 mM glycine- 0.1% SDS). The proteins were transferred onto an Immobilon-P membrane (1 h at 100 V in 10 mM CAPS-10% methanol). The membrane was blocked with 5% nonfat dry milk in TBST (10 mM Tris-HCl, pH 7.5, 100 mM NaCl, 0.1% Tween 20), and mouse PrPSc was detected by immunoblotting with the antibody SAF75 (a generous gift from J. Grassi) in conjunction with a peroxidase-conjugated goat anti-mouse secondary antibody. The blot was developed by the use of enhanced chemiluminescence.
(iii) ScN2a cells.
ScN2a cells were incubated with 0.2 μM quinacrine for 6 days, after which the cells were washed three times in PBS, lysed, treated with extraction buffer, and then treated with proteinase K (protein from 2 × 107 cells per ml digested with 1 μg of proteinase K per ml for 1 h at 37°C; this is 10 times the concentration required to digest PrPc in this system). The reaction was stopped with phenylmethylsulfonyl fluoride, and the amount of PrPres present was determined using an enzyme immunoassay with a polyclonal antibody as the capture antibody (R528.7; a gift from J. Langeveld, Pepscan System BV, Lelystad, The Netherlands) and the MAb SAF83 (from J. Grassi) and an alkaline phosphatase kit as the detection system. PrPres immunodetection was carried out by optic densitometry measured at 414 nm. Recombinant murine PrP (Prionics) was used to calibrate the amounts of PrPres present in the treated and untreated ScN2a cells.
Infection of animals.
C57BL6 mice (age, 8 weeks; weight, 20 g) were obtained from Harlan (Gannat, France). The animals received water and food ad libitum. The mice were injected intraperitoneally with the 6PB1 mouse-adapted BSE strain. One hundred microliters of a 2% (wt/vol) brain homogenate (titrated as 107.2 50% lethal doses/ml) were injected intraperitoneally. Ten mice were inoculated with 100 μl of negative mouse brain homogenate as a control group.
Treatment of animals.
The protocol applied here allows a rapid estimation of the peripheral accumulation of PrPres during the incubation period. Animals were treated with the different drugs (5 animals/group) by the intraperitoneal route for 3 weeks starting the day following the day of inoculation. The drugs used were quinacrine (10 mg/kg), chlorpromazine (5 mg/kg), the two drugs in combination, and the amphotericin B derivatives MS-8209 (25 mg/kg) and MS-1191 (10 mg/kg). The mice in the treatment control group (10 mice) were treated with a sterile glucose solution (5% [wt/vol]). The mice were sacrificed 30 days after infection, the time when spleen PrPres reaches a plateau in untreated animals (3).
Protein analysis (enzyme immunoassay).
Mice were killed by cervical disruption 30 days after inoculation. The spleens were frozen immediately in liquid nitrogen and kept at −80°C until they were used. The spleen homogenates were suspended at 10% (wt/vol) in a 5% glucose solution. PrPres purification was performed with the BSE purification kit (Bio-Rad) (11) adapted to the murine spleen. Four-hundred-microliter aliquots were digested with a final concentration of 20 μg of proteinase K/ml for 10 min at 37°C. One-hundred-microliter aliquots (representing one-third of the resuspended pellet and 6.67 mg of spleen equivalent) were added to duplicate wells on enzyme immunoassay plates (CEA) coated with the mouse-specific MAb SAF53 (16). The plates were incubated for 1 h at room temperature in the dark and washed three times, 100 μl of MAb 11C6-G4 (10) was added, and the plates were incubated for 1 h. After several washes, 100 μl of Ellman solution (CEA) (29) was added, and the plates were incubated for at least 30 min. PrPres immunodetection was carried out by optic densitometry measured at 414 nm.
RESULTS
Quinacrine binds to PrP peptides.
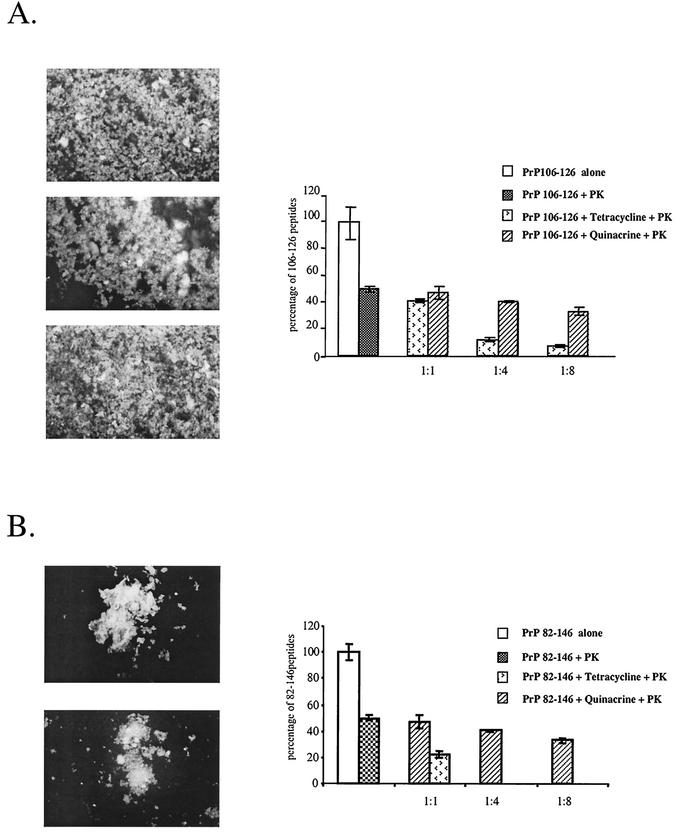
The ability of quinacrine to interact with PrP aggregates was investigated using synthetic peptides homologous to residues 82 to 146 and 106 to 126 of human PrP that have a high propensity to adopt a β-sheet secondary structure and to form amyloid fibrils (14, 36). The intrinsic fluorescence of quinacrine and tetracycline allowed us to directly observe their binding capacities by coincubation with the amyloid fibrils. Thioflavin T, an amyloid-binding fluorochrome, was used as a positive control. As shown in Fig. 1, fluorescence microscopy revealed identical labeling with quinacrine, tetracycline, and thioflavin T. These results indicate that quinacrine has the ability to interact with the PrP106-126 peptide (Fig. 1A, top) and the PrP 82-146 peptide (Fig. 1B, top).
FIG. 1.
Binding of quinacrine, tetracycline, and thioflavin T to PrP106-126 and PrP82-146 peptides and their effects on proteinase K (PK) resistance of these peptides. (A) Fluorescence microscopy of PrP106-126 aggregates incubated with quinacrine (top), tetracycline (middle), or thioflavin T (bottom) and effects of quinacrine and tetracycline on proteinase K resistance of the peptide (graph). (B) Fluorescence microscopy of PrP82-146 aggregates incubated with quinacrine (top) or tetracyline (bottom) and effects of quinacrine and tetracycline on proteinase K resistance of the peptide (graph). The extents of proteolysis of PrP106-126 (A) and PrP82-146 (B) in the absence or presence of quinacrine or tetracycline were calculated as the percentage of peptide present in the pellet compared to the total amount originally present.
Quinacrine does not modify the proteinase K resistance properties of PrP peptides.
Once synthetic PrP peptides are allowed to polymerize into amyloid fibrils, they are characterized by high resistance to proteinase K. As quinacrine has been shown to bind to PrP peptides, we investigated whether it could affect the proteinase K resistance properties of the peptides. The results shown in the graphs in Fig. 1 indicated that the addition of quinacrine to the peptides up to a ratio of 8 to 1 had no significant effect on their proteinase K resistance. In contrast, tetracycline reduced the protease resistances of both the PrP106-126 and PrP82-146 peptides in a dose-dependent manner (34). These results probably reflect the inability of quinacrine to disaggregate the fibrils of synthetic peptides once they are formed.
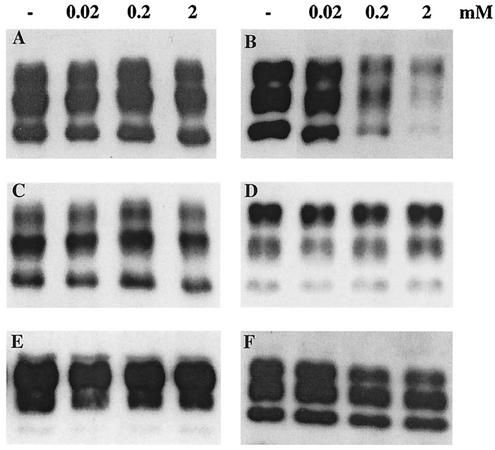
Quinacrine cannot reverse the protease resistance of PrPres.
To test the ability of quinacrine to affect the protease resistance of disease-specific PrP, PrPres was partially extracted from brain tissue of sporadic- and variant-CJD patients, 263K-infected hamsters, and 139A-infected mice and incubated with 20 μM to 2 mM quinacrine or tetracycline for 48 h prior to proteinase K digestion. While treatment of the samples with tetracycline resulted in a decrease in PrPres to an extent which was dependent on the drug concentration, as described previously (15, 34), quinacrine had no effect (Fig. 2). Again, this was a reflection of the inability of quinacrine to disaggregate bona fide PrPres from infectious brains.
FIG. 2.
Effects of quinacrine (A and C to F) and tetracycline (B) on the protease resistance of a variety of PrPres isoforms. Partially purified PrPres was incubated with increasing concentrations of the compounds (indicated at the top; −, absence of treatment) and then digested with proteinase K. (A and B) Sporadic CJD with type 1 PrPres; (C) sporadic CJD with type 2 PrPres; (D) variant CJD; (E) 263K-infected hamster; (F) 139A-infected mouse. The immunoblot analysis was carried out using the MAb 3F4 (A to E), and the polyclonal antibody PrP95-108 (F).
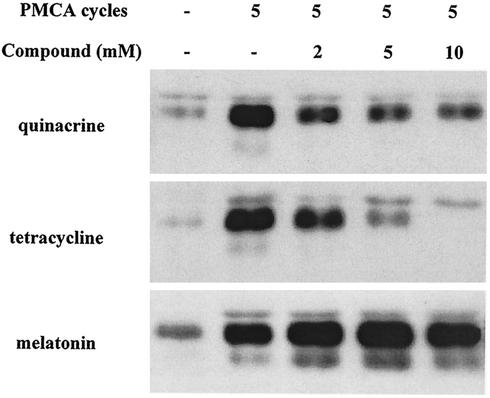
Quinacrine reduces cyclic amplification of PrPres.
It has been reported that PrPres can be amplified in the presence of an excess of PrPc by cycles of incubation and sonication (30). To verify whether PrPc-to-PrPres conversion was affected by quinacrine, the compound was added to the reaction mixture (i.e., a 1:300 dilution of 263K scrapie-infected hamster brain homogenate in normal hamster brain homogenate) at concentrations ranging from 2 to 10 mM before cycling. Control experiments were carried out using tetracycline (as a molecule that interacts with and affects the physicochemical properties of PrPres) and melatonin (as a molecule binding to an amyloid protein that is irrelevant here, namely, Aβ of Alzheimer's disease). Quinacrine reduced and tetracycline inhibited PrPres amplification. By contrast, melatonin had no effect (Fig. 3).
FIG. 3.
Effects of quinacrine, tetracycline, and melatonin on cyclic amplification of PrPres from scrapie-infected hamster brain. The number of cycles of incubation-sonication and the concentrations of the compounds are indicated. −, none; PMCA, protein misfolding cyclic amplification.
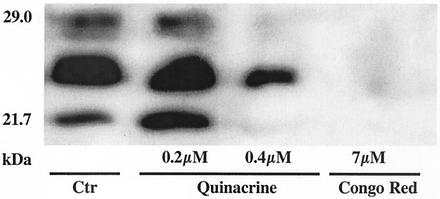
Quinacrine inhibits PrPres accumulation in N2a58/22L and ScN2a cells.
The inefficiency of quinacrine in disrupting PrP aggregates and reverting the protease resistance phenotype led us to investigate its ability to inhibit PrPres accumulation in cellular models. When scrapie-infected cells were treated once a day for 3 days with quinacrine at 0.4 μM concentration, the PrPres signal decreased significantly without any toxicity (Fig. 4) (cytotoxicity was tested from 0.02 to 200 μM and began at 1 μM [data not shown]). Congo red (7 μM), used as a positive control, completely abolished the PrPres signal (22). Similar data were obtained using conventional ScN2a cells (data not shown).
FIG. 4.
Quinacrine inhibits PrPres accumulation in N2a58/22L cells. Anti-PrP immunoblots with SAF75 of N2a58/22L cells that were incubated with quinacrine and Congo red (three administrations) for 3 days are shown. Control (Ctr) corresponds to the absence of treatment.
Quinacrine reduces microglia-mediated killing of ScN2a cells.
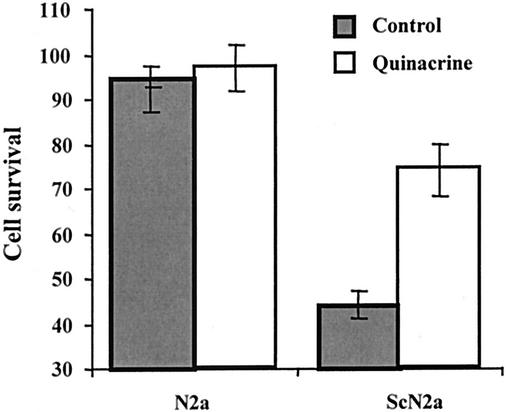
Previous studies showed that microglia selectively kill neuroblastoma cells actively producing PrPres (2). In this assay, microglia cells were cocultured with either ScN2a cells or the uninfected N2a control cells in the presence or absence of 0.2 μM quinacrine. The number of microglia added was not sufficient to reduce the survival of N2a cells. In contrast, microglia reduced the survival of ScN2a cells, an effect that was partially blocked by the presence of quinacrine (Fig. 5).
FIG. 5.
Quinacrine protects ScN2a cells against microglia-mediated killing. The survival of prion-infected (ScN2a) and uninfected (N2a) neuroblastoma cells cocultured with microglia in the presence (open bars) or absence (shaded bars) of 0.2 μM quinacrine is illustrated. The values given are the means ± standard deviations of triplicate experiments repeated twice (six observations).
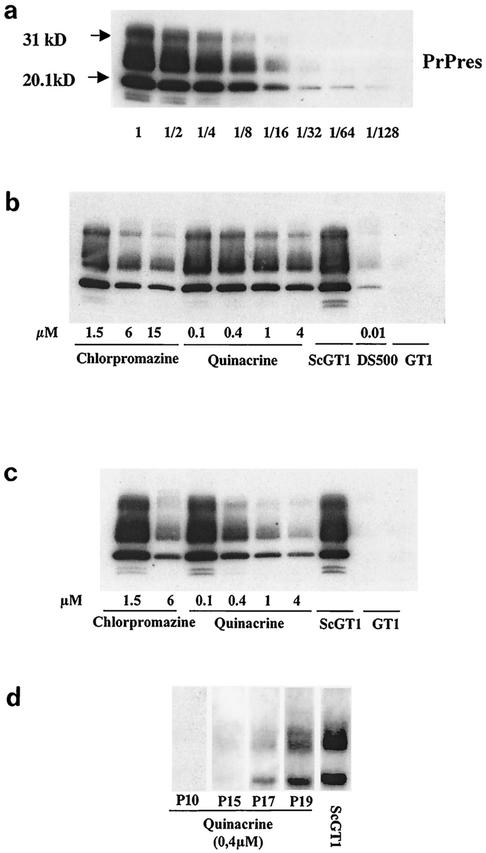
Quinacrine is less efficient in inhibiting PrPres accumulation in ScGT1 cells. (i) Single treatment lasting 3 days.
In ScGT1 cells, quinacrine did not entirely inhibit the accumulation of PrPres, even at 4 μM (2 μg/ml), which corresponds to the threshold of cytotoxicity (Fig. 6b). At this concentration, a twofold decrease in the PrPres signal was observed compared to that in untreated cells. When the concentration was increased to 10 μM, to try to achieve a more efficient PrPres inhibition as described by Korth et al. (18), complete cytotoxicity was observed. Chlorpromazine at 15 μM (5 μg/ml) induced a higher (threefold) PrPres signal decrease with limited toxicity. As a comparison, a 10-fold decrease in the PrPres signal was observed in the absence of toxicity with high-molecular-weight dextran sulfate (DS500 at 0.01 μM, or 5 μg/ml), used here as a positive treatment control.
FIG. 6.
In vitro evaluation by Western blotting (with SAF83) of the efficacy of treatment with quinacrine, chlorpromazine, and DS500 on PrPres accumulation in ScGT1 cells. (a) Dilution scale of ScGT1 cells (lane 1 corresponds to 300 μg of total protein). (b) Effects of a range of quinacrine and chlorpromazine concentrations and of DS500 (0.01 μM) after a unique 4-day treatment. (c) Same doses of chlorpromazine, quinacrine, and DS500 applied three times in 6 days. (d) Effects of 0.4 μM quinacrine treatment every day for 3 weeks and at several passages (P) after treatment was stopped.
(ii) Three treatments over 6 days.
With repeated treatments, a 16-fold decrease in the PrPres signal was observed with a 4 μM quinacrine treatment, whereas a 0.4 μM treatment decreased the signal 8-fold (Fig. 6c). The maximum concentration of chlorpromazine used in this treatment regimen was 6 μM, which decreased PrPres accumulation 10-fold. Repeated administration of DS500 was cytotoxic—effects on cell adherence were observed even at 0.01 μM; therefore, it could not be used to evaluate PrPres accumulation.
(iii) Long-term treatment.
ScGT1 cells were treated with 0.4 μM quinacrine every day for 3 weeks (21 doses). With this treatment, PrPres accumulation was entirely inhibited over 12 passages posttreatment (i.e., 12 weeks) but reappeared after 15 passages (Fig. 6d).
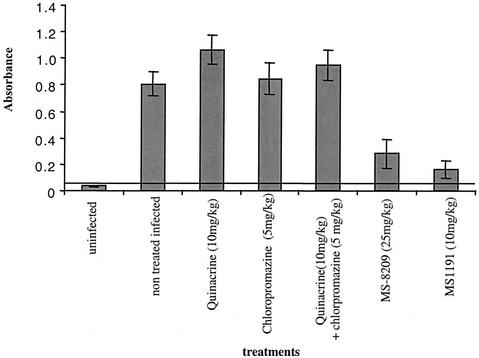
Quinacrine and chlorpromazine do not inhibit PrPres accumulation in vivo.
A rapid model based on the detection of PrPres accumulation in the spleens of intraperitoneally scrapie-infected mice was used as previously described (3). The choice of the quinacrine concentration administered (10 mg/kg of body weight) was based on the doses given to humans in compassionate CJD treatment (300 mg/day). It corresponded to a twofold dose administered to humans. No curative effect of quinacrine was observed at 10 mg/kg/day, and indeed, a slightly increased PrPres signal in the spleen was seen in animals infected with an experimental mouse-adapted BSE strain (6PB1) (Fig. 7). This was confirmed by immunohistochemistry, where at this early stage of infection (30 days postinfection), PrPres aggregates were detected in the spleens of quinacrine-treated animals and not in infected untreated controls (data not shown).
FIG. 7.
Evaluation by enzyme immunoassay of the efficacies of different drugs in reducing PrPres accumulation in the spleens of scrapie-infected mice. Mice infected intraperitoneally with the 6PB1 strain were treated intraperitoneally each day for 3 weeks with quinacrine alone (10 mg/kg), chlorpromazine alone (5 mg/kg), both drugs, and MS-8209 (25 mg/kg) or MS1191 (10 mg/kg). The PrPres concentrations in the spleens of the animals after 30 days were evaluated by optical densitometry. Day 30 is the time when the scrapie PrP concentration reaches a plateau in this organ (3). The horizontal line at an absorbance of 0.08 corresponds to the cutoff, calculated as 2.5 times the mean absorbance of the negative controls.
The dose of chlorpromazine administered by the intraperitoneal route was limited to 5 mg/kg because of toxicity, and no effect on PrPres was observed.
We also sought to determine whether a synergic effect of quinacrine and chlorpromazine could be obtained by combining the two drugs, but even in association, quinacrine did not induce any decrease in the PrPres signal.
In contrast, a ≥3-fold decrease in the PrPres signal was observed with a daily intraperitoneal administration of the polyene antibiotics MS-8209 (25 mg/kg) and MS-1191 (10 mg/kg), used as a positive treatment control and for which clinical efficiency had been previously reported in several in vivo models (1). Moreover, preliminary experiments on a limited number of mice infected by the experimental BSE strain and treated at the beginning of the clinical phase (three animals per group) did not show any clinical improvement or increase in survival time with quinacrine and chlorpromazine used either alone or in association (data not shown).
DISCUSSION
To date, there is no effective therapy for prion diseases. Recently, Korth et al. presented quinacrine as a potentially good candidate for human treatment based on its ability to efficiently inhibit PrPres accumulation in ScN2a cells. The published results indicated that quinacrine “cured” the cells (i.e., it abolished the PrPres signal) at 0.4 μM (0.2 μg/ml) with three repeat treatments (18). Previous studies had shown similar results with ScNB cells, another scrapie-infected neuroblastoma cell line; the efficient concentration with a unique treatment was 2 μM (1 μg/ml) (12). The use of this molecule for the treatment of unrelated human disorders and its ability to penetrate the blood-brain barrier have led to its use for human CJD treatment (20). Twenty patients clinically affected with CJD have been included in the French human quinacrine treatment cohort since August 2001, but no significant improvement in clinical status could be observed for any of them (A. Alperovitch, personal communication).
We have investigated the possible mechanism of action of quinacrine on prion replication to assess the relevance of such treatment by using different experimental models. The efficiency of quinacrine was tested in cell-free systems (PrP binding, proteinase K resistance, and PrPres amplification), in different cellular models (ScN2a and ScGT1 cells), and in vivo. On the whole, both in vitro and in vivo studies led to a reassessment of the efficiency described both for this drug and for chlorpromazine, a drug also proposed for compassionate treatment but rapidly abandoned because of its adverse effects (i.e., it leads to a therapeutical coma). In ScN2a cells, we reproduced the data described by Doh-Ura et al. and by Korth et al. (12, 18). Moreover, we observed potentially important indirect effects of quinacrine, namely, that neuroblastoma cells treated with high concentrations of quinacrine were resistant to the toxicity of PrP peptides (data not shown) and increased survival of quinacrine-treated ScN2a cells cocultured with microglia (Fig. 5); the latter suggested that quinacrine treatment and inhibition of PrPres formation reversed the infection-induced changes in the cell membrane that are recognized by microglia. Conversely, in ScGT1 cells, only subtoxic doses of quinacrine (4 μM, i.e., 10 times higher than the dose described as being effective in ScN2a cells [18]) and chlorpromazine efficiently decreased PrPres accumulation after a single treatment over 3 days, or even with repeated treatments over 6 days, in contrast with other molecules, such as DS500 and MS8209, which were effective with a single treatment at nontoxic doses. Nevertheless, ScGT1 cells could be cured without toxicity by a long treatment (every day for 3 weeks) with 0.4 μM quinacrine, but this effect was not permanent, as PrPres reappeared 4 months after the treatment was stopped. Following these different results in the various tissue culture models, we wanted to investigate in detail the mechanism by which this molecule could interfere with prion replication, and in particular its binding to, and interaction with, PrP. We showed that this molecule, as opposed to controls, exhibits binding to peptides qualitatively similar to that observed with tetracycline or thioflavin T. Thus, we expected an effect of quinacrine on the protease resistance of PrP aggregates. However, it did not display any defibrillogenic effect on preformed synthetic peptide fibrils, nor did it reduce PrPres proteinase K resistance in different brain tissues taken from CJD patients or experimentally infected rodents. This suggests that if quinacrine cannot disrupt preformed PrPres aggregates, its effects during the course of the disease may be weak, as degradation is very slow in the brain, where the aggregates cause neuronal damage. However, using the protein misfolding cyclic amplification method, we found that quinacrine decreased de novo PrPres synthesis. In this regard, we noted that this effect could not be previously detected with a one-step PrPres in vitro conversion (12), which is in line with the necessity of repeated in vitro treatments to observe a curing effect. Thus, once aggregates exist, quinacrine would have no effect, although it would decrease de novo formation of PrPres.
We then investigated the relevance of a quinacrine treatment in an experimental infected-animal model. We first investigated its efficiency in decreasing PrPres replication in the lymphoreticular system by using a rapid in vivo model. Following such studies, we also wanted to know if we could prevent PrPres propagation to the central nervous system. The absence of efficiency in reducing the accumulation of PrPres in the spleens of mice after a 3-week treatment was consistent with the in vitro results obtained in ScGT1 cells. Furthermore, our preliminary results treating mice in early stages of clinical disease with 10 mg of quinacrine/kg were consistent with recent data which indicated that oral treatment of a murine model of CJD with quinacrine (also at 10 mg/kg/day) after intracerebral inoculation did not lead to any increased survival compared to controls (8). The dose of 10 mg/kg, twice the dose currently used in humans that leads to hepatotoxicity and cessation of treatment in some patients, appeared to be tolerated by mice in this study. In the present studies, not only did we fail to find a curative effect of this drug on PrPres accumulation in the lymphoreticular system, but the accumulation even increased somewhat. This suggests that quinacrine induces an imbalance between the synthesis and catabolism of PrPres. Quinacrine is known to have a tropism for lysosomes, which are suspected to play a role in the synthesis and/or accumulation of PrPres (21). However, the complexity of intercellular relations between different cell populations of the spleen and the distribution of the drug might explain the discrepancy between our in vitro and in vivo data. Moreover, the existence of different physical states of PrPres in cultured cells and in the organs could also explain in part these divergent observations. Our investigations confirmed the capacity of quinacrine to interfere with PrPres formation, with varying efficiencies in different cell lines. The drug did not affect resistance to proteinase K digestion of PrP peptides and PrPres from various sources and failed to show an effect on PrPres accumulation in the spleens of scrapie-inoculated mice. Taken together, these data do not support the potential therapeutic efficiency of quinacrine and underline the urgency of developing new therapeutic approaches with a mechanistic analysis and the necessity to test potential antiprion drugs in animal models once they have been screened in vitro.
Acknowledgments
This work was supported in part by the European Community (QLK3-2001-00283 and BMH4-CT98-6011) and the Department of Social Services, Italian Ministry of Health (RF 2001-96).
We are grateful to S. Lehmann for N2a58/22L and ScGT1 cells, to J. Grassi for the antibodies SAF75, SAF53, and SAF83, and to J. Langeveld for the antibody R528.7.
REFERENCES
- 1.Adjou, K. T., N. Privat, S. Demart, J. P. Deslys, M. Seman, J. J. Hauw, and D. Dormont. 2000. MS-8209, an amphotericin B analogue, delays the appearance of spongiosis, astrogliosis and PrPres accumulation in the brain of scrapie-infected hamsters. J. Comp. Pathol. 122:3-8. [DOI] [PubMed] [Google Scholar]
- 2.Bate, C., S. Reid, and A. Williams. 2001. Killing of prion-damaged neurones by microglia. Neuroreport 12:2589-2594. [DOI] [PubMed] [Google Scholar]
- 3.Beringue, V., F. Lamoury, K. T. Adjou, T. Maignien, M. Demoy, P. Couvreur, and D. Dormont. 2000. Pharmacological manipulation of early PrPres accumulation in the spleen of scrapie-infected mice. Arch. Virol. Suppl. 16:39-56. [DOI] [PubMed] [Google Scholar]
- 4.Bolton, D. C., M. P. McKinley, and S. B. Prusiner. 1982. Identification of a protein that purifies with the scrapie prion. Science 218:1309-1311. [DOI] [PubMed] [Google Scholar]
- 5.Caughey, B., D. Ernst, and R. E. Race. 1993. Congo red inhibition of scrapie agent replication. J. Virol. 67:6270-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caughey, B., G. J. Raymond, D. A. Kocisko, and P. T. Lansbury, Jr. 1997. Scrapie infectivity correlates with converting activity, protease resistance, and aggregation of scrapie-associated prion protein in guanidine denaturation studies. J. Virol. 71:4107-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caughey, W. S., L. D. Raymond, M. Horiuchi, and B. Caughey. 1998. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc. Natl. Acad. Sci. USA 95:12117-12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, S. J., V. Lewis, M. Brazier, A. F. Hill, A. Fletcher, and C. L. Masters. 2002. Quinacrine does not prolong survival in a murine Creutzfeldt-Jakob disease model. Ann. Neurol. 52:503-506. [DOI] [PubMed] [Google Scholar]
- 9.Demaimay, R., J. Harper, H. Gordon, D. Weaver, B. Chesebro, and B. Caughey. 1998. Structural aspects of Congo red as an inhibitor of protease-resistant prion protein formation. J. Neurochem. 71:2534-2541. [DOI] [PubMed] [Google Scholar]
- 10.Demart, S., J. G. Fournier, C. Creminon, Y. Frobert, F. Lamoury, D. Marce, C. Lasmezas, D. Dormont, J. Grassi, and J. P. Deslys. 1999. New insight into abnormal prion protein using monoclonal antibodies. Biochem. Biophys. Res. Commun. 265:652-657. [DOI] [PubMed] [Google Scholar]
- 11.Deslys, J. P., E. Comoy, S. Hawkins, S. Simon, H. Schimmel, G. Wells, J. Grassi, and J. Moynagh. 2001. Screening slaughtered cattle for BSE. Nature 409:476-478. [DOI] [PubMed] [Google Scholar]
- 12.Doh-Ura, K., T. Iwaki, and B. Caughey. 2000. Lysosomotropic agents and cysteine protease inhibitors inhibit scrapie-associated prion protein accumulation. J. Virol. 74:4894-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farquhar, C. F., and A. G. Dickinson. 1986. Prolongation of scrapie incubation period by an injection of dextran sulphate 500 within the month before or after infection. J. Gen. Virol. 67:463-473. [DOI] [PubMed] [Google Scholar]
- 14.Forloni, G., N. Angeretti, R. Chiesa, E. Monzani, M. Salmona, O. Bugiani, and F. Tagliavini. 1993. Neurotoxicity of a prion protein fragment. Nature 362:543-546. [DOI] [PubMed] [Google Scholar]
- 15.Forloni, G., S. Iussich, T. Awan, L. Colombo, N. Angeretti, L. Girola, I. Bertani, G. Poli, M. Caramelli, M. G. Bruzzone, L. Farina, L. Limido, G. Rossi, G. Giaccone, J. W. Ironside, O. Bugiani, M. Salmona, and F. Tagliavini. 2002. Tetracyclines affect prion infectivity. Proc. Natl. Acad. Sci. USA 9:10849-10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grassi, J., C. Creminon, Y. Frobert, P. Fretier, I. Turbica, H. Rezaei, G. Hunsmann, E. Comoy, and J. P. Deslys. 2000. Specific determination of the proteinase K-resistant form of the prion protein using two-site immunometric assays. Application to the post-mortem diagnosis of BSE. Arch. Virol. Suppl. 16:197-205. [DOI] [PubMed] [Google Scholar]
- 17.Kimberlin, R. H., and C. A. Walker. 1986. Suppression of scrapie infection in mice by heteropolyanion 23, dextran sulfate, and some other polyanions. Antimicrob. Agents Chemother. 30:409-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korth, C., B. C. May, F. E. Cohen, and S. B. Prusiner. 2001. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl. Acad. Sci. USA 98:9836-9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladogana, A., P. Casaccia, L. Ingrosso, M. Cibati, M. Salvatore, Y. G. Xi, C. Masullo, and M. Pocchiari. 1992. Sulphate polyanions prolong the incubation period of scrapie-infected hamsters. J. Gen. Virol. 73:661-665. [DOI] [PubMed] [Google Scholar]
- 20.Love, R. 2001. Old drugs to treat new variant Creutzfeldt-Jakob disease. Lancet 358:563.. [DOI] [PubMed] [Google Scholar]
- 21.Lullmann-Rauch, R., R. Pods, and B. von Witzendorff. 1996. The antimalarials quinacrine and chloroquine induce weak lysosomal storage of sulphated glycosaminoglycans in cell culture and in vivo. Toxicology 110:27-37. [DOI] [PubMed] [Google Scholar]
- 22.Milhavet, O., A. Mange, D. Casanova, and S. Lehmann. 2000. Effect of Congo red on wild-type and mutated prion proteins in cultured cells. J. Neurochem. 74:222-230. [DOI] [PubMed] [Google Scholar]
- 23.Nishida, N., D. A. Harris, D. Vilette, H. Laude, Y. Frobert, J. Grassi, D. Casanova, O. Milhavet, and S. Lehmann. 2000. Successful transmission of three mouse-adapted scrapie strains to murine neuroblastoma cell lines overexpressing wild-type mouse prion protein. J. Virol. 74:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccardo, P., J. P. Langeveld, A. F. Hill, S. R. Dlouhy, K. Young, G. Giaccone, G. Rossi, M. Bugiani, O. Bugiani, R. H. Meloen, J. Collinge, F. Tagliavini, and B. Ghetti. 1998. An antibody raised against a conserved sequence of the prion protein recognizes pathological isoforms in human and animal prion diseases, including Creutzfeldt-Jakob disease and bovine spongiform encephalopathy. Am. J. Pathol. 152:1415-1420. [PMC free article] [PubMed] [Google Scholar]
- 25.Pocchiari, M., S. Schmittinger, and C. Masullo. 1987. Amphotericin B delays the incubation period of scrapie in intracerebrally inoculated hamsters. J. Gen. Virol. 68:219-223. [DOI] [PubMed] [Google Scholar]
- 26.Priola, S. A., A. Raines, and W. S. Caughey. 2000. Porphyrin and phthalocyanine antiscrapie compounds. Science 287:1503-1506. [DOI] [PubMed] [Google Scholar]
- 27.Prusiner, S. B. 1991. Molecular biology of prion diseases. Science 252:1515-1522. [DOI] [PubMed] [Google Scholar]
- 28.Prusiner, S. B., M. P. McKinley, K. A. Bowman, D. C. Bolton, P. E. Bendheim, D. F. Groth, and G. G. Glenner. 1983. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 35:349-358. [DOI] [PubMed] [Google Scholar]
- 29.Rodolfo, K., R. Hassig, K. L. Moya, Y. Frobert, J. Grassi, and L. Di Giamberardino. 1999. A novel cellular prion protein isoform present in rapid anterograde axonal transport. Neuroreport 10:3639-3644. [DOI] [PubMed] [Google Scholar]
- 30.Saborio, G. P., B. Permanne, and C. Soto. 2001. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411:810-813. [DOI] [PubMed] [Google Scholar]
- 31.Soto, C., R. J. Kascsak, G. P. Saborio, P. Aucouturier, T. Wisniewski, F. Prelli, R. Kascsak, E. Mendez, D. A. Harris, J. Ironside, F. Tagliavini, R. I. Carp, and B. Frangione. 2000. Reversion of prion protein conformational changes by synthetic beta-sheet breaker peptides. Lancet 355:192-197. [DOI] [PubMed] [Google Scholar]
- 32.Supattapone, S., H. O. Nguyen, F. E. Cohen, S. B. Prusiner, and M. R. Scott. 1999. Elimination of prions by branched polyamines and implications for therapeutics. Proc. Natl. Acad. Sci. USA 96:14529-14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Supattapone, S., H. Wille, L. Uyechi, J. Safar, P. Tremblay, F. C. Szoka, F. E. Cohen, S. B. Prusiner, and M. R. Scott. 2001. Branched polyamines cure prion-infected neuroblastoma cells. J. Virol. 75:3453-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tagliavini, F., G. Forloni, L. Colombo, G. Rossi, L. Girola, B. Canciani, N. Angeretti, L. Giampaolo, E. Peressini, T. Awan, L. De Gioia, E. Ragg, O. Bugiani, and M. Salmona. 2000. Tetracycline affects abnormal properties of synthetic PrP peptides and PrP(Sc) in vitro. J. Mol. Biol. 300:1309-1322. [DOI] [PubMed] [Google Scholar]
- 35.Tagliavini, F., R. A. McArthur, B. Canciani, G. Giaccone, M. Porro, M. Bugiani, P. M. Lievens, O. Bugiani, E. Peri, P. Dall'Ara, M. Rocchi, G. Poli, G. Forloni, T. Bandiera, M. Varasi, A. Suarato, P. Cassutti, M. A. Cervini, J. Lansen, M. Salmona, and C. Post. 1997. Effectiveness of anthracycline against experimental prion disease in Syrian hamsters. Science 276:1119-1122. [DOI] [PubMed] [Google Scholar]
- 36.Tagliavini, F., F. Prelli, L. Verga, G. Giaccone, R. Sarma, P. Gorevic, B. Ghetti, F. Passerini, E. Ghibaudi, G. Forloni, et al. 1993. Synthetic peptides homologous to prion protein residues 106-147 form amyloid-like fibrils in vitro. Proc. Natl. Acad. Sci. USA 90:9678-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]