Abstract
The DNA sequence of the 15,155-bp O-antigen gene cluster of Escherichia coli O121 was determined, and 14 open reading frames were identified (all had the same transcriptional direction). Analyses of results indicated that the wzx (O-antigen flippase) and wzy (O-antigen polymerase) genes were E. coli O121 specific, so regions in these two genes were chosen for development of PCR assays. The PCR assays using DNA from 99 E. coli O121 strains, strains representative of non-O121 E. coli serogroups, and strains of other bacterial genera and PCR assays using DNA from seven enrichments of swine fecal samples naturally contaminated with E. coli O121 showed specificity for E. coli O121. Thus, the PCR assay can be employed to reliably identify E. coli O121 and to potentially detect the organism in food, fecal, and environmental samples.
More than 200 Escherichia coli serotypes isolated from humans have been identified as Shiga toxin-producing E. coli (STEC), and more than 100 of these serotypes have been associated with human illness (24). E. coli O157:H7 is the most common STEC. In the United States, E. coli O157:H7 is more often associated with hemorrhagic colitis and hemolytic-uremic syndrome (HUS) than any of the other STEC serogroups. However, in other countries, such as Argentina, Germany, and Australia, non-O157 STEC strains have become an important public health problem (2, 5, 7, 9). Unlike E. coli O157:H7, which generally does not ferment sorbitol or have β-glucuronidase activity, the non-O157 STEC strains do not have identifiable biochemical markers to facilitate screening for and identification of these pathogens. Detection of non-O157 STEC requires testing for the Shiga toxins or for genes which encode Shiga toxins, followed by serotyping using antisera produced against the ca. 179 different E. coli serogroups. Thus, due to the lack of simple and rapid methods for detection and identification of non-O157 STEC, the incidence of disease caused by these organisms is likely underestimated.
Shiga toxin-producing E. coli O121 strains are classified as enterohemorrhagic E. coli (EHEC), since they have been isolated from patients with hemorrhagic colitis or HUS (3, 11, 12, 18, 24, 25). Additionally, strains of E. coli O121 serogroup, possessing virulence characteristics similar to those of Shigella and enteroinvasive E. coli, have caused shigellosis-like illnesses (8, 10). In 1999, E. coli O121:H19 was associated with an outbreak of HUS at a lake in Connecticut (11). Due to the public health concern over E. coli O121 infection, assays specific for this serogroup are needed to rapidly and reliably detect this pathogen and to further define its role in causing human illness. Tarr et al. (19) characterized 24 isolates of E. coli O121:H19 and nonmotile variants using multilocus enzyme electrophoresis and multilocus sequencing and found that the isolates represented a single bacterial clone. The isolates possessed a virulence gene profile typical of EHEC clones; however, the results of sequencing analyses showed that the O121:H19 clone did not fall into any of the classical EHEC or enteropathogenic E. coli groups. Tarr et al. suggested that E. coli O121:H19 independently acquired virulence genes and represents a distinct EHEC clone.
The O antigen is the surface polysaccharide side chain of lipopolysaccharide present in gram-negative bacteria, and the H antigen is found on the flagellar protein. Typing E. coli isolates is traditionally performed by serotyping, which relies on agglutination reactions using antisera raised against the 179 O and 56 H serogroup antigens. Serotyping, however, can generally only be performed in specialized laboratories, is labor-intensive, and may require several days to complete, and cross-reactivity of antisera with multiple O or H serogroups frequently occurs. Characteristically, genes specific to O-antigen synthesis are located in the O-antigen gene cluster between the galF and gnd genes on the E. coli chromosome. Determination of the sequence of the genes in the cluster permits identification of unique genes or sequences that can be used to design serogroup-specific PCR assays. These assays can be employed for detection, as well as typing, of E. coli as an alternative to serotyping. Several O-antigen gene clusters have been sequenced, including O55, O91, O104, O111, O113, and O157, and serogroup-specific PCR assays based on genes in the respective O-antigen clusters have been developed (15, 16, 20-23). In the present study, the O-antigen gene cluster of an E. coli serogroup O121 strain was sequenced, and PCR assays using primers based on the wzx and wzy genes in the cluster were designed and used to detect E. coli O121 strains in swine feces.
E. coli O121:H19 strain 96-1585, obtained from the Health Canada Laboratory Centre for Disease Control, Ottawa, Ontario, Canada, was used for sequencing. The PCR results targeting the stx1 and stx2 genes showed that this strain harbors only stx2 (data not shown). Bacteria used to test for specificity of the PCR included 99 E. coli serogroup O121 strains and one or more representative strains from each of the remaining different E. coli serogroups isolated from humans, animals, food, and water. These serogroups included E. coli serogroups O1 to O173, excluding O14, O31, O47, O67, O72, O93, O94, and O122, since these serogroup designations have been eliminated (13) and OX3, OX6, OX7, OX9, OX10, OX13, OX18, OX19, OX21, OX23, OX25, OX28, OX38, and OX43. In addition, strains representative of other bacterial genera including Shigella (three Shigella sonnei strains, three Shigella flexneri strains, two Shigella boydii strains, and two Shigella dysenteriae strains), Salmonella (three Salmonella enterica serovar Typhimurium strains, one S. enterica serovar Enteriditis strain, and one S. enterica serovar Worthington strain), Yersinia (one Yersinia enterocolitica strain), Vibrio (one Vibrio cholerae strain), Pseudomonas (one Pseudomonas fluorescens strain), Erwinia (one Erwinia carotovora strain), Serratia (one Serratia liquefaciens strain), Klebsiella (one Klebsiella pneumoniae strain), Citrobacter (two Citrobacter freundii strains and one Citrobacter braakii strain), and Listeria (one Listeria monocytogenes strain) were tested. The bacteria were from the strain collections at the Gastroenteric Disease Center at Pennsylvania State University in State College and the Microbial Food Safety Research Unit at the Eastern Regional Research Center in Wyndmoor, Pa.
Sequence and analyses.
E. coli O121:H19 strain 96-1585 was grown for 18 h in Luria-Bertani broth (Difco, Detroit, Mich.) at 37°C, and genomic DNA was extracted using the High Pure PCR template preparation kit (Roche Applied Science, Indianapolis, Ind.). Long PCR was performed using the Expand Long Template PCR system (Roche Applied Science) and JUMPSTART (named for just upstream of many polysaccharide-associated gene starts) and 6-phosphogluconate dehydrogenase (GND) primers (23), resulting in a ca. 15,000-bp PCR product. The JUMPSTART sequence is a conserved 39-bp region present upstream of a number of polysaccharide gene clusters, and the gnd locus, which encodes an enzyme of the pentose phosphate shunt, GND, is found downstream of the O-antigen gene clusters of E. coli and Salmonella. The JUMPSTART sense primer sequence was 5′-ATTGGTAGCTGTAAGCCAAGGGCGGTAGCGT-3′, and the GND antisense primer was 5′-CACTGCCATACCGACGACGCCGATCTGTTGCTTGG-3′ (Invitrogen, Carlsbad, Calif.).
The PCR mixture consisted of 5 μl of 10× buffer 2, 0.5 mM each of the four deoxynucleotide triphosphates (dNTPs), 0.4 μM each of the JUMPSTART and GND primers, 0.75 μl of enzyme mix, and 15 μl of template DNA. The PCR was performed in a GeneAmp PCR System 9600 thermal cycler (Applied Biosystems, Foster City, Calif.) using a total reaction mixture volume of 50-μl. The temperature cycling protocol consisted of the following steps: (i) an initial step of 2 min at 94°C; (ii) 30 cycles, with 1 cycle consisting of 10 s at 94°C, 30 s at 60°C, and 15 min at 68°C; and (iii) a final extension step of 7 min at 68°C.
The long PCR products were cleaned using the Quickstep II PCR purification kit (Edge Biosystems, Gaithersburg, Md.), and all of the DNA was used in a single DNase I digestion experiment. DNase I digestion was performed using the DNase Shotgun cleavage kit (Novagen, Madison, Wis.) according to the manufacturer's instructions. The DNA sized from 2 to 3.5 kb was excised from each lane of an agarose gel using a scalpel and purified from the gel using the QIAEX II gel cleanup kit (Qiagen, Valencia, Calif.). The DNA from a single lane was resuspended in 19 μl of water. Using the Single dA cloning kit (Novagen, Madison, Wis.), the flushing reaction was performed, followed by the single A tailing reaction. After end tailing was finished, the enzymes were deactivated by incubation at 70°C for 20 min, and the DNA was then extracted with 75 μl of chloroform. Ligations were performed following the manufacturer's instructions using the pGEM-T Easy kit (Promega, Madison, Wis.). The pGEM-T vector contains single 3′-T overhangs at the insertion site.
Transformation of ligated DNA into library-efficiency, chemically competent E. coli DH5α cells was performed using the manufacturer's instructions (Invitrogen). Plasmids were purified using a Qiagen BioRobot 9600 and Qiagen 96 Turbo protocol. The plasmids were sequenced using an Applied Biosystems 3700 automated DNA sequencer and the Big Dye Terminator sequencing kit (Applied Biosystems). One hundred ninety-two clones were sequenced (two 96-well plates). The sequence data were imported into Sequencher software (Genecodes, Ann Arbor, Mich.) for quality assessment, vector trimming, and assembly. Primers were designed to fill gaps not covered by the random shotgun clones, and PCR products were sequenced until all gaps were filled. The assembled sequences were imported into Artemis (17), the open reading frames were located, and the putative coding regions were analyzed using the National Center for Biotechnology Information (NCBI) BLASTP program against the nonredundant database (1).
Fourteen open reading frames were identified as encoded by the 15,155-bp O-antigen gene cluster of E. coli O121 with all having the same postulated transcriptional direction (GenBank accession no. AY208937). The genes within the cluster, identified with various degrees of precision, are shown in Table 1. There were seven transferase genes and O-antigen flippase (wzx) and O-antigen polymerase (wzy) genes. The putative wzx ORF of E. coli O121:H19 strain 96-1585 had only 21% identity with the published homolog of E. coli O55:H7, and the wzy ORF of E. coli O121:H19 had 20% identity with wzy of E. coli O7:K1. Open reading frame 6 had 35% identity with a hypothetical protein from Bordetella, so the function of this gene in E. coli O121 is currently unknown. Gene names were assigned on the basis of the Bacterial Polysaccharide Gene Nomenclature scheme (http://www.microbio.usyd.edu.au/BPGD/big_paper.pdf).
TABLE 1.
Summary of genes in E. coli O121 O-antigen gene cluster
| ORFa | Proposed gene name | Locationb | No. of amino acids | Putative function | Most significant homolog (accession no.)c | % Identity/% similarityd |
|---|---|---|---|---|---|---|
| 1 | wbqA | 153-1433 | 426 | UDP-glucose-6-dehydrogenase | WbgT from Shigella sonnei (AAG17418) | 71/84 |
| 2 | wbqB | 1449-2498 | 349 | UDP-glucose-4-epimerase | WbgU from Shigella sonnei (AAG17419) | 70/82 |
| 3 | rmlB | 2501-3589 | 362 | dTDP-glucose-4,6-dehydratase | RmlB from Salmonella enterica (AAG09513) | 71/81 |
| 4 | rmlA | 3556-4455 | 299 | Glucose-1-phosphate thymidylyltransferase | RmlA from Aeromonas hydrophila (AAM22545) | 72/85 |
| 5 | vioA | 4455-5558 | 367 | dTDP-4-amino-4,6-dideoxyglucose aminotransferase | VioA from Pseudomonas aeruginosa (AAK15326) | 58/75 |
| 6 | wbqC | 5555-6271 | 208 | Unknown function | Hypothetical protein from Bordetella (CAA07645) | 35/51 |
| 7 | wbqD | 6261-6683 | 140 | Acetyltransferase | Acetyltransferase from Thermoanaerobacter tengcongensis (AAM23813) | 37/53 |
| 8 | wzx | 6793-8187 | 464 | O-antigen flippase | Wzx from E. coli O55:H7 (AAL67558) | 21/42 |
| 9 | wbqE | 8193-9083 | 296 | Glycosyltransferase | Glycosyltransferase from Clostridium acetobutylicum (AAK80301) | 35/59 |
| 10 | wbqF | 9086-9607 | 173 | Acetyltransferase | Acetyltransferase from Thermotoga maritima (AAD35754) | 39/59 |
| 11 | wzy | 9639-10829 | 396 | O-antigen polymerase | Wzy from E. coli O7:K1 (AAD44158) | 20/47 |
| 12 | wbqG | 10837-12729 | 630 | Asparagine synthase | WbpS from Pseudomonas aeruginosa (AAF24002) | 55/70 |
| 13 | wbqH | 12684-13841 | 385 | Glycosyltransferase | WbpT from Pseudomonas aeruginosa (AAF23993) | 46/68 |
| 14 | wbqI | 13842-14966 | 374 | Glycosyltransferase | WbpU from Pseudomonas aeruginosa (AAF23992) | 53/70 |
ORF, open reading frame.
Location of the open reading frame in nucleotides.
Sequences can be accessed via Entrez at the NCBI website (http://www.ncbi.nih.gov/).
Percent identity and percent similarity of the open reading frame to the most significant homolog.
The structure of the E. coli O121 O-specific polysaccharide has been determined (14) and shown to be very similar to that of S. dysenteriae type 7. The chemical structure consists of the following repeating unit: →4)-α-d-GalpNAcAN-(1→4)-α-d-GalpNAcA-(1→3)-α-d-GlcpNAc-(1→3)-β-d-Quip4NAcGly-(1→ and having an O-acetyl group located on O-3 of the GalNAcAn. The only difference between the chemical structures of the O antigen of S. dysenteriae type 7 and that of E. coli O121 was that O acetylation in the repeating unit of S. dysenteriae type 7 was stoichiometric (14). The structure of the E. coli O121 repeating unit was not confirmed in this study using E. coli O121:H19 strain 96-1585.
Selection of PCR primers and specificity testing.
In other studies, PCR assays using primers based on the wzx and wzy genes were serogroup specific (20, 21, 23). Sequence similarity analyses were performed comparing the E. coli O121 wzx and wzy genes to similar genes in other E. coli serogroups, and results demonstrated that there were unique regions in the E. coli O121 genes. Therefore, two sets of oligonucleotide primers complementary to each of the genes were designed and used in PCR assays to determine the specificity for E. coli O121 (Table 2). The PCR testing was performed at the Gastroenteric Disease Center. Template DNA from the bacteria was prepared by mixing a colony in sterile distilled water and heating at 100°C for 20 min. The PCR was performed in a RapidCycler (Idaho Technology, Inc., Salt Lake City, Utah) using total reaction mixture volumes of 11 μl. The PCR mixture consisted of 3 μl of template DNA, 0.5 μM (each) primers (Integrated DNA Technologies, Inc., Coralville, Iowa) (Table 2), 0.18 mM each of the four dNTPs, 3.0 mM MgCl2, 0.4 U of Taq DNA polymerase (PGC Scientifics, Frederick, Md.), 50 mM Tris (pH 8.3), 250 μg of bovine serum albumin per ml, 2% sucrose, and 0.1 mM cresol red (Idaho Technology, Inc.). One primer set was used for each of the PCR assays. The thermal cycling protocol was performed using the rapid cycle DNA amplification method and consisted of the following steps: (i) an initial denaturation step of 30 s at 94°C; (ii) 30 cycles, with 1 cycle consisting of template denaturation at 94°C for 0 s, primer annealing (at 57°C for 0 s for the O121wzx1 and O121wzx2 primer sets, 63°C for 0 s for the O121wzy1 set, and 58°C for 0 s for the O121wzy2 set), and primer extension at 72°C for 13 s, and a final extension step of 30 s at 72°C. The PCR products were visualized following electrophoresis through 1% agarose gels stained with ethidium bromide.
TABLE 2.
Oligonucleotide primers used for amplification of the E. coli O121 wzx and wzy genes
| Oligonucleotide primer set | Sequence (5′ to 3′) | Target gene | Base position of primers | Expected size of PCR product (bp) |
|---|---|---|---|---|
| O121wzx1F | AGGCGCTGTTTGGTCTCTTA | wzx | 152-461 | 310 |
| O121wzx1R | TCGCTACCGCTAATGATTCC | |||
| O121wzx2F | TGGCTAGTGGCATTCTGATG | wzx | 821-1142 | 322 |
| O121wzx2R | TGATACTTTAGCCGCCCTTG | |||
| O121wzy1F | AGCCGGTAGTGTTGAAAGGA | wzy | 327-625 | 299 |
| O121wzy1R | CTTCAATGAGTGCAGGCAAA | |||
| O121wzy2F | GCAATGAGGACCGGTATATCTC | wzy | 634-951 | 318 |
| O121wzy2R | CACGCCCGTGTTAATATTCC |
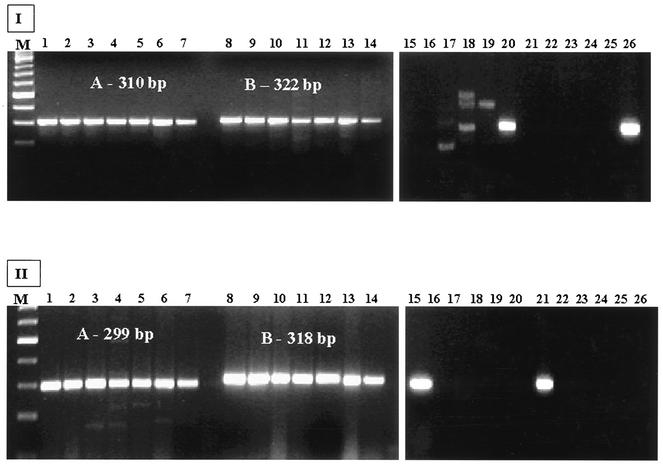
Ninety-nine E. coli strains serogrouped as E. coli O121 at the Gastroenteric Disease Center isolated from humans, water, cider, and different animal species were tested by PCR using the primer sets designed in this study targeting the E. coli O121 wzx and wzy genes. PCR results showed high specificity for E. coli O121 with no amplification of wzx and wzy genes from other E. coli serogroups and no amplification with DNA of other bacterial genera (Fig. 1). However, when primer set O121wzx2F-O121wzx2R was used, nonspecific bands were visible on agarose gels using DNA from C. braakii ATCC 43162, E. coli O111:NM strain 91.1030, and S. flexneri ATCC 12022. Although of lower intensity, a band of ca. 322 bp in length, the size of the expected amplicon using this primer set, was visible using DNA from E. coli O111:NM strain 91.1030. Although this band was not visible following PCR using DNA from eight other E. coli O111 strains tested (data not shown), it may be preferable to employ primer set O121wzx1F-O121wzx1R, O121wzy1F-O121wzy1R, or O121wzy2F-O121wzy2R rather than O121wzx2F-O121wzx2R for detection or typing of E. coli O121. Although there may be similarities between the O antigen of S. dysenteriae and E. coli O121 (14), amplification products of the expected sizes of 310, 322, 299, or 318 bp were not obtained using DNA from S. dysenteriae, S. flexneri, S. boydii, and S. sonnei. These findings indicate that the PCR assays are suitable for DNA-based typing of E. coli O121. It may be possible to combine the primer sets for the wzx or wzy genes with primers for the Shiga toxin genes and/or other virulence genes in E. coli O121 in a multiplex PCR format.
FIG. 1.
Agarose gel showing PCR results of DNA from seven E. coli O121-positive swine fecal samples using two primer sets for the wzx gene and two primer sets for the wzy gene. (I) Lanes 1 to 7 and 8 to 14, PCR products using DNA from isolates K84-9 O121:H10, K84-11 O121:H10, K84-12 O121:H10, K84-36 O121:H10, K84-40 O121:H10, K102-27 O121:H−, and K150-1 O121:H−, respectively, and primer sets O121wzx1F-O121wzx1R (A) and O121wzx2F-O121wzx2R (B). Lanes 15 to 20 and 21 to 26, PCR products using DNA from E. coli O103:H3 93-0626, C. freundii ATCC 33128, C. braakii ATCC 43162, E. coli O111:NM 91.0130, S. flexneri ATCC 12022, and E. coli O121:H19 96-1585, respectively, and primer sets O121wzx2F-O121wzx2R and O121wzx1F-O121wzx1R, respectively. (II) Lanes 1 to 7 and 8 to 14, PCR products using DNA from the same swine fecal isolates described above for panel I and primer sets O121wzy1F-O121wzy1R (A) and O121wzy2F-O121wzy2R (B), respectively. Lanes 15 to 20 and 21 to 26, PCR products using DNA from E. coli O121:H19 96-1585, E. coli O103:H3 93-0626, C. freundii ATCC 33128, C. braakii ATCC 43162, E. coli O111:NM 91.0130, and S. flexneri ATCC 12022, respectively, and primer sets O121wzy1F-O121wzy1R and O121wzy2F-O121wzy2R, respectively. Lanes M, 100-bp ladder molecular size standards (Invitrogen).
PCR to detect E. coli O121 in swine fecal samples.
Since swine can harbor non-O157 STEC that could potentially cause human illness (4, 6), a study is currently being conducted in our laboratory to examine the prevalence of STEC in swine in the United States. Briefly, fecal samples collected from swine operations from 13 of the top 17 producing states were subjected to enrichment by adding 10 g of feces to 90 ml of tryptic soy broth (TSB; BD Diagnostics Systems, Sparks, Md.) and incubating at 37°C for 12 h at 150 rpm. Shiga toxin-producing bacteria, isolated by colony hybridization using digoxigenin-labeled DNA probes for the stx1 and stx2 genes, were sent to the Gastroenteric Disease Center for serotyping. Seven isolates from seven different fecal samples were serogrouped as E. coli O121. The seven enrichments that had been stored at −80°C were then tested by the E. coli O121-specific PCR assays designed in this study. A portion (500 μl) of the frozen enrichment was added to 5 ml of TSB, and the samples were incubated at 37°C for 4 h. One milliliter of this enrichment was subjected to DNA extraction using the PrepMan Ultra reagent (Applied Biosystems) according to the manufacturer's instructions.
The PCR was performed in a GeneAmp PCR system 9600 thermal cycler (Applied Biosystems) using a total reaction mixture volume of 50 μl. The PCR mixture consisted of 5 μl of template DNA, 0.5 μM (each) primer (Invitrogen), 0.2 mM each of the four dNTPs, 3.0 mM MgCl2, 1.25 U of Taq DNA polymerase (Invitrogen), 20 mM Tris-HCl (pH 8.4), and 50 mM KCl. The thermal cycling protocol consisted of the following steps: (i) an initial denaturation step of 2 min at 94°C; (ii) 35 cycles, with 1 cycle consisting of 20 s at 94°C, annealing (1 min at 60°C), and extension (1 min at 72°C); and (iii) a final extension step of 10 min at 72°C. The PCR products were visualized following electrophoresis through 1.5% agarose gels stained with ethidium bromide.
All seven swine fecal enrichment samples that contained E. coli O121, determined by colony hybridization using DNA probes complementary to the stx1 or stx2 gene and serotyping of the isolates, were positive by PCR using the four primer sets designed in this study for amplification of portions of the wzx and wzy genes in the O-antigen gene cluster of E. coli O121 (Fig. 1). The strains and serotypes of the seven isolates follow: K84-9 O121:H10, K84-11 O121:H10, K84-12 O121:H10, K84-36 O121:H10, K84-40 O121:H10, K102-27 O121:H−, and K150-1 O121:H−. In addition, to rapidly identify an isolate as an enterohemorrhagic E. coli O121 strain, multiplex PCR assays targeting the Shiga toxin genes and genes in the O121 O-antigen gene cluster can be employed. This type of identification cannot easily be performed using serotyping methods.
In conclusion, in addition to providing information regarding the evolution of the O-antigen locus genes in E. coli and correlating chemical diversity with the genetic diversity (22), the DNA sequences of the genes in the O-antigen gene clusters can be utilized to design PCR-based assays for the detection or identification of specific E. coli serogroups. In this study, PCR assays were developed to detect or identify E. coli serogroup O121 on the basis of the wzx and wzy genes in the E. coli O121 O-antigen gene cluster. The PCR assays were used to detect E. coli O121 in swine fecal samples. Thus, use of the PCR assays provides the ability to detect, identify, and type this serogroup, eliminating the use of the more labor-intensive serotyping procedure.
Acknowledgments
Mention of a brand and/or firm name is not an endorsement by the U.S. Department of Agriculture over others of a similar nature not mentioned.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutin, L., S. Zimmermann, and K. Gleier. 1998. Human infections with Shiga toxin-producing Escherichia coli other than serogroup O157 in Germany. Emerg. Infect. Dis. 4:635-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornu, G., W. Proesmans, A. Dediste, F. Jacobs, J. Van DeWalle, A. Mertens, J. Ramet, and S. Lauwers. 1999. Hemolytic uremic syndrome in Belgium: incidence and association with verocytotoxin-producing Escherichia coli infection. Clin. Microbiol. Infect. 5:16-22. [DOI] [PubMed] [Google Scholar]
- 4.DesRosiers, A., J. M. Fairbrother, R. P. Johnson, C. Desautels, A. Letellier, and S. Quessy. 2001. Phenotypic and genotypic characterization of Escherichia coli verotoxin-producing isolates from humans and pigs. J. Food Prot. 64:1904-1911. [DOI] [PubMed] [Google Scholar]
- 5.Elliott, E. J., R. M. Robins-Browne, E. V. O'Loughlin, V. Bennett-Wood, J. Bourke, P. Henning, G. G. Hogg, J. Knight, H. Powell, D. Redmond, and Contributors to the Australian Paedriatic Surveillance Unit. 2001. Nationwide study of haemolytic uraemic syndrome: clinical, microbiological, and epidemiological features. Arch. Dis. Child. 85:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garbal, J. I., E. A. Gonzalez, F. Vazquez, J. Blanco, M. Blanco, and J. E. Blanco. 1996. Serogroups of Escherichia coli isolated from piglets in Spain. Vet. Microbiol. 48:113-123. [DOI] [PubMed] [Google Scholar]
- 7.Gerber, A., H. Karch, F. Allerberger, H. M. Verweyen, and L. B. Zimmerhackl. 2002. Clinical course and the role of Shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997-2000, in Germany and Austria: a prospective study. J. Infect. Dis. 186:493-500. [DOI] [PubMed] [Google Scholar]
- 8.Hiruta, N., M. Himori, M. Habutsu, N. Okamura, M. Ogawa, S. Matsushita, and Y. Kudoh. 1991. Enteroinvasive Escherichia coli O121:H85− isolated from traveller's diarrhea. Kansenshogaku Zasshi 65:537-539. [DOI] [PubMed] [Google Scholar]
- 9.López, E. L., M. M. Contrini, and M. F. De Rosa. 1998. Epidemiology of Shiga toxin-producing Escherichia coli in South America, p. 30-37. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
- 10.Matsushita, S., S. Yamada, A. Kai, and Y. Kudoh. 1993. Invasive strains of Escherichia coli belonging to serotype O121:NM. J. Clin. Microbiol. 31:3034-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy, T. A., N. L. Barrett, J. L. Hadler, B. Salsbury, R. T. Howard, D. W. Dingman, C. D. Brinkman, W. F. Bibb, and M. L. Carter. 2001. Hemolytic-uremic syndrome and Escherichia coli O121 at a lake in Connecticut, 1999. Pediatrics 108:E59.. [DOI] [PubMed] [Google Scholar]
- 12.Novicki, T. J., J. A. Daly, S. L. Mottice, and K. C. Carroll. 2000. Comparison of sorbitol MacConkey agar and a two-step method which utilizes enzyme-linked immunosorbent assay toxin testing and a chromogenic agar to detect and isolate enterohemorrhagic Escherichia coli. J. Clin. Microbiol. 38:547-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ørskov, I., F. Ørskov, B. Jann, and K. Jann. 1977. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol. Rev. 41:667-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parolis, H., L. A. S. Parolis, and G. Olivieri. 1997. Structural studies on the Shigella-like Escherichia coli O121 O-specific polysaccharide. Carbohydr. Res. 303:319-325. [DOI] [PubMed] [Google Scholar]
- 15.Paton, A. W., and J. C. Paton. 1999. Molecular characterization of the locus encoding biosynthesis of the lipopolysaccharide O antigen of Escherichia coli serotype O113. Infect. Immun. 67:5930-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perelle, S., F. Dilasser, J. Grout, and P. Fach. 2002. Identification of the O-antigen biosynthesis genes of Escherichia coli O91 and development of a O91 PCR serotyping test. J. Appl. Microbiol. 93:758-764. [DOI] [PubMed] [Google Scholar]
- 17.Rutherford, K., J. Parkhill, J. Crook, T. Horsnell, P. Rice, M.-A. Rajandream, and B. Barrell. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944-945. [DOI] [PubMed] [Google Scholar]
- 18.Stock, K. J., M. A. Scott, S. F. Davis, R. N. Pierson, and J. S. Dummer. 2001. Hemorrhagic colitis due to a novel Escherichia coli serotype (O121:H19) in a transplant patient. Transpl. Int. 14:44-47. [DOI] [PubMed] [Google Scholar]
- 19.Tarr, C. L., T. M. Large, C. L. Moeller, D. W. Lacher, P. I. Tarr, D. W. Acheson, and T. S. Whittam. 2002. Molecular characterization of a serotype O121:H19 clone, a distinct Shiga toxin-producing clone of pathogenic Escherichia coli. Infect. Immun. 70:6853-6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, L., C. E. Briggs, D. Rothemund, P. Fratamico, J. B. Luchansky, and P. R. Reeves. 2001. Sequence of the E. coli O104 antigen gene cluster and identification of O104 specific genes. Gene 270:231-236. [DOI] [PubMed] [Google Scholar]
- 21.Wang, L., H. Curd, W. Qu, and P. R. Reeves. 1998. Sequencing of Escherichia coli O111 O-antigen gene cluster and identification of O111-specific genes. J. Clin. Microbiol. 36:3182-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, L., S. Huskic, A. Cisterne, D. Rothemund, and P. R. Reeves. 2002. The O-antigen gene cluster of Escherichia coli O55:H7 and identification of a new UDP-GlcNAc C4 epimerase. J. Bacteriol. 184:2620-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, L., and P. R. Reeves. 1998. Organization of Escherichia coli O157 O-antigen gene cluster and identification of its specific genes. Infect. Immun. 66:3545-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. 1998. Zoonotic non-O157 Shiga toxin-producing Escherichia coli (STEC). Report of a WHO Scientific Working Group Meeting, 23 to 26 June 1998, Berlin, Germany. [Online.] http://www.who.int/emc-documents/zoonoses/docs/whocsraph988.html/shigaindex.html.
- 25.Yatsuyanagi, J., Y. Kinouchi, S. Saito, Y. Suzuki, H. Sato, and Y. Miyajima. 1999. Epidemiological characteristics and virulence factor of verotoxin-producing Escherichia coli O121:H19 isolated in Akita Prefecture in July 1997. Kansenshogaku Zasshi 73:218-224. [DOI] [PubMed] [Google Scholar]