Abstract
We assessed the requirement of the host cytoskeleton for the intracytosolic transport of incoming human cytomegalovirus (HCMV) capsids. Treatments with microtubule (MT)-depolymerizing drugs nocodazole and colchicine led to a drastic decrease in levels of IE1 antigen, whereas cytochalasin B had no effect on the level of IE1 as determined by Western blot analyses. Sequential treatment including nocodazole washout and removal of cell surface virion revealed that HCMV entry into the cells occurred normally in the absence of the MT network. This finding was also supported by data obtained by monitoring pUL83 signals with an immunofluorescent assay (IFA). Furthermore, we demonstrated a close association of incoming HCMV capsids with MTs by IFA and ultrastructural analyses. In the absence of the MT network, the capsids which had entered the cytoplasm did not move to close proximity of the nucleus. These data suggest that HCMV capsids associate with the MT network to facilitate their own movement to the nucleus before the onset of immediate-early (IE) gene expression and that this association is required to start efficient IE gene expression.
Human cytomegalovirus (HCMV) is a prevalent pathogen responsible for significant morbidity and mortality in immunosuppressed individuals. It is a member of the betaherpesvirus family containing a double-stranded 230-kbp DNA genome, which is predicted to encode over 200 open reading frames (15). Like other herpesviruses, HCMV comprises three structural elements, an icosahedral capsid containing the DNA genome, a tegument, and an envelope. The dense tegument layer is distinguished from those of other herpesvirus families and is composed of a large number of proteins, but little is known about its structure or function (9). The infectious cycle of HCMV begins with multistep binding of virus to the cell surface, followed by penetration. Although release and dissociation of some tegument components occur upon entry (19), unlike those of alphaherpesviruses, the capsids are assumed to retain abundant structural tegument proteins and supposed to move towards the nuclear envelope (NE). Among the many unresolved processes, the mechanism of HCMV capsid transport in the cytoplasm remains unknown. Since movement of virus through the cytosol is not likely to occur by free diffusion but rather by a cellular transport system, it is conceivable that interactions of viral tegument proteins with the host machinery involved in cellular transport systems permits an active process (22). It has been reported that the efficient transport of herpes simplex virus type 1 (HSV-1) within cells in culture requires the microtubule (MT)-dependent motor proteins (8, 23), and interaction of tegument components with the motor proteins has been reported (7). MTs are organized in a polarized fashion with minus ends at the MT organization center (MTOC) and plus ends at the cell periphery; therefore, an interaction with MTs could facilitate proper movement of the cytosolic capsids to the NE, as has been shown for other viruses, including adenovirus and parvovirus (24, 25). In this study, we have examined the role of the host cytoskeleton in the intracytosolic transport of incoming HCMV capsids. Here we report that efficient onset of immediate-early (IE) gene expression depends on an intact MT network and also demonstrate the close association of HCMV capsids with the MT network during the postentry phase.
Human embryonic lung (HEL) fibroblasts between passages 15 and 25 were inoculated with HCMV (Towne strain) as previously described (16). Prior to inoculation, the cells grown on 6-cm-diameter dishes were pretreated in the medium containing drugs (10 μM nocodazole, 10 μM Taxol, 0.5 μM cytochalasin B, and 5 μM colchicines; all purchased from Sigma, St. Louis, Mo.) for 1 h and were maintained in the media containing each drug during infection. In some experiments, HCMV-inoculated cells were washed twice with cold phosphate-buffered saline (PBS) and treated with 1 ml of a 1.5-mg/ml concentration of proteinase K (Roche Diagnostics, Mannheim, Germany) in PBS on ice. After 30 min of incubation, 1 ml of PBS containing 3% bovine serum albumin and 0.5 mM phenylmethylsulfonyl fluoride was added to stop further proteolysis. The cells were collected and centrifuged in the medium containing 10% fetal bovine serum, replated onto the dishes, and cultured at 37°C in a 5% CO2 incubator for various times. Lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting analysis with a monoclonal antibody (MAb) specific for the IE gene product of HCMV (MAB810; Chemicon, Temecula, Calif.) and detected by an ECL system (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Signal intensity was measured by using Image Gauge software (Fujifilm, Kanagawa, Japan). Immunofluorescence (IF) analysis was carried out using a confocal laser-scanning microscope (LSM 410; Carl Zeiss, Oberkochen, Germany) as described previously (17), with the following antibodies: MAbs against α-tubulin (Oncogene Research Products, Boston, Mass.), HCMV pUL83 (Goodwin Institute, Plantation, Fla), and the nuclear pore complex (MAb414; BabCO, Richmond, Calif.) and rabbit polyclonal antibody against HCMV pUL32 (12). Secondary antibodies were anti-mouse immunoglobulin G antibody or anti-rabbit immunoglobulin antibody labeled with Alexa Fluor 488 or 568 (Molecular Probes, Eugene, Oreg.). Nuclei were visualized with TOPRO3 (Molecular Probes). For transmission electron microscopy (TEM) analysis, cells grown in 2-cm-diameter dishes were inoculated with HCMV at a multiplicity of infection (MOI) of 20 PFU/cell in the presence of 0.5 mM cycloheximide. At 4 h postinfection (hpi), cells were treated with the medium containing 10 μM Taxol for 3 min at 37°C and prefixed with 4% paraformaldehyde in PBS containing 10 mM EGTA and 2 mM MgCl2 for 20 min at 37°C, followed by a conventional fixation procedure for TEM as described previously (17). Ultra-thin sections were cut parallel to the substrate. MTs were identified by the characteristic tubular structure with a diameter of 25 nm.
Efficient HCMV IE1 gene expression requires an intact MT network.
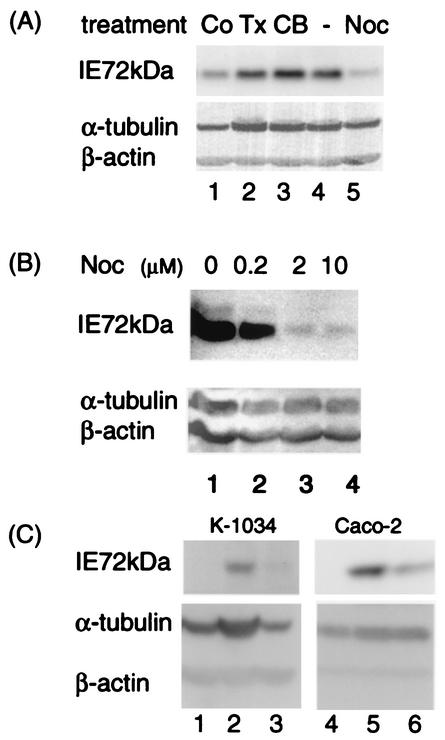
To determine the role of cytoskeleton structures in the IE phase of HCMV infection, the effects of various drugs on expression levels of IE1 antigen were examined in the HCMV-infected HEL cells at 12 hpi (Fig. 1A). Among these effects, treatments with MT-depolymerizing agents nocodazole and colchicine resulted in remarkable reduction of IE1 expression compared to that in untreated cells. This finding contrasted to that observed with an actin filament-depolymerizing drug, cytochalasin B, which showed no significant effects. Since Taxol did not change the IE1 level under the same conditions, the dynamic instability of MTs seemed not to be required. Even at 18 hpi, the inhibitory effects of nocodazole were observed in a dose-dependent manner (Fig. 1B). A very low but distinct level of IE1 antigen was detected with 10 μM nocodazole (Fig. 1A and B), although the MT network was completely disrupted in IF analysis (data not shown). The inhibitory effect of nocodazole was also observed in other HCMV-permissive epithelial cells, K-1034 and Caco-2 cells (Fig. 1C). Thus, in the absence of an intact MT network, significantly less IE1 was synthesized both in fibroblastic and epithelial cells.
FIG. 1.
Efficient IE1 antigen expression requires an intact MT network. (A and B) HEL cells were infected with HCMV at an MOI of 3 PFU/cell in the presence of various drugs, and effects on expression levels of the IE1 antigen (72 kDa) were examined at 12 (A) and 18 (B) hpi. (A) Lane 1, 5 μM colchicine (Co); lane 2, 10 μM Taxol (Tx); lane 3, 0.5 μM cytochalasin B (CB); lane 4, no drug; lane 5, 10 μM nocodazole (Noc). (B) Lane 1, no drug; lane 2, 0.2 μM nocodazole; lane 3, 2 μM nocodazole; lane 4, 10 μM nocodazole. (C) K-1034 and Caco-2 cells were mock infected (lanes 1 and 4) or HCMV-infected (lanes 2, 3, 5, and 6) at an MOI of 3 PFU/cell. Effects of nocodazole on expression levels of the IE1 antigen (72 kDa) were examined at 24 hpi in the absence (lanes 2 and 5) or presence (lanes 3 and 6) of 10 μM nocodazole.
HCMV enters the cytoplasm in the absence of the MT network.
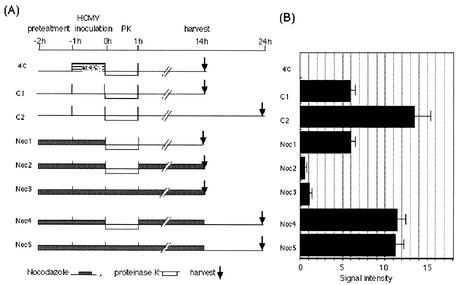
To determine whether the virus entry process depends on the MT network, we examined sequential treatments including nocodazole washout, removal of cell surface-absorbed virions by proteinase K, and incubation with or without the MT network as shown in Fig. 2A. IF analysis revealed that removal of nocodazole led to the complete reorganization of the MT network within 60 min in HEL cells (data not shown). Cell surface-attached virions that had not entered the cytoplasm during the inoculation at 4°C were completely removed by proteinase K digestion (Fig. 2B, 4°C). The effects of nocodazole washout on IE1 expression were examined at 14 hpi after incubation either in the absence or in the presence of nocodazole. Drug removal after inoculation (Fig. 2B, Noc1) led to a level of IE1 similar to that observed in the control experiment without the drug (Fig. 2B, C1). Similarly, when the drug was removed at 14 hpi and cells were incubated for a further 10 h in the presence of the MT network, IE1 antigen was recovered at a level similar to that observed in the second control experiment at 24 hpi (Fig. 2B, Noc4 and C2). These data suggest that virus had already entered into the cell before proteinase K treatment in the absence of the MT network.
FIG. 2.
HCMV enters into the cytoplasm in the absence of the MT network. Effects of nocodazole removal on expression levels of IE1 were examined with HEL cells infected with HCMV at an MOI of 3 PFU/cell. (A) Schematic representation of sequential treatments with nocodazole, treatment with proteinase K (PK), and inoculation. C1 and C2, controls 1 and 2. (B) Lysates were taken at the times indicated in panel A and subjected to Western blotting analysis to compare IE1 antigens. Relative signal intensities of IE1 normalized by signals of beta-actin are shown. Values represent means ± standard deviations (n = 3 or 4).
FIG.4.
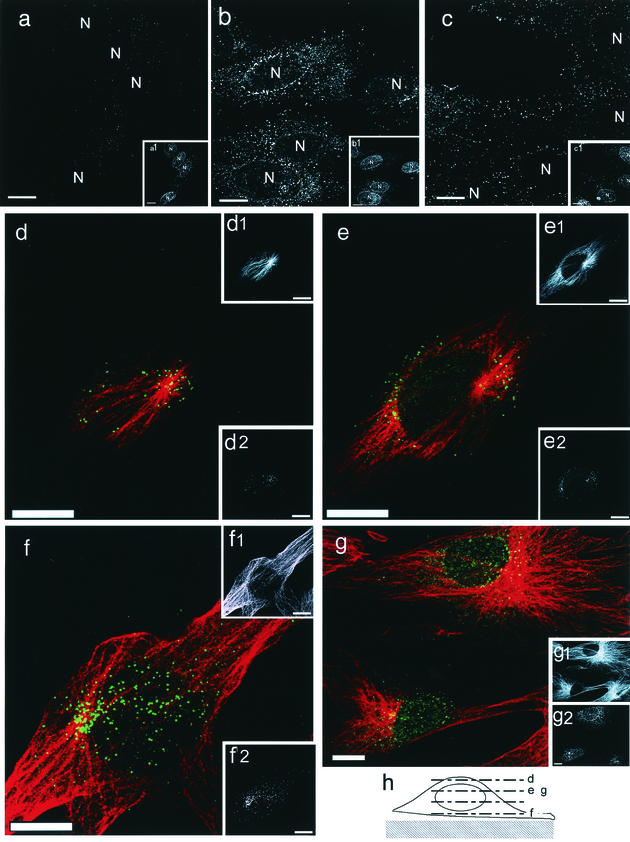
IF analyses of incoming HCMV capsids labeled with anti-pUL32 antibody. (a to c) Untreated (a and b) or nocodazole-treated (c) HEL cells were mock infected (a) or HCMV-infected (b and c) in the presence of 0.5 mM cycloheximide at an MOI of 5 PFU/cell. At 4 hpi, the cells were fixed and costained with anti-pUL32 antibody. (Insets a1 to c1) The nucleus (N) was stained with MAb against the nuclear pore complex. (d to g) HEL cells were infected at an MOI of 5 PFU/cell in the presence of cycloheximide, fixed at 2 (g) or 4 (d to f) hpi, and double labeled with anti-pUL32 antibody (green) and MAb against α-tubulin (red). The laser-scanning microscopy images were sequentially obtained with 1-μm intervals. (d to g) Merged signals. (Insets d1 to g1) α-Tubulin. (Insets d2 to g2) pUL32. (h) Schematic representation of levels of sectioning. The sectioning levels are indicated by dotted lines marked with letters d to g, which correspond to the merged images shown in panels d to g. Colocalization of pUL32-labeled capsids with MTs was clearly demonstrated, especially beneath the nucleus and on the top surface near the MTOC. Bars, 10 μm.
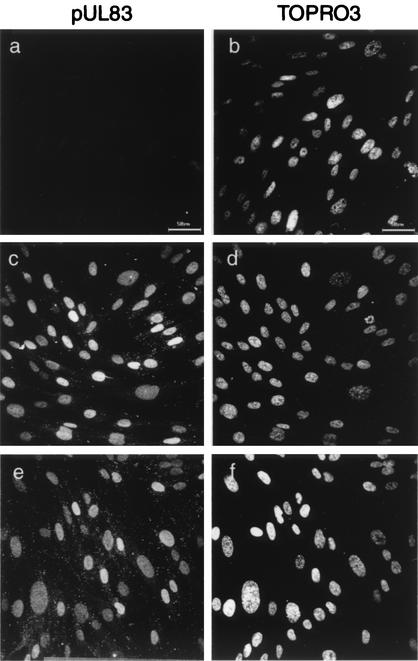
The notion of virus entry in the absence of the MT network was also supported by monitoring pUL83 of the infected cells, which is one of the most abundant tegument components and is released from the capsid upon entry and translocated to the nucleus before IE gene expression (19). IF assays revealed that the pUL83 signal was not changed by nocodazole treatment: pUL83 of the infected cells localized to the nucleus by 2 hpi regardless of MTs (Fig. 3). Thus, HCMV entry into the cells appeared to occur normally without the MT network.
FIG. 3.
pUL83 release in nocodazole-treated HCMV-infected cells. HEL cells were mock infected (a and b) or infected with HCMV at an MOI of 5 PFU/cell in the absence (c and d) or presence (e and f) of 10 μM nocodazole. At 2 hpi, the cells were fixed and stained for pUL83 (a, c, and e). Nuclei were visualized with TOPRO3 (b, d, and f). Bars, 50 μm.
Close association of incoming HCMV capsids with MTs.
It is assumed that the association of incoming HCMV capsids with MTs facilitates transport to the NE, which thereby permits efficient start of IE gene expression. To assess whether the capsids associate with MTs, HEL cells were infected in the presence of cycloheximide and IF assays were carried out. The cytoplasmic capsids were detected by antibodies against pUL32, as has been reported for immunostaining of cytoplasmic HCMV particles (20). pUL32 is one of the major structural tegument proteins which are tightly associated with capsids (2, 5, 9) and is supposed to be retained within the tegument layer of capsids during translocation to the nucleus. The NE was visualized with MAb against the nuclear pore complex. The capsids in the cytoplasm were detected as intensely labeled small spots, as has been reported for HSV-1 capsids (23). Their distribution changed from the cell periphery to the perinuclear region with increasing time. By 4 hpi, most of the pUL32 signal had accumulated in the vicinity of the NE in untreated cells (Fig. 4b). This was in contrast to that in nocodazole-treated cells, where the pUL32 signal was not observed to accumulate around the nucleus but remained throughout the cytoplasm (Fig. 4c). Double staining with tubulin and pUL32 revealed a clear association of virions with MTs (Fig. 4d to g). At 2 hpi, the pUL32 signal began to accumulate around the MTOC, but numerous spots were still distributed in the cytoplasm (Fig. 4g). Accumulation of the pUL32 signal around the nucleus became more prominent at 4 hpi (Fig. 4d to f). Sequential sectioning of confocal laser-scanning microscopy images from the bottom to the top levels of cells as illustrated in Fig. 4h revealed that pUL32 signal association with MTs was most prominent beneath the nucleus and on the top surface near the MTOC.
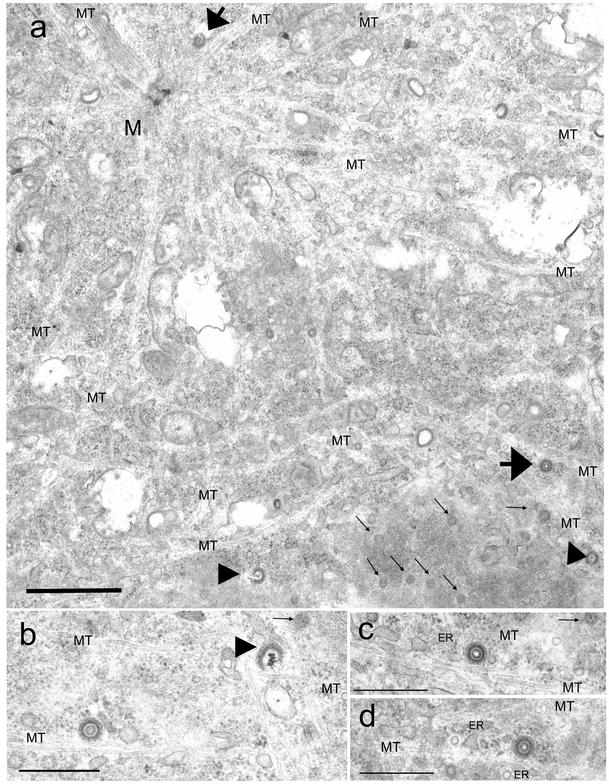
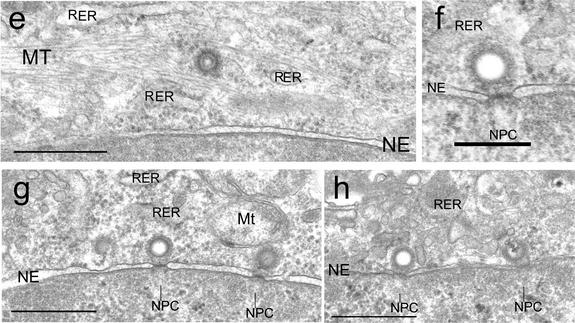
To further assess whether incoming HCMV capsids associate directly with MTs, we analyzed ultrastructurally HCMV-infected cells under conditions of intact MT networks. In agreement with IF analysis data, most cytoplasmic capsids were observed in the vicinity of the NE by 4 hpi (Fig. 5). Notably, some part of MTs directly projected very close to the nuclear pores from the MTOC (Fig. 5a and b) (11). As expected, the incoming capsids were surrounded by a fibrillar tegument coating (Fig. 5a to d), and three types of capsids were observed, that is, capsids with core densities, those without core densities, and broken capsids. In the cytosolic perinuclear region but not at the NE, capsids possessing core densities were closely associated with MTs (Fig. 5b to d) and were sometimes within MT bundles near the nucleus (Fig. 5e). Thus, close association of MTs and cytosolic capsids could be clearly demonstrated ultrastructurally. At the NE, many capsids without core densities directly docked to the nuclear pore complexes, which appears to show that they had already released their contents (Fig. 5f), as has been reported previously for HCMV (4) and for alphaherpesviruses (10, 18, 23). Broken capsids under releasing their contents were observed occasionally in the vicinity of the NE (Fig. 5a and b) or at the nuclear pores (Fig. 5g and h). At 4 hpi, capsids with core densities, those without core densities, and broken capsids were estimated to be about 30, 52, and 18% of the total of 143 capsids examined, respectively.
FIG. 5.
The incoming HCMV capsids closely associated with MTs and an endoplasmic reticulum (ER)-like membrane structure. HEL cells were infected with HCMV in the presence of 0.5 mM cycloheximide at an MOI of 20 PFU/cell and processed for TEM at 4 hpi as described in the text. (a to d) The cytoplasmic capsids were surrounded by a fibrillar tegument layer. In the perinuclear region, MTs projected very close to the nuclear pores (small arrows) from the MTOC (M). The cytoplasmic capsids with core densities (large arrows) were associated with MTs in the vicinity of the nucleus. Three types of capsids, those with core densities, those without core densities, and broken capsids (large arrowheads), were observed. (e) Capsid located in MT bundles near the NE. (f to g) At the NE, capsids without core densities directly docked to the nuclear pore complexes. (g and h) Occasionally, capsids under releasing their contents were also observed. RER, rough endoplasmic reticulum; Mt, mitochondria; NPC, nuclear pore complexes. Bars, 1 μm (a); 0.5 μm (b to e); 0.2 μm (f); 0.5 μm (g and h).
The mechanism of betaherpesvirus transport in the cytoplasm is unknown to date. Here we demonstrate that an intact MT network but not actin filament is required for an efficient onset of the replication cycle of HCMV, as has been previously reported for alphaherpesviruses. In the absence of the MT network, HCMV entry into the cell occurred normally but failed to start efficient IE gene expression. Thus, it appears that an MT-dependent process occurs between viral entry and the onset of IE gene expression, and the most probable one is capsid transport to the nucleus. This is also supported by our morphological studies which clearly demonstrated the close association of incoming HCMV capsids with MTs both by IF analysis and TEM. From our IF analysis data, we know that HCMV capsids began to accumulate at 2 hpi around the MTOC and that by 4 hpi most of the capsids had concentrated in the vicinity of the nucleus, which is similar to the reported kinetics of the nuclear targeting of HSV-1 capsids (23). We propose that HCMV capsids also utilize the MT network to facilitate their own movement to the NE after entry and before IE gene expression. Interestingly, the step between postentry and before IE gene expression was reported as a key step for HCMV replication in endothelial cells (3, 20, 21). It is noteworthy that mutant HCMV lacking major tegument components pUL47 and pUL48 exhibited a defect after entry and before IE gene expression (1). Either or both tegument proteins could function in the capsid movement to the nucleus (1).
An incomplete inhibitory effect of nocodazole on capsid transport has been reported previously for other viruses (13, 23). Our data also showed that a very small but distinct level of IE gene expression occurred in the absence of MTs. There may be a second mechanism for nuclear transport of incoming HCMV. Alternatively, the capsids are transported on short nocodazole-resistant MT filaments (13), whereas our IF assay detected no MT network. Reasons for an incomplete inhibitory effect of nocodazole on IE gene expression remain to be further studied. Since an MT-stabilizing drug, Taxol, showed no effect on IE gene expression, as previously reported for adenoviruses and HSV-1 (23, 24), dynamic instability of MTs was not required for capsid transport.
Little has been studied about ultrastructures of incoming betaherpesvirus capsids upon entry (4), while a number of electron microscopy studies have shown that other incoming viruses associate with MTs (6, 10, 14, 23). This is the first report, to our knowledge, showing the TEM images of incoming HCMV capsids in the cytosol, which presumably represent the way to the NE. Our protocol for preparing TEM samples while keeping an intact MT permitted us to observe easily the accumulated capsids in the perinuclear region. Unlike those of alphaherpesviruses (10, 23), the incoming HCMV capsids in the cytosol retain dense tegument layers similar to those observed in the late replication cycle (9). Unexpectedly, many broken capsids were observed in the perinuclear region, although their origins and characterization remain to be assessed to determine whether they were in a normal process or an abortive one. Recently, specific interaction of tegument components with the motor proteins has been reported for HSV-1 (7). The HCMV-encoded protein(s) (or protein complex) responsible for the association with MTs remains to be identified and is probably located in the tegument layer.
Acknowledgments
We thank W. Hall (Department of Medical Microbiology, University College Dublin) for his critical reading of the manuscript.
REFERENCES
- 1.Bechtel, J. T., and T. Shenk. 2002. Human cytomegalovirus UL47 tegument protein functions after entry and before immediate-early gene expression. J. Virol. 76:1043-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benko, D. M., R. S. Haltiwanger, G. W. Hart, and W. Gibson. 1988. Virion basic phosphoprotein from human cytomegalovirus contains O-linked N-acetylglucosamine. Proc. Natl. Acad. Sci. USA 85:2573-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolovan-Fritts, C., and J. A. Wiedeman. 2001. Human cytomegalovirus strain Toledo lacks a virus-encoded tropism factor required for infection of aortic endothelial cells. J. Infect. Dis. 184:1252-1261. [DOI] [PubMed] [Google Scholar]
- 4.Cavallo, T., K. Graves, N. L. Cole, and T. Albrecht. 1981. Cytomegalovirus: an ultrastructural study of the morphogenesis of nuclear inclusions in human cell culture. J. Gen. Virol. 56:97-104. [DOI] [PubMed] [Google Scholar]
- 5.Chen, D. H., H. Jiang, M. Lee, F. Liu, and Z. H. Zhou. 1999. Three-dimensional visualization of tegument/capsid interactions in the intact human cytomegalovirus. Virology 260:10-16. [DOI] [PubMed] [Google Scholar]
- 6.Dales, S., and Y. Chardonnet. 1973. Early events in the interaction of adenoviruses with HeLa cells. IV. Association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology 56:465-483. [DOI] [PubMed] [Google Scholar]
- 7.Diefenbach, R. J., M. Miranda-Saksena, E. Diefenbach, D. J. Holland, R. A. Boadle, P. J. Armati, and A. L. Cunningham. 2002. Herpes simplex virus tegument protein US11 interacts with conventional kinesin heavy chain. J. Virol. 76:3282-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dohner, K., A. Wolfstein, U. Prank, C. Echeverri, D. Dujardin, R. Vallee, and B. Sodeik. 2002. Function of Dynein and dynactin in herpes simplex virus capsid transport. Mol. Biol. Cell 13:2795-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson, W. 1996. Structure and assembly of the virion. Intervirology 39:389-400. [DOI] [PubMed] [Google Scholar]
- 10.Granzow, H., F. Weiland, A. Jons, B. G. Klupp, A. Karger, and T. C. Mettenleiter. 1997. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J. Virol. 71:2072-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray, E. G., and L. E. Westrum. 1976. Microtubules associated with nuclear pore complexes and coated pits in the CNS. Cell Tissue Res. 168:445-453. [DOI] [PubMed] [Google Scholar]
- 12.Greis, K. D., W. Gibson, and G. W. Hart. 1994. Site-specific glycosylation of the human cytomegalovirus tegument basic phosphoprotein (UL32) at serine 921 and serine 952. J. Virol. 68:8339-8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabit, H., M. Y. Nakano, U. Prank, B. Saam, K. Dohner, B. Sodeik, and U. F. Greber. 2002. Intact microtubules support adenovirus and herpes simplex virus infections. J. Virol. 76:9962-9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miles, B. D., R. B. Luftig, J. A. Weatherbee, R. R. Weihing, and J. Weber. 1980. Quantitation of the interaction between adenovirus types 2 and 5 and microtubules inside infected cells. Virology 105:265-269. [DOI] [PubMed] [Google Scholar]
- 15.Mocarski, E. S. 1996. Cytomegaloviruses and their replication, p. 2447-2492. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 16.Ogawa-Goto, K., Y. Arao, Y. Ito, T. Ogawa, T. Abe, T. Kurata, S. Irie, and H. Akanuma. 1998. Binding of human cytomegalovirus to sulfated glucuronyl glycosphingolipids and their inhibitory effects on the infection. J. Gen. Virol. 79:2533-2541. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa-Goto, K., S. Irie, A. Omori, Y. Miura, H. Katano, H. Hasegawa, T. Kurata, T. Sata, and Y. Arao. 2002. An endoplasmic reticulum protein, p180, is highly expressed in human cytomegalovirus-permissive cells and interacts with the tegument protein encoded by UL48. J. Virol. 76:2350-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ojala, P., B. Sodeik, M. Ebersold, U. Kutay, and A. Helenius. 2000. Herpes simplex virus type 1 entry into host cells: reconstitution of capsid binding and uncoating at the nuclear pore complex in vitro. Mol. Cell. Biol. 20:4922-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmolke, S., P. Drescher, G. Jahn, and B. Plachter. 1995. Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J. Virol. 69:1071-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinzger, C., M. Kahl, K. Laib, K. Klingel, P. Rieger, B. Plachter, and G. Jahn. 2000. Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus. J. Gen. Virol. 81:3021-3035. [DOI] [PubMed] [Google Scholar]
- 21.Slobbe-van Drunen, M. E., A. T. Hendrickx, R. C. Vossen, E. J. Speel, M. C. van Dam-Mieras, C. A. Bruggeman, and M. Terasaki. 1998. Nuclear import as a barrier to infection of human umbilical vein endothelial cells by human cytomegalovirus strain AD169. Virus Res. 56:149-156. [DOI] [PubMed] [Google Scholar]
- 22.Sodeik, B. 2000. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 8:465-472. [DOI] [PubMed] [Google Scholar]
- 23.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suomalainen, M., M. Y. Nakano, S. Keller, K. Boucke, R. P. Stidwill, and U. F. Greber. 1999. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J. Cell Biol. 144:657-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vihinen-Ranta, M., W. Yuan, and C. R. Parrish. 2000. Cytoplasmic trafficking of the canine parvovirus capsid and its role in infection and nuclear transport. J. Virol. 74:4853-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]