Abstract
Transport of the recycling marker transferrin was analysed in polarized hepatic HepG2 cells using quantitative fluorescence microscopy and mathematical modelling. A detailed map and kinetic model for transport of transferrin in hepatic cells was developed. Fluorescent transferrin was found to be transported sequentially through basolateral SE (sorting endosomes) to a SAC/ARC (subapical compartment/apical recycling compartment). DiI (di-indocarbocyanine) lipid probes of different acyl chain length (DiIC12 and DiIC16) co-localized with transferrin in basolateral SE and in the SAC/ARC. By kinetic comparison of hepatic transport of transferrin and labelled HDL (high-density lipoprotein), it is shown that transport of transferrin from SE to the SAC/ARC follows a default pathway together with HDL. Kinetic modelling of fluorescence data provides an identical half-time for SE-to-SAC/ARC transport of transferrin and fluorescent HDL (t½=4.2 min). Fluorescent transferrin was found to recycle with a half-time of t½=12.9 min from the SAC/ARC to the basolateral cell surface of HepG2 cells. In contrast with HDL, targeting of labelled transferrin from the SAC/ARC to the apical biliary canaliculus was negligible. The results indicate that transport from basolateral hepatic SE to the SAC/ARC represents a bulk flow process and that polarized sorting occurs mainly at the level of the SAC/ARC.
Keywords: endocytic recycling, endocytosis, mathematical model, hepatic cell, protein sorting, transferrin
Abbreviations: Alexa488, Alexa Fluor® 488; Alexa488-HDL, Alexa488-labelled HDL; Alexa546-Tf, Alexa546-labelled transferrin; BC, biliary canaliculus; C6-NBD-SM, 6-[N-(7-nitro-2,1,3-benzoxadiazol-4-yl) amino]hexanoylsphingosyl-phosphorylcholine; DiI, di-indocarbocyanine; DMEM, Dulbecco's modified Eagle's medium; DOF, depth of field; ERC, endocytic recycling compartment; FCS, fetal calf serum; Fl-dextran, fluorescein-labelled dextran; HDL, high-density lipoprotein; LE/LYS, late endosomes and lysosomes; MDCK, Madin–Darby canine kidney; NA, numerical aperture; PAFA, paraformaldehyde; pIgA-R, polymeric IgA receptor; ROI, regions of interest; SAC/ARC, subapical compartment/apical recycling compartment; SE, sorting endosomes
INTRODUCTION
The plasma membrane of epithelial cells is divided into morphologically and functionally distinct regions or domains. The apical membrane faces the external environment like the lumen of the gastrointestinal tract, the lateral membrane binds to adjacent cells by cell–cell contacts and the basal membrane attaches epithelial cells to the substratum and provides contact to the capillary system and blood vessels. The functional diversity of the plasma membrane domains of polarized cells is accompanied by large differences in the protein and lipid composition, which are maintained by active transport processes. Efficient sorting mechanisms must exist to ensure maintenance of a polarized lipid and protein distribution.
Hepatocytes are a specific type of epithelial cell mediating biliary secretion of proteins and lipids as well as of toxic compounds at their apical (canalicular) membrane into the bile. In vitro cell-culture models of hepatocytes have been extensively used to study intrahepatic transport. WIF-B and HepG2 cells are well-polarized hepatocytic cells which form both an apical vacuole resembling closely the BC (biliary canaliculus) of hepatocytes [1,2]. Apical membrane proteins like the pIgA-R (polymeric IgA receptor), 5′-nucleotidase, APN (aminopeptidase N) or dipeptidyl peptidase IV become delivered to the canalicular membrane via a SAC/ARC (subapical compartment/apical recycling compartment) [1,3]. Recently we found evidence for transcytosis of a portion of HDL (high-density lipoprotein) to the BC in polarized HepG2 cells [4]. Alexa488-HDL [Alexa488 (Alexa Fluor® 488)-labelled HDL] became internalized into transferrin-containing vesicles and accumulated in the SAC/ARC within 10 min. Fluorescent sphingolipids, analogues of phosphatidylcholine and the natural cholesterol analogue dehydroergosterol also co-localized with fluorescent transferrin in this subapically located compartment [5,6].
Transferrin is a well-known marker for SE (sorting endosomes) and the ERC (endocytic recycling compartment), and it has been used previously to characterize the kinetics of endocytic transport in non-polarized (hepatic) cells [7–10]. From studies in non-polarized Chinese-hamster ovarian cells expressing the human transferrin receptor (called TRVb-1 cells) a detailed trafficking scheme and kinetic map for endocytosis and recycling of transferrin has been developed using quantitative fluorescence microscopy and image analysis [11–13]. Similarly, in polarized MDCK (Madin–Darby canine kidney) cells, endocytic transport routes have been quantitatively assessed using transferrin as marker combined with mathematical modelling [14]. It has been suggested that transferrin recycles from basolateral SE as well as from apical recycling endosomes, while it co-localizes with the transcytotic marker pIgA-R in both compartments in MDCK cells [14,15]. It was proposed that the preferred basolateral distribution of transferrin (∼70% of total) compared with pIgA-R (∼10% of total) is maintained by default recycling of transferrin but not pIgA-R from the SAC/ARC to the basolateral cell surface of MDCK cells [13,14]. In contrast, transport of pIgA-R and transferrin from the SAC/ARC to the apical membrane should occur with similar kinetics. This conclusion was questioned in other studies where a separate apical recycling endosome accessible for pIgA-R but not for transferrin has been proposed for the same cell line [16,17].
Compared with this detailed knowledge of transport routes in polarized kidney cells, insight into kinetics of polarized transport in hepatic cells is very limited. This is partly caused by the fact that the BC, due to its closed geometry, cannot be accessed in tracer studies, and no apical chase medium is present. Therefore analysis of hepatic transport in cell-culture experiments largely relies on (quantitative) fluorescence microscopy [3–6,18–20]. Using this approach combined with mathematical modelling it is shown here that fluorescent transferrin is sequentially transported from basolateral SE to the SAC/ARC in polarized hepatic HepG2 cells. This transport occurs with very similar kinetics like that of HDL as verified by fluorescence ratio imaging of labelled HDL versus transferrin in endosome populations. Moreover, transferrin co-localizes with fluorescent lipid probes of different acyl chain length along the endocytic recycling pathway. Together, these results indicate that the pathway from basolateral SE to the SAC/ARC resembles a default bulk flow process as previously shown for non-polarized cells [21]. A small percentage of transferrin is slowly exported from the SAC/ARC to the BC with an apparent half-time of t½=157 min. A sequential kinetic model previously developed for polarized trafficking of HDL was extended to include recycling of transferrin from SE as well as from the SAC/ARC to the basolateral cell surface. Based on these results it is suggested that the slow exit of transferrin from the SAC/ARC to the apical (canalicular) membrane and its recycling from the SAC/ARC to the basolateral cell surface determine the polarized distribution of transferrin in hepatic cells.
MATERIALS AND METHODS
Reagents
DiIC12(3) (1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate), DiIC16(3) (1,1′-dihexadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate), succinimidyl esters of Alexa488 and Alexa546 were purchased from Molecular Probes (Eugene, OR, U.S.A.). Buffer medium contained 150 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM glucose and 20 mM Hepes (pH 7.4). Release medium is buffer medium supplemented with 25.5 mM citric acid, 24.5 mM sodium citrate and 100 mM deferoxamine mesylate; it was adjusted to pH 5.2 and contained 280 mM sucrose instead of glucose (see above). Fl-dextran (fluorescein-labelled dextran) (70 kDA) was dissolved in PBS and repeatedly dialysed before use to remove unconjugated dye. FCS (fetal calf serum) and DMEM (Dulbecco's modified Eagle's medium) were from Gibco BRL (Life Technologies, Paisley, Scotland, U.K.). All other chemicals were from Sigma (St. Louis, MO, U.S.A.). Transferrin was iron-loaded as previously described [7]. Succinimidyl ester of Alexa546 was then conjugated to the iron-loaded transferrin following the manufacturer's instructions. Human HDL3 was kindly provided by Dr David Silver and Dr Ira Tabas (Columbia University, New York, U.S.A.). It was labelled with Alexa488 following the manufacturer's instructions. Alexa488-HDL was purified by gel filtration on a Sephadex B column and dialysed three times against PBS at 4 °C overnight.
Cell culture
HepG2 cells were grown in DMEM with 4.5 g/l glucose, supplemented with 10% (v/v) heat-inactivated FCS and antibiotics. Cells were routinely passaged in plastic tissue culture dishes. For experiments, cells were plated on to glass coverslips coated with poly(D-lysine) and used after reaching the highest degree of polarization as described previously [6].
Transport experiments
Co-localization of Alexa488-HDL with Alexa546-Tf
Cells were co-labelled with 2 μg/ml Alexa488-HDL and with 5 μg/ml Alexa546-Tf (Alexa546-labelled transferrin) for 1 min at 37 °C, washed and chased at 37 °C in buffer medium for the indicated time points.
Co-localization of DiI (di-indocarbocyanine) lipids with Alexa488-Tf
Labelling solutions for DiI lipids were prepared as previously described [5,22]. Cells were co-labelled with 5 μg/ml Alexa488-Tf and either DiIC12 or DiIC16 for 1 min at 37 °C. Cells were washed and chased in the presence of Alexa488-Tf for 1, 15 or 30 min at 37 °C. Cells were washed and imaged as described below. The same approach was used to determine transport kinetics of Alexa488-Tf to intracellular compartments. Here, DiIC12 was used as a mask to identify the BC and the SAC/ARC as described in [4]. Cells were washed, incubated with 5 μg/ml Alexa488-Tf and DiIC12 for 1 min at 37 °C, washed and chased at 37 °C in buffer medium for the indicated time points.
Co-localization of DiI lipids with Fl-dextran
HepG2 cells were prelabelled with 4 mg/ml Fl-dextran for 1 h at 37 °C to label the lysosomal pathway. Cells were washed with buffer medium and labelled with either DiIC12 or DiIC16 for 1 min at 37 °C. Cells were washed again and chased for 30 min at 37 °C. Cells were fixed with 2% (w/v) PAFA (paraformaldehyde) for 10 min on ice and imaged on a confocal microscope as described below.
Recycling kinetics of Alexa488-Tf in polarized HepG2 cells
Cells were labelled for 5 min at 37 °C with 10 μg/ml Alexa488-Tf, washed with buffer medium and chased for 30 min at 37 °C. Cells were chilled with ice-cold buffer medium and incubated in release medium for 10 min on ice to remove surface-bound transferrin by a mild acid-wash [5,23]. Cells were warmed to 37 °C, chased for the indicated time points and imaged on a wide field microscope.
Fluorescence microscopy and image analysis
Wide field fluorescence microscopy and digital image acquisition were routinely carried out using a Leica DMIRB microscope with a 63×1.4 NA (numerical aperture) oil immersion objective (Leica Lasertechnik, Wetzlar, Germany) equipped with a Princeton Instruments cooled CCD (charge-coupled-device) camera driven by Image-1/MetaMorph imaging system software. DiI lipids and Alexa546-Tf were imaged using a standard rhodamine filter set [535 nm (50 nm bandpass) excitation filter, 565 nm longpass dichromatic filter, and 610 nm (75 nm bandpass) emission filter], while Alexa488-HDL and Alexa488-Tf were imaged using a standard fluorescein filter set [470 nm (20 nm bandpass) excitation filter, 510 nm longpass dichromatic filter, and 537 nm (23 nm bandpass) emission filter]. Image analysis was carried out using the software packages Image-1/MetaMorph imaging system (Universal Imaging, Downington, PA, U.S.A.), ImagePro Plus (Media Cybernetics, Silver Spring, MD, U.S.A.) or NIH Image in the form of ImageJ (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/ij). For presentation purposes and contrast adjustment Adobe Photoshop (Adobe Systems Inc.) was used. Determination and subtraction of crossover of fluorescence between the channels was performed as described in [22,24].
Confocal microscopy was performed using an Axiovert 100 M inverted microscope equipped with a 63×1.4 NA plan Apochromat objective (Carl Zeiss). Cells labelled with DiI lipids were excited with a 1.0 mW helium/neon laser emitting at 543 nm, while a 560 nm longpass filter was used for collecting emissions. Fl-dextran was excited with a 25 mW argon laser emitting at 488 nm. A 505–530 nm bandpass filter was used for emissions. The two channels were scanned sequentially in a line-by-line mode, having only one laser line and one detector channel on at each time.
In polarized HepG2 cells stained with Alexa488-Tf and co-labelled with DiIC12, fluorescence in the SAC/ARC and BC was quantified by defining ROI (regions of interest) for these compartments based on the fluorescence of DiIC12 (for the BC and SAC/ARC). Fluorescence intensity in all ROI was measured after background subtraction and normalized to total cell-associated fluorescence of Alexa488-Tf in the two cells forming a BC, and plotted as a function of time [4,6]. For quantification of transport through SE and SAC/ARC, images of cells double-labelled with Alexa488-HDL and Alexa546-Tf were background-corrected, and the intensity ratio was calculated for both compartments. SE were identified by size and shape of intensity profile (10–40 pixels in area; 1 pixel is 0.15 μm at ×63 magnification) [21,25,26]. The SAC/ARC was identified by being located close (2–5 μm) to the BC of two neighbouring cells, while the size of this endocytic compartment was >150 pixels in area. The ratios of Alexa488-HDL and Alexa546-Tf were then determined independently for SE and SAC/ARC [21].
In an independent set of experiments, images of fluorescent beads were acquired with identical settings as used for imaging of Alexa488-HDL and Alexa546-Tf respectively (see above). To this end, serial focal plane images of 0.1 μm TetraSpec fluorescent beads (Molecular Probes) mounted in gelvatol on a glass coverslip were acquired as described in [5]. The fluorescence intensity was measured for red and green channels and the ratio calculated for different focal positions along the optical axis. A valid approximation for the DOF (depth of field) along the optical path giving the width of sharp focus in the z-direction is given by [27]:
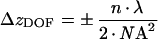 |
(1) |
Here, n is the refractive index, NA the numerical aperture and λ the wavelength of emitted light. Thus it is expected that for a ×63 oil immersion objective, as used here, for red and green fluorescence the DOF is 0.207 and 0.182 μm respectively. The measurements reveal that the fluorescence ratio is constant over a much larger range of z-positions (see Supplementary material at http://www.BiochemJ.org/bj/400/bj4000267add.htm) in accordance with previously published results [27]. This is due to the incoherent light used in wide field fluorescence microscopy [27]. To independently verify this conclusion for living HepG2 cells, three-dimensional stacks of cells double-labelled with Alexa488-HDL and Alexa546-Tf were acquired by starting from a focal position 2 μm above the largest diameter of the cells [5,28]. Fluorescence ratio in endosomes and correlation coefficients were calculated as described above and were found to be largely independent of the focal position (see the Results section).
Data analysis
Curve-fitting of transport equations for fluorescent transferrin (see Appendix A) to experimental data was done by a multicompartment non-linear regression procedure implemented in the SAAM software (SAAM Institute, Seattle, WA, U.S.A.) as described in [18]. Based on the covariance matrix of estimated parameters, random sets of kinetic parameters that follow a normal distribution were generated by Monte Carlo simulation as described in [18]. Time-dependent solutions of a differential equation system presented in Appendix A were solved using Mathematica 2.2. (Wolfram Research). All simulations were performed on a 2 GHz Linux or Windows personal computer having a Dual Core Athlon processor. Sensitivity of models to determined kinetic parameters was obtained from the respective sensitivity matrix (see Appendix B). Data plotting was performed using SigmaPlot 4.0 (SPSS Inc., Chicago, IL, U.S.A.). Co-localization of DiI lipid probe with Alexa488-Tf was determined from wide field images of double-labelled cells using the ImagePro Plus software (Media Cybernetics, Silver Spring, MD, U.S.A.). Pearson's correlation coefficient, rp, is given by:
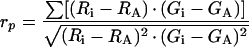 |
(2) |
where Ri and Gi are the fluorescence intensities in red and green per pixel i respectively. The average intensities in red and green in an image are given by RA and GA respectively [17].
This parameter was calculated for DiI lipids and Alexa488-Tf and plotted as a function of time, as well as for Alexa488-HDL and Alexa546-Tf as a function of z-position in image stacks.
RESULTS
DiI lipids co-localize with Alexa488-Tf in SE and in the SAC/ARC
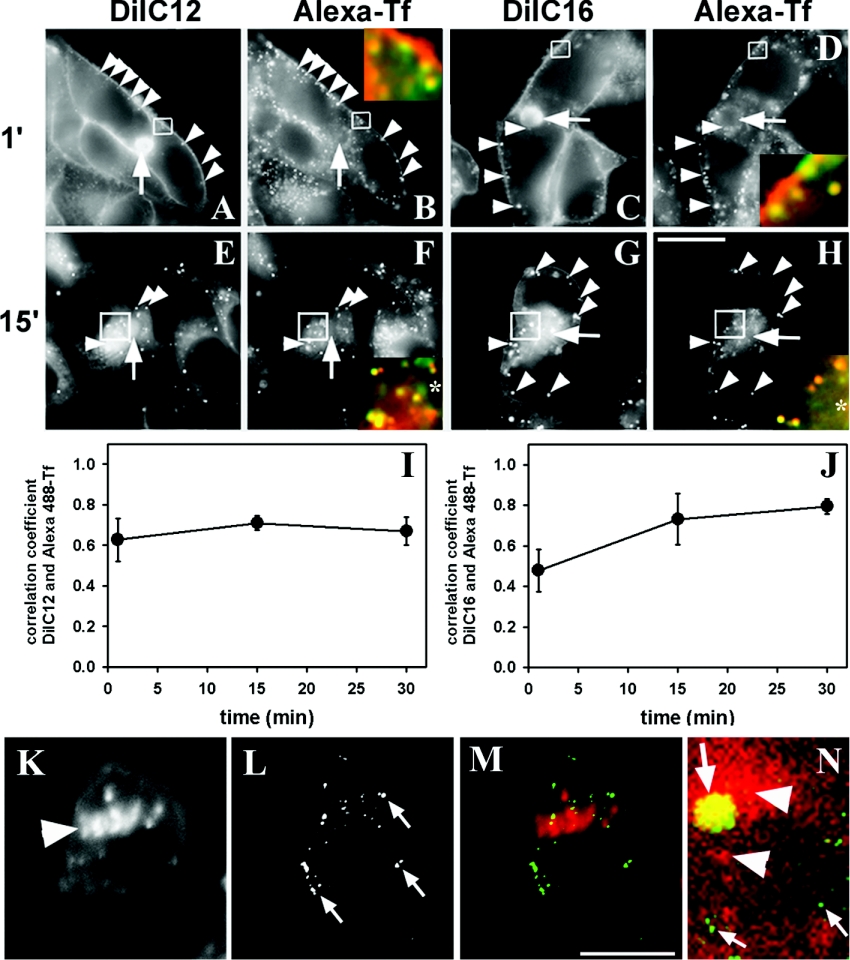
DiI lipids are prominent probes to investigate endocytic lipid sorting in non-polarized cells [22]. Here, transport of those analogues was compared with that of fluorescent transferrin in polarized hepatic HepG2 cells. Cells were co-labelled with Alexa488-Tf and DiIC12 or DiIC16 respectively for 1 min at 37 °C, washed and imaged. For both lipid analogues bright staining of both plasma membrane domains was found (Figures 1A–1D). Also the canalicular membrane appeared labelled with DiIC12 as well as with DiIC16 but this membrane domain did not contain Alexa488-Tf (arrows). Initial staining of the canalicular membrane is due to non-vesicular transport of DiI lipids to the BC [18]. In fact, ATP depletion, which blocks endocytosis and vesicular transport, could not interfere with canalicular staining of DiI analogues (results not shown). DiI lipids co-localized with fluorescent transferrin in endocytic vesicles along the basolateral membrane after 1 min chase (Figures 1A–1D, insets and arrowheads). The zoomed regions outlined by a box show that most vesicles located close to the basolateral membrane containing DiI also have Alexa488-Tf (colour-merged panels with DiI lipid in red and Alexa488-Tf in green; note that intensive staining of the membrane by DiI after 1 min chase creates a red appearing background). Double-labelled vesicles are mostly SE, as we have shown previously by measuring transport kinetics of fluorescent ASOR (asialoorosomucoid) [6,29]. DiIC12 as well as DiIC16 accumulated in the subapical region in vesicles which contained also Alexa488-Tf after 15 min chase (Figures 1E–1H). This is clearly seen in the colour-merged zoomed regions. The results suggest transport of both lipids to an SAC/ARC as found previously for analogues of phosphatidylcholine and for C6-NBD-SM {6-[N-(7-nitro-2,1,3-benzoxadiazol-4-yl) amino]hexanoylsphingosylphosphorylcholine} [6]. Co-localization of DiIC12 as well as of DiIC16 with Alexa488-Tf is almost constant over a 30 min chase as indicated by the Pearson correlation coefficient (Figures 1I and 1J). DiI lipids do not co-localize with Fl-dextran, a marker for LE/LYS (late endosomes and lysosomes), neither in non-polarized nor in polarized HepG2 cells, as shown for DiIC16 (Figures 1K–1N) [22]. Together, these results demonstrate that both DiI lipids, DiIC12 as well as DiIC16, are transported from the basolateral membrane to the SAC/ARC in the same endocytic vesicles like fluorescent transferrin.
Figure 1. DiIC12 and DiIC16 co-localize with Alexa488-Tf in polarized HepG2 cells.
Cells were labelled for 1 min with DiIC12 (A, E) or DiIC16 (C, G) and 5 μg/ml Alexa488-Tf (B, D, F, H) at 37 °C. Cells were chased for 1 min (A–D) or 15 min (E–H) at 37 °C. DiIC12 and DiIC16 stained the basolateral as well as the canalicular membrane, i.e. apical BC (arrows). Both DiI lipids co-localized in vesicles with Alexa488-Tf after 1 min chase (arrowheads) (see inset, B, D). Both lipid probes co-localized with Alexa488-Tf in the subapical region after 15 min chase, indicating enrichment of DiIC12 and DiIC16 in the SAC/ARC (arrowheads and zoomed insets, F, H). This can be clearly seen in the colour-merged zoom panels with DiI in red and Alexa488-Tf in green. (I, J) Quantification of co-localization of DiI lipids and Alexa488-Tf. From images as shown in (A–H) Pearson's correlation coefficient was calculated for DiIC12 or DiIC16 and Alexa488-Tf in endosomes respectively. Results shown represent the means±S.E.M. for five to ten fields of cells with at least two measurements (i.e. BC forming cell couplets) per field. (K–N) HepG2 cells were pre-incubated for 1 h with 4 mg/ml Fl-dextran, washed and labelled for 1 min with DiIC16 at 37 °C. Cells were washed and chased for 30 min at 37 °C, washed, slightly fixed with 2% PAFA and imaged with a confocal microscope. DiIC16 accumulated in the perinuclear region in non-polarized cells (arrowhead, K). Fl-dextran-containing LE/LYS (arrows, L). (M) Colour overlay with DiIC16 (red) and Fl-dextran (green). In polarized HepG2 cells (N), DiIC16 (red) is enriched in vesicles close to the BC (large arrow), while DiIC16 was not found in Fl-dextran-containing LE/LYS (small arrows pointing to green dots, N). Subapical vesicles containing DiIC16 belong to the SAC/ARC (arrowheads). The BC (large arrow) has DiIC16 as well as Fl-dextran and therefore appears yellow. (K–M) Sum projection of five corresponding planes. (N) Single confocal plane. Scale bar, 20 μm.
Kinetic analysis of polarized trafficking of transferrin in HepG2 cells
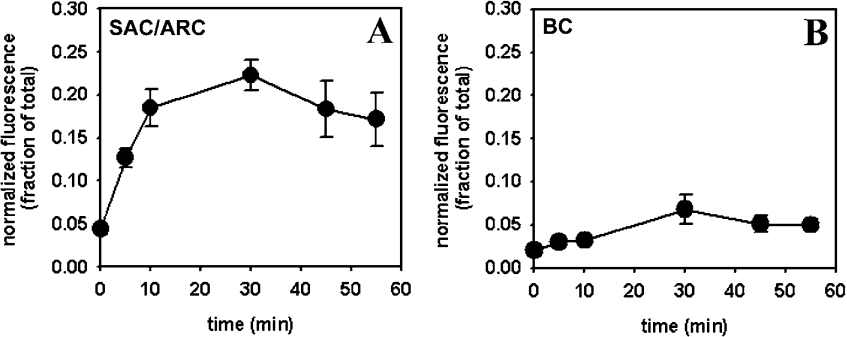
To measure time courses of enrichment of fluorescent transferrin in intracellular compartments, HepG2 cells were pre-incubated with DiIC12 to identify the BC and SAC/ARC respectively, washed, pulse-labelled with Alexa488-Tf and chased for various times. Fluorescence of Alexa488-Tf increased first in the SAC/ARC with a maximum after approx. 30 min chase (Figure 2A). Subsequently, a decrease in fluorescence of Alexa488-Tf associated with the SAC/ARC was found. This fluorescence decrease was paralleled by a very low fluorescence increase of Alexa488-Tf in the BC. Fluorescence of Alexa488-Tf in the BC reached a plateau after approx. 30–60 min chase (Figure 2B). For these experiments fluorescence of Alexa488-Tf in the SAC/ARC and BC respectively was normalized to total cell-associated fluorescence of labelled transferrin (see the Materials and methods section).
Figure 2. Time course of intracellular transport of Alexa488-Tf in polarized HepG2 cells.
Cells were labelled for 1 min with DiIC12 and 5 μg/ml Alexa488-Tf at 37 °C. Cells were washed and chased for the indicated time points. Fluorescence of Alexa488-Tf in the SAC/ARC (A) and in the BC (B) was measured and normalized to total cell-associated fluorescence of Alexa488-Tf as described in the Materials and methods section. Results shown represent the means±S.E.M. for five to ten fields of cells with at least two measurements (i.e. BC forming cell couplets) per field. Approximately 85% of measurements were performed in the same cell pairs for (A, B).
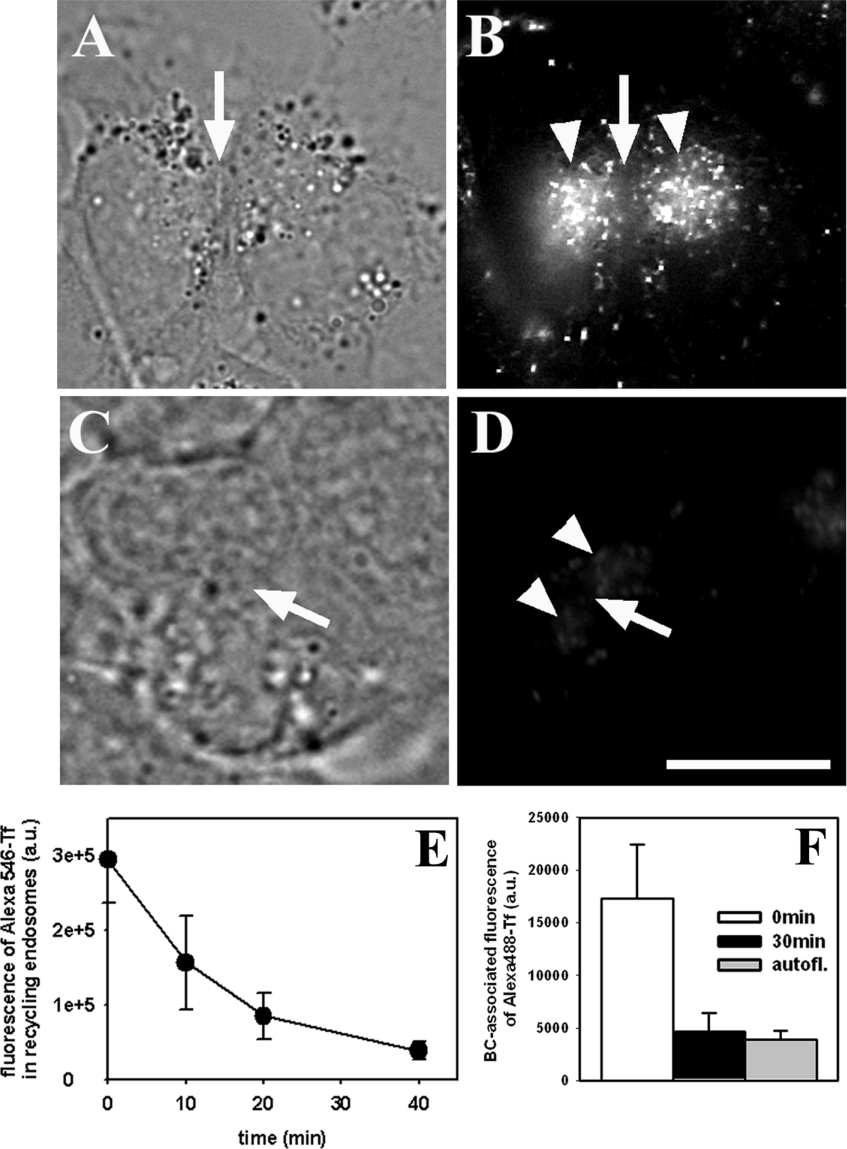
To measure the kinetics of recycling of Alexa488-Tf from the SAC/ARC, cells were labelled and chased to reach the steady-state distribution of fluorescent transferrin (Figures 3A and 3B). HepG2 cells were washed with release medium to remove any surface-bound transferrin as described in [23]. Fluorescence of Alexa488-Tf in the SAC/ARC was measured during a chase as a function of time (without normalization to total cell-associated fluorescence). There is a strong decrease in total cell-associated fluorescence of Alexa488-Tf (Figures 3C and 3D). By this method a complete chase-out of fluorescent transferrin from the cells could be obtained (Figures 3D and 3E). Quantification of fluorescence of Alexa488-Tf shows that essentially all transferrin is released from the SAC/ARC and the cells in a chase time of approx. 40 min (i.e. kinetics of fluorescence decrease of Alexa488-Tf in the SAC/ARC paralleled fluorescence loss from cells). Quantification of BC-associated fluorescence of Alexa488-Tf from this experiment demonstrates that after prolonged chase-out of transferrin from HepG2 cells, almost no fluorescence of Alexa488-Tf can be detected in the BC (Figure 3F, black bar). In fact, the intensity of Alexa488-Tf is hardly above autofluorescence background (measured in unlabelled cells) at the end of the chase-out experiment (Figure 3F, grey bar). Unfortunately, the low extent of BC-associated fluorescent transferrin at steady state does not allow one to measure a time course of chase-out from the BC.
Figure 3. Kinetics of recycling of Alexa488-Tf in polarized HepG2 cells.
Cells were labelled for 5 min at 37 °C with 10 μg/ml Alexa488-Tf, washed and chased for 30 min at 37 °C. Cells were chilled with ice-cold buffer medium and incubated in release medium for 10 min on ice to remove surface-bound transferrin by a mild acid-wash. Cells were warmed to 37 °C, chased for the indicated time points and imaged on a wide field microscope. (A, B) At the start of the chase, Alexa488-Tf was highly enriched in subapical vesicles resembling the SAC/ARC (arrowheads), while fluorescence intensity of Alexa488-Tf in the BC was low (arrow). (C, D) After 40 min chase, cell-associated fluorescence of Alexa488-Tf was strongly reduced, indicating chase-out of the probe from cells. (E) Quantification of fluorescence of Alexa488-Tf in the SAC/ARC from wide field fluorescence images like those shown in (B, D). (A, C) Bright field images of the fields shown in (B) and (D) respectively. (F) Quantification of BC-associated fluorescence of Alexa488-Tf during the chase out. White bar, start of experiment; black bar, after 30 min chase; grey bar, autofluorescence in the BC of cells not labelled with Alexa488-Tf. Scale bar, 20 μm. Results shown represent the means±S.E.M. for five to ten fields of cells with at least two measurements (i.e. BC forming cell couplets) per field and time point.
Quantitative comparison of intrahepatic transport of HDL and transferrin
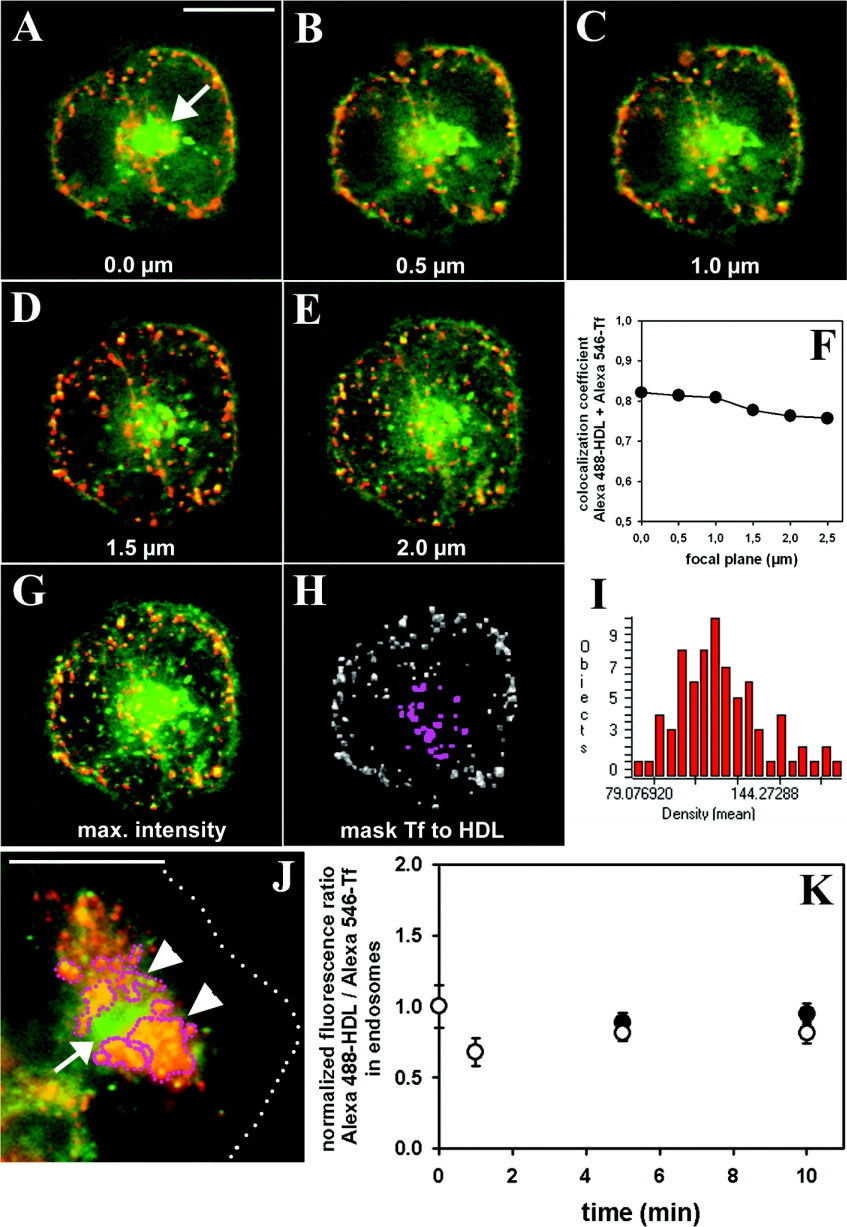
Recently, we found that HDL co-localizes with transferrin in basolateral SE and in the SAC/ARC of polarized hepatic cells [4]. To obtain further insight into hepatic transport of HDL and transferrin, the trafficking of both proteins was compared by ratio imaging. HepG2 cells were double-labelled with Alexa488-HDL and Alexa546-Tf, chased and imaged either as single planes or in a z-stack. Images acquired in both channels along the optical axis with 0.5 μm step size after 5 min chase reveal that Alexa488-HDL and Alexa546-Tf co-localize in basolateral endosomes as well as in the subapical region (Figures 4A–4E). In fact, it was found that the Pearson correlation coefficient is higher than rp=0.75 in all image planes acquired in the z-direction at a particular chase time (Figure 4F). A maximum intensity projection of the colour-merged stacks reveals high co-localization of both probes in endosomes, whereas only Alexa488-HDL but almost no Alexa546-Tf is found in the BC at that time point (Figure 4G). Note that BC-associated fluorescence of Alexa488-HDL appears over-emphasized due to the colour-merging procedure. From a projected z-stack of Alexa546-Tf, a binary mask was generated after thresholding to include approx. 93% of all endosomes (based on pixel area as criteria). This mask was applied to an Alexa488-HDL z-stack projection image and endosome fluorescence was measured (exemplified in Figures 4H and 4I). It was found that approx. 88% of all endosomes having Alexa546-Tf contain also Alexa488-HDL. This was found irrespective of the endosome population (i.e. basolateral SE versus subapical SAC/ARC). The SAC/ARC was defined based on its close proximity to the apical BC and the mean size of the endosome population (see the Materials and methods section). At the particular chase time of 5 min most endosome fluorescence comes from SE, while only a minor fraction belongs to the SAC/ARC based on the defined criteria to distinguish both endosome fractions (indicated in pink in Figure 4H). At later time points the SAC/ARC forms a prominent accumulated vesicle pool in the subapical area (outlined by pink dots and arrowheads in Figure 4J; compare Figures 1 and 3B). From single images of Alexa546-Tf and Alexa488-HDL acquired in the central focal position (compare Figure 4A), the fluorescence ratio of both probes was measured as function of time in basolateral SE and in the SAC/ARC (Figure 4K) [21]. The fluorescence ratio of both labelled proteins remained constant in the basolateral SE as well as in the SAC/ARC over a prolonged chase. Moreover, the ratio of Alexa488-HDL to Alexa546-Tf in the SAC/ARC was found to be equal to that in basolateral SE. Slight defocus in the z-position by approximately three times the theoretical depth of field of approx. 0.2 μm has no impact on the measured fluorescence ratio as inferred from images of multi-labelled beads having a diameter of 0.1 μm (see the Materials and methods section and Supplementary material) [27]. Similarly, z-stacks measured for cells double-labelled with Alexa488-HDL and Alexa546-Tf gave the same ratio as single plane images (results not shown). The results demonstrate that Alexa488-HDL and Alexa546-Tf traffic with indistinguishable kinetics from basolateral SE to the SAC/ARC, but also that they arrive in the same proportion in the SAC/ARC in hepatic cells.
Figure 4. Ratio of Alexa488-HDL and Alexa546-Tf in various endosomes of HepG2 cells.
Cells were co-labelled with 2 μg/ml Alexa488-HDL and with 5 μg/ml Alexa546-Tf for 1 min at 37 °C, washed and chased at 37 °C in buffer medium for the indicated time points. (A–E) Individual planes of z-stack of polarized cells double-labelled with Alexa488-HDL (green) and Alexa546-Tf (red) after 5 min chase. (F) Pearson's co-localization coefficient calculated from the double-labelled stack as a function of z-position. (G) Maximum intensity projection of the planes shown in (A–E). (H) Intensity of Alexa488-HDL in endosomes defined by applying a binary mask generated by thresholding of the maximum intensity projection image of Alexa546-Tf. Pink defines endosomes belonging to the SAC/ARC as defined due to their size and proximity to the BC. (I) Histogram of measured intensities of Alexa488-HDL in endosomes defined by Alexa546-Tf fluorescence from the image in (H). (J) Single plane image of HepG2 cells double-labelled with both probes after 30 min chase. Arrows point to the central BC containing mainly Alexa488-HDL; arrowheads and pink outline indicate the SAC/ARC. White dots indicate the cell border. Colour-merged images show co-localization in yellow to orange. (K) Fluorescence ratio of Alexa488-HDL and Alexa546-Tf measured from fluorescence per endosome as described above in basolateral SE (○) and in the SAC/ARC (●) (see also the Materials and methods section). Calculated ratios were normalized to the initial ratio at t=0 in SE. Results shown represent the means±S.E.M. for five to ten fields of cells with at least two measurements (i.e. BC forming cell couplets) per field and time point.
Kinetic modelling of polarized trafficking of transferrin in hepatic cells
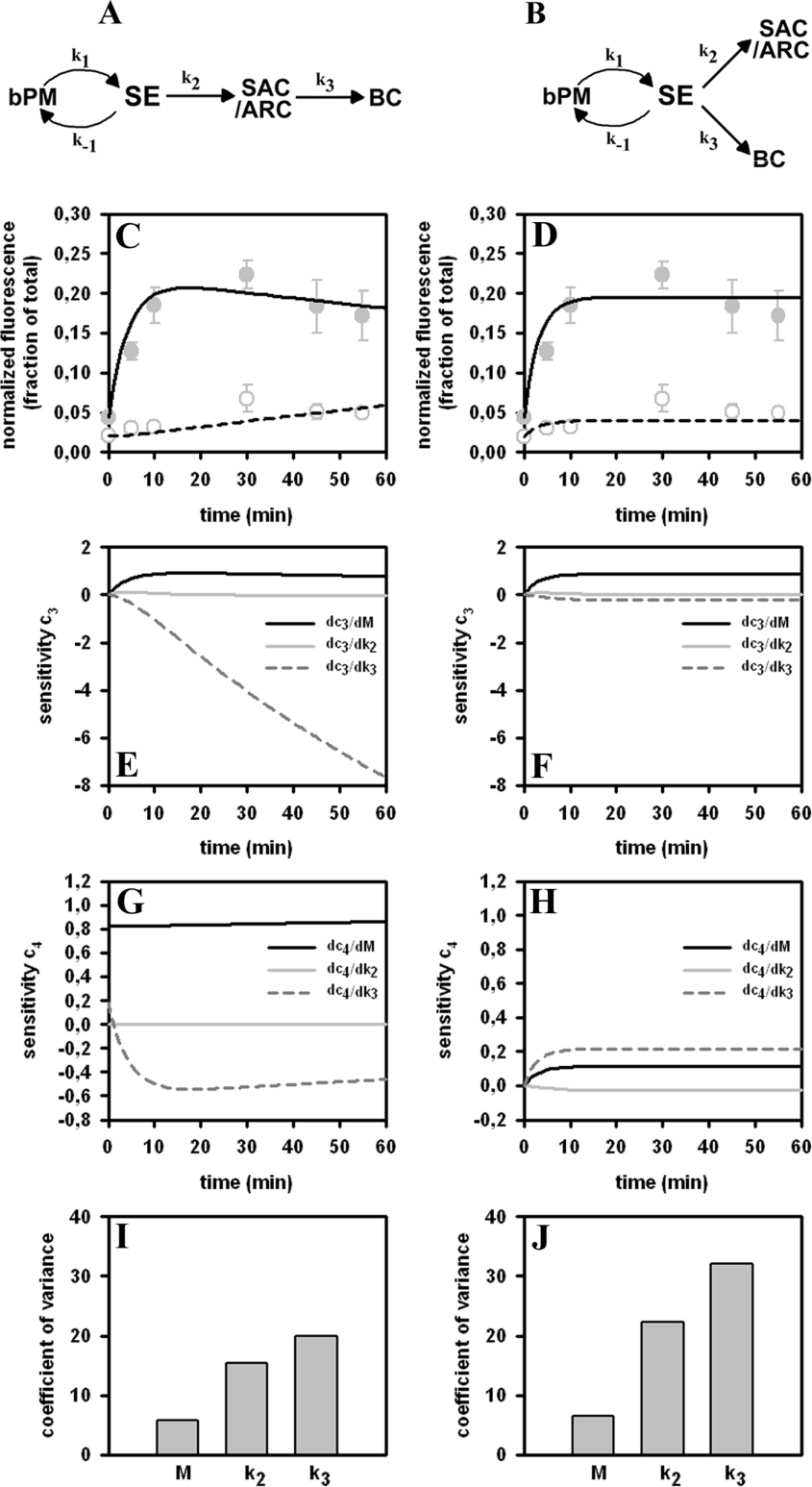
Measured transport kinetics of Alexa488-Tf were further analysed by kinetic modelling. As a start model equations previously derived for the analysis of hepatic transport of HDL were used [18]. This is reasonable based on the observed similarities in transport of both ligands in HepG2 cells (compare Figure 4 and [4]). Rapid recycling of labelled transferrin from basolateral SE was included in a sequential and a parallel transport model respectively (Figures 5A and 5B and [18]). The ratio q describing internalization of transferrin (rate constant k1) versus recycling of transferrin from SE (rate constant k−1) was set to q=0.85. This value is higher than that used previously to model rapid recycling of fluorescent HDL from hepatic SE (q=0.5) [18]. It resembles a recycling rate of transferrin from basolateral SE of k−1=0.412, well in accordance with literature values [10,23,24]. While the sequential model assumes that transferrin being exported from SE traffics through the SAC/ARC to the BC, the parallel model considers transport of transferrin simultaneously to the SAC/ARC and the BC respectively. The non-linear regression of both models to transferrin transport data shows that the sequential model describes the data more accurately than the parallel model (Figures 5C and 5D). This is also reflected by the larger uncertainty (given by a higher coefficient of variance) in determining the kinetic parameters in the parallel compared with the sequential model (Figures 5I and 5J).
Figure 5. Kinetic modelling reveals sequential transport of transferrin in HepG2 cells.
Kinetic models of sequential (A, C, E, G, I) or parallel (B, D, F, H, J) transport of Alexa488-Tf were fitted to experimental time courses of Alexa488-Tf transport in polarized HepG2 cells (see Figures 2 and 3 and [18]). Both models assume that fluorescent transferrin recycles rapidly between the basolateral membrane (bPM) and the SE. In the sequential model, Alexa488-Tf exported from SE is transported via the SAC/ARC to the BC, whereas in the other model, transport of transferrin occurs in parallel to SAC/ARC and BC respectively. Model fit to time courses of transferrin transport to the SAC/ARC (closed grey symbols) and to the BC (open grey symbols) is shown as straight and dashed lines in (C) and (D) respectively. A sensitivity analysis representing the derivations of system output with respect to the kinetic parameters is shown for the model output of compartments c3 (i.e. SAC/ARC; E, F) and c4 (i.e. BC; G, H). (I, J) Coefficient of variance for the determined parameters for the sequential (I) and the parallel model (J).
Sensitivity analysis is a useful tool to assess how exactly one can determine kinetic parameters from given experimental data. One calculates for a given model the derivation of system output (i.e. the solution of a kinetic model's differential equation system) with respect to the kinetic parameters being estimated from the experiments (see Appendix B). High sensitivity means that small variations in the parameters are associated with drastic changes in system output. Consequently, one can determine kinetic parameters for a given data set, and measurement error with higher accuracy, if the system output of the used model is more sensitive to parameter perturbations. A sensitivity analysis reveals that the sequential model is very sensitive to changes in the parameter k3 (dashed lines in Figures 5E and 5G). The time-dependent decrease in fluorescence of Alexa488-Tf in the SAC/ARC and the accompanying intensity increase in the BC at prolonged chase times provide a lot of information for determining the parameter k3 in the sequential model (dashed line in Figures 5E and 5G). Both processes are not accurately described by the parallel transport model. The parallel model is relatively insensitive to changes in the parameter k3 (dashed line in Figures 5F and 5H). Together, the sensitivity analysis supports that the measured slow decline of fluorescence of Alexa488-Tf in the SAC/ARC after prolonged chase is reflected by the sequential but not by the parallel model. It can be concluded that fluorescent transferrin escaping basolateral recycling from SE is transport to the SAC/ARC from which compartment a small portion can be exported to the BC.
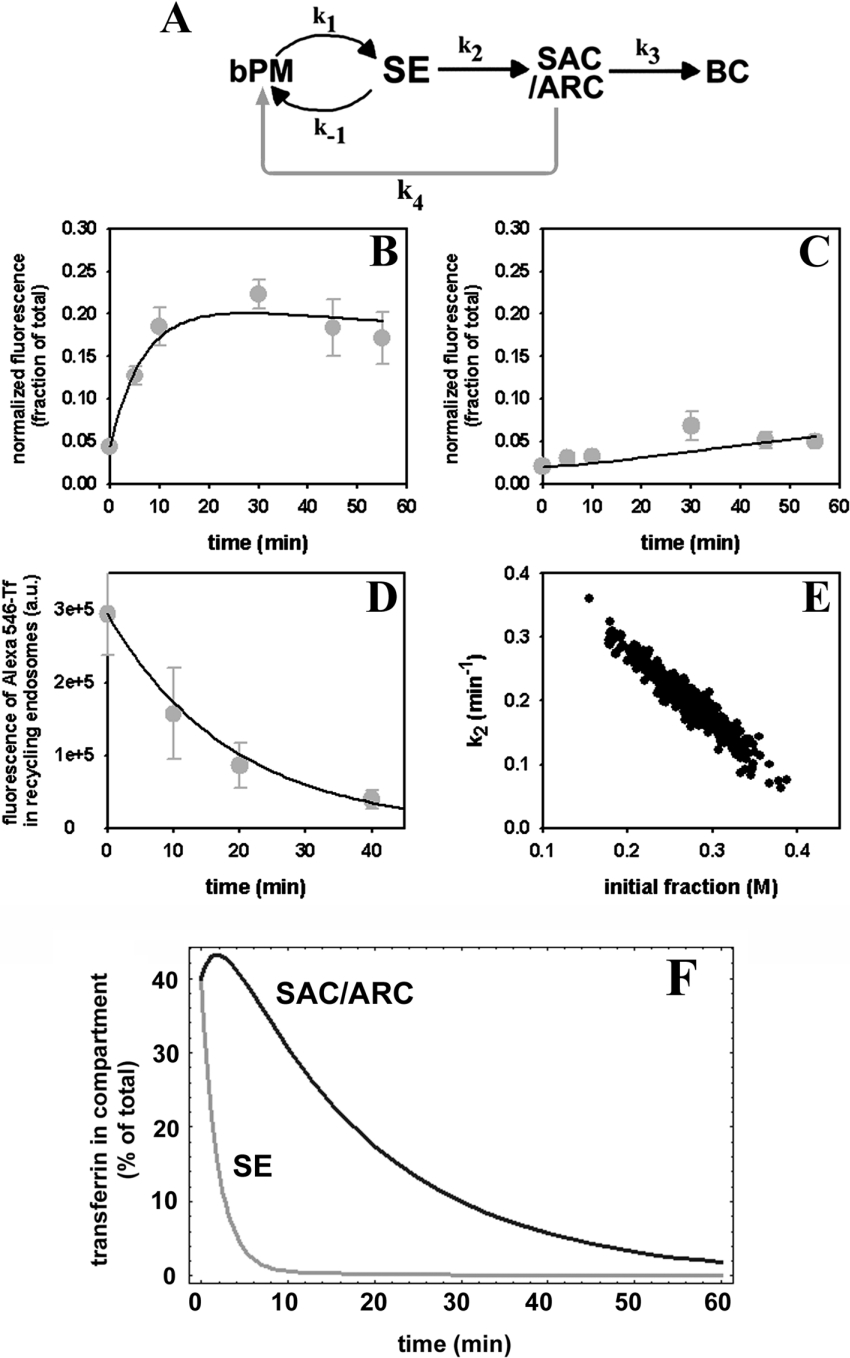
The measured kinetics of release of fluorescent transferrin from cells indicates that Alexa488-Tf recycles from the SAC/ARC to the basolateral cell surface (Figure 3E). To include this observation into the model analysis the sequential model was extended: a mono-exponential decay function was fitted to the recycling kinetics of transferrin from the SAC/ARC in HepG2 cells (rate constant k4, grey arrow in Figure 6A pointing to the added kinetic step compared with the model shown in Figure 5A). The recycling rate constant k4 was estimated from all three time courses in parallel (see Figures 6B–6D and compare Figures 2 and 3). The regression gave a half-time of t½=12.9 min for recycling of fluorescent transferrin from the SAC/ARC (see Table 1). On the other hand, the half-time of transport of Alexa488-Tf from the SAC/ARC to the BC is t½=147.0 min (k3=0.0047 min−1, see Table 1). Thus there is a very slow transport of some Alexa488-Tf from the SAC/ARC to the BC. As shown in Figures 6(B)–6(D), this model is in very good agreement with the experimental kinetic data. Analysis of the estimated rate constants obtained using SAAM software (SAAM Institute) indicates that transport of Alexa488-Tf from basolateral SE to the SAC/ARC occurs with a half-time of t½=3.47 min (k2=0.20 min−1). This value is almost identical with what was found previously for transport of Alexa488-HDL from SE to the SAC/ARC [18]. There is some redundancy in the parameter set as inferred from the covariance ellipse by plotting the initial amount of transferrin in the basolateral membrane, M, against k2 (Figure 6E). Covariance ellipses plotted for other parameter combinations revealed no correlations (results not shown). In fact, by setting the rate constant k2 to the value previously measured for Alexa488-HDL in HepG2 cells, the fit could even be improved (see Table 1). The model analysis thereby supports the results from the ratio image analysis (Figure 4), namely that fluorescent transferrin and HDL traffic with indistinguishable kinetics from basolateral SE to the SAC/ARC.
Figure 6. Kinetic modelling of polarized transport of fluorescent transferrin in HepG2 cells.
(A) An extended sequential model was derived (see Appendix A). Fluorescent transferrin can recycle in two circuits: rapid recycling from SE (rate constant k−1) and slow recycling from the SAC/ARC (rate constant k4, grey arrow). This model was fitted simultaneously to the experimental time courses of Alexa488-Tf in the SAC/ARC (B), in the BC (C) and to the recycling kinetics of transferrin from the SAC/ARC (chase out, D; see Figure 3) by a multi-compartment non-linear regression. For modelling release of Alexa488-Tf from the SAC/ARC, a mono-exponential decay function was used in the fit (D). Straight lines, model; grey symbols, data. (E) Covariance ellipse for the initial fraction of Alexa488-Tf in the basolateral membrane versus rate constant k2 modelling transport of fluorescent transferrin from SE to the SAC/ARC, as obtained from a Monte Carlo simulation of the model parameters (see the Materials and methods section). (F) Numerical simulation of the model shown in (A) with an additional release step of transferrin from the transferrin receptor at the basolateral membrane (release rate constant k0 being k0=2.16 min−1, resembling dissociation of apotransferrin from the transferrin receptor [10]). Initial conditions were set to simulate the chase out experiment shown in Figure 3(E) with 40% transferrin in basolateral SE (grey lines) and the SAC/ARC (black lines) respectively and 20% Alexa488-Tf in the BC (results not shown, given as percentage of total).
Table 1. Rate constants and coefficients of variance for transport of transferrin in polarized hepatic cells.
Kinetic rate constants for non-linear multi-compartment regression of the model for transport of transferrin in HepG2 cells are given as obtained from time-dependent compartmental fluorescence of Alexa488-Tf. Rate constants as obtained by allowing all parameters to be fitted to transferrin transport data (free) or, alternatively, to transport data of Alexa488-Tf using a fixed rate constant k2 as obtained previously for transport of HDL from basolateral SE to the SAC/ARC (restricted) [18]. For the latter case, the value of k2 as obtained from the analysis of HDL transport is given in italics [18].
| Parameter | Value-free | % Variance-free | Value-restricted | % Variance-restricted |
|---|---|---|---|---|
| M | 0.27191±0.03810 | 14.01 | 0.30409±0.01475 | 4.85 |
| k2 | 0.19926±0.04606 | 23.11 | 0.16443 | − |
| k3 | 0.00467±0.00103 | 21.96 | 0.00471±0.00099 | 21.02 |
| k4 | 0.05326±0.00285 | 5.35 | 0.05357±0.00275 | 5.13 |
The assumptions made in the above given kinetic analysis can be tested in a numerical simulation of the model shown in Figure 6(A) by adding a release step of fluorescent transferrin from its receptor after recycling to the basolateral membrane (rate constant k0=2.6 min−1, measured for release of apotransferrin from the transferrin receptor [10]). One can assume a steady-state distribution of Alexa488-Tf after removing the fraction being associated with the basolateral membrane (by mild acid-wash, compare Figure 3) with 40% in SE and the SAC/ARC respectively and 20% in the BC (in percentage of total). The numerical analysis shows that basolateral recycling of transferrin from the SAC/ARC (black line) is much slower than that from the SE (Figure 6F, grey line). After a short initial fluorescence rise due to transport of transferrin from SE to the SAC/ARC, the time course of Alexa488-Tf fluorescence in the SAC/ARC is well described by a mono-exponential decay function. Thus the model assumption made for recycling of transferrin from the SAC/ARC (mono-exponential fit with rate constant k4, see above) is valid.
DISCUSSION
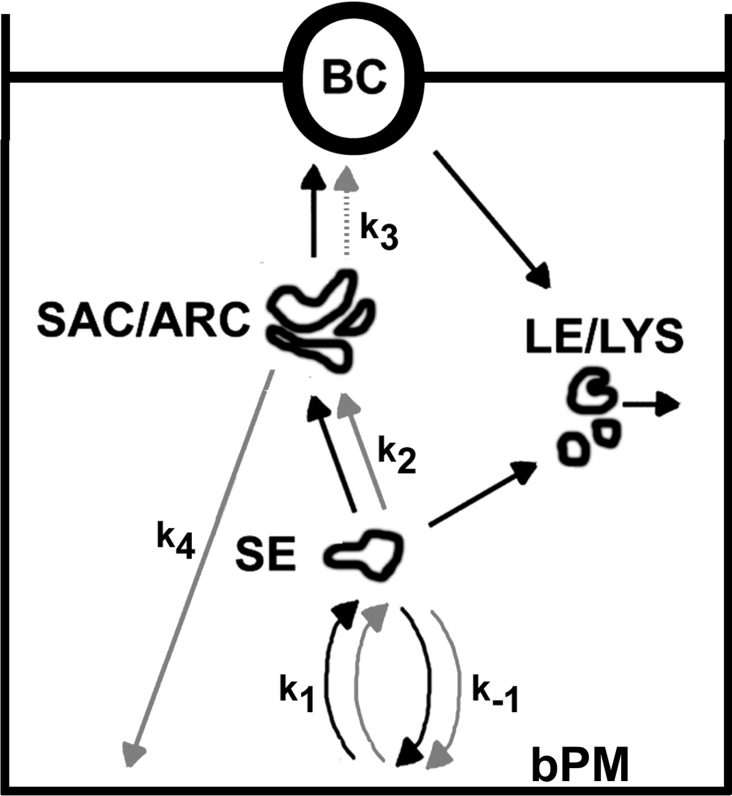
In the present paper, the transport pathways of the recycling marker transferrin through hepatic endosomes have been investigated and compared with trafficking of labelled HDL and fluorescent lipid probes. Evidence is provided that fluorescent transferrin (i) recycles from basolateral SE as well as from the SAC/ARC, (ii) is sequentially transported from the basolateral membrane via SE to the SAC/ARC and from there to a low extent to the BC, and (iii) follows a bulk flow pathway together with fluorescent lipid probes and HDL from basolateral SE to the SAC/ARC (Figure 7). Export of transferrin from the SAC/ARC to the BC is significantly slower compared with that of HDL, supporting the function of the SAC/ARC as a sorting organelle [3,14]. It has long been recognized that polarized epithelial cells are able to sort proteins and lipids at different locations. Two recycling circuits were reported in polarized MDCK cells, i.e. sorting from basolateral SE as well as recycling from a SAC/ARC [14]. In non-polarized TRVb1 cells, rapid recycling from SE as well as slower recycling from the SAC/ARC-related ERC has been demonstrated [12,23,24]. The estimated half-time of t½≈12.9 min for recycling of Alexa488-Tf from the SAC/ARC of polarized HepG2 cells is very close to that measured previously for recycling of transferrin from this compartment in polarized MDCK cells [14]. It is well in accordance with recycling kinetics of transferrin from the ERC in non-polarized cells as well as of radioactively labelled transferrin in HepG2 cell suspension [10,23]. Using calmodulin antagonists, Hoekstra and co-workers [31] distinguished two recycling circuits for C6-NBD-SM in polarized HepG2 cells: rapid calmodulin-dependent recycling from transferrin-containing early endosomes and slow calmodulin-independent recycling from the transferrin-positive SAC/ARC. In non-polarized cells it has been demonstrated that transport from SE to the ERC as well as endocytic recycling occurs by a bulk flow process [12,21]. This means that there is no specific signal required for regulating transferrin transport kinetics between endosomes compared with other ligands. Instead, several ligands travel with the same kinetics between endocytic organelles, which is often referred to as a so-called ‘sorting by default’ process in the literature [15,21]. It was shown that fluorescent lipid probes like C6-NBD-SM and phosphatidylcholine travel with indistinguishable kinetics like transferrin from SE to the ERC in non-polarized TRVb1 cells [21]. Based on the results presented in the present paper it can be concluded that also in polarized (hepatic) cells, transport from (basolateral) SE to subapical recycling endosomes resembles a bulk flow process. Lipid probes of different acyl chain length, DiIC12 and DiIC16, co-localize with labelled transferrin in basolateral SE and in the SAC/ARC (see Figure 1). The lipoprotein HDL is shuttled within the same endosomes to the SAC/ARC as is Alexa546-Tf, supporting that SE-to-SAC/ARC transport is a default pathway (see Figure 4). Based on modelling results it can be confirmed that the transport step from SE to the SAC/ARC has the same rate constant for both proteins (see Figure 6). Co-transport from basolateral SE to the SAC/ARC was shown previously in primary hepatocytes for pIgA-R and transferrin as well as for the apical membrane protein B10 [15,32]. Different apical membrane proteins like pIgA-R as well as P-glycoproteins were identified in the same transcytotic vesicles being attached to microtubule tracks in primary hepatocytes [33]. By subcellular fractionation, Mostov and co-workers [15] have shown that the density of the transferrin receptor and transcytotic polymeric IgA in early endosomes and in tubular recycling endosomes was comparable, suggesting receptor sorting by default. These observations are fully in line with the results presented here on polarized HepG2 cells using quantitative fluorescence microscopy and kinetic modelling.
Figure 7. Model for trafficking of transferrin and HDL in polarized hepatic cells.
Based on the results presented in the present paper, it is assumed that fluorescent transferrin and HDL are commonly internalized and traffic together from basolateral SE to the SAC/ARC (rate constant k2=0.164 min−1). Also fluorescent lipid probes like DiIC12 and DiIC16 travel through SE to the SAC/ARC with the same kinetics as transferrin, suggesting that this transport step resembles a default (bulk flow) pathway. Both probes, transferrin and HDL, can recycle from the basolateral SE (rate constant k−1). Fluorescent transferrin but not HDL can exit the SAC/ARC to the basolateral membrane, thereby creating a polarized, preferentially basolateral, distribution of transferrin (rate constant k4=0.054 min−1). Transport of fluorescent transferrin from the SAC/ARC to the BC is very slow (rate constant k3=0.005 min−1). Based on previous measurements, it is evident that fluorescent HDL can become delivered to LE/LYS probably from both plasma membrane domains where the lipoprotein gets degraded [4,18]. In contrast, fluorescent transferrin is not transported to LE/LYS. Black arrows, HDL; grey arrows, transferrin; dotted grey arrow, very slow transport step for transferrin (t½>100 min). See text for further details.
In polarized kidney-derived MDCK cells, it was found that sorting of pIgA-R from transferrin is mediated by recycling of the latter but not of pIgA-R from the SAC/ARC to the basolateral cell surface [34]. Indeed, Sheff et al. [14] provided evidence that both proteins are commonly transported from the SAC/ARC to the apical membrane [14]. The results presented here indicate that the steady-state distribution between the SAC/ARC and the BC differ for various proteins: fluorescent HDL but almost no transferrin is found in the BC after prolonged chase in HepG2 cells [18]. The results indicate that the different steady-state distributions of fluorescent transferrin and HDL between the SAC/ARC and the BC are maintained by differing export kinetics of both proteins from the SAC/ARC and recycling of transferrin but not HDL from the SAC/ARC to the basolateral membrane. In contrast with transferrin, fluorescent HDL is targeted to LE/LYS after internalization from the BC where the lipoprotein is probably degraded [18]. It is possible that some transferrin is internalized from the BC but transported back to the SAC/ARC instead. This is in fact suggested by some additional modelling (results not shown). Apical recycling would indicate that apical and basolateral endocytic pathways of transferrin merge in the SAC/ARC of hepatic cells as has been reported for MDCK cells [35,36]. In fact, fluorescent sphingolipids recycle between the BC and the SAC/ARC in polarized HepG2 cells as reported by Hoekstra and co-workers [3,37]. Similarly, time-lapse microscopy experiments show vesicle transport of fluorescent phosphatidylcholine and DiI analogues towards the SAC/ARC region (results not shown but see [18]). Apical recycling could contribute to keep the fluorescence of Alexa488-Tf low in the BC and would explain why fluorescence of Alexa488-Tf completely vanishes from the BC in the chase out experiment (compare Figure 3F). Based on the experimentally available data, however, it is not possible to get a reliable rate constant for recycling of fluorescent transferrin from the SAC/ARC to the BC.
By combining quantitative fluorescence microscopy with kinetic modelling and statistical assessment of derived kinetic parameters, evidence for default transport of ligands and membrane lipids between the basolateral SE and the SAC/ARC of hepatic cells is provided. The results presented here describing a detailed kinetic map of transferrin transport in polarized hepatocyte-like cells also set the stage for applications directed towards transferrin receptor-based delivery of drugs against liver diseases [38].
Online data
Acknowledgments
I thank Dr Frederick R. Maxfield (Weill Medical College of Cornell University, New York, NY, U.S.A.) who gave me the opportunity to use his fluorescence microscopy instrumentation, reagents and cell-culture facility for some of the experiments described in this paper, Dr Heinz Sklenar (Max Delbrück Center for Molecular Medicine, Berlin, Germany) for providing computer resources and Dr David L. Silver (Columbia University, New York, NY, U.S.A.) for kindly providing human HDL3. I acknowledge funding by a postdoctoral fellowship of the Max-Delbrück Center and by grants of the Danish Heart Association Hjerteforeningen and Diabetes Foundation Diabetesforeningen.
APPENDIX A
The sequential model shown in Figure 6(A) gives the following system of differential equations with basolateral membrane (c1), SE (c2), SAC/ARC (c3) and BC (c4):
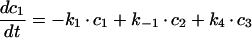 |
(A1) |
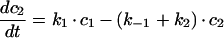 |
(A2) |
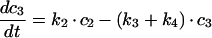 |
(A3) |
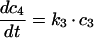 |
(A4) |
The rapid equilibrium approximation, which makes the assumption that uptake and recycling of transferrin is much faster than export of transferrin from SE to the SAC/ARC was applied [39]. This gives:
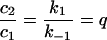 |
(A5) |
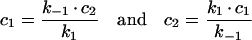 |
(A6) |
Due to the rapid equilibration between c1 and c2 and using equation (A6) one will obtain:
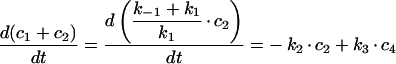 |
(A7) |
The differential equation (A2) for c2(t) will therefore be:
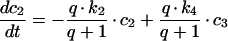 |
(A8) |
The differential equation system consisting of equations (A3), (A4) and (A8) was solved with the initial conditions c2(0)=(M·q)/(1+q), c3(0)=c4(0)=0, yielding:
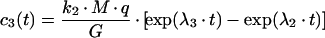 |
(A9) |
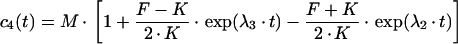 |
(A10) |
with
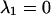 |
(A11) |
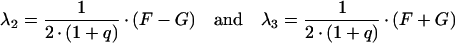 |
(A12, A13) |
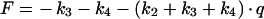 |
(A14) |
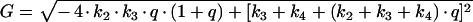 |
(A15) |
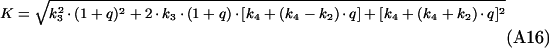 |
(A16) |
APPENDIX B
In order to determine how much information about estimated kinetic parameters can be obtained from a given mathematical model, a sensitivity analysis is performed. This type of analysis allows one to assess the change of system output in response to changes of the measured parameters by defining a sensitivity matrix S. The method was employed here to analyse the suitability of sequential versus parallel transport models in describing the time courses of transferrin transport to the SAC/ARC [c3(t)] and BC [c4(t)] respectively. Model equations were derived in a previous publication [18], while kinetic parameters were obtained in a fit to data (see Figure 5). The sensitivity matrix becomes in this case:
 |
(B1) |
For the sequential model, one gets the following derivations for the SAC/ARC with respect to the experimentally determined kinetic parameters:
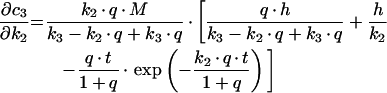 |
(B2) |
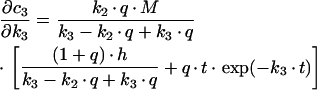 |
(B3) |
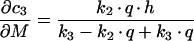 |
(B4) |
with
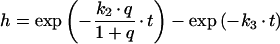 |
(B5) |
Derivations for the BC with respect to parameters become:
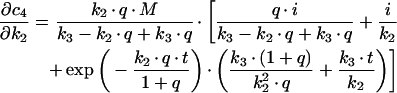 |
(B6) |
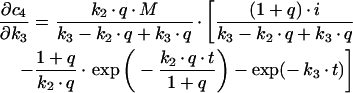 |
(B7) |
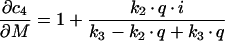 |
(B8) |
with
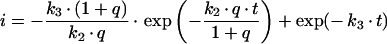 |
(B9) |
Similarly, one gets the following derivations for the SAC/ARC in the parallel transport model:
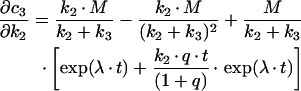 |
(B10) |
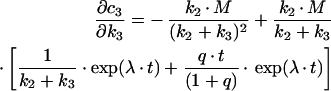 |
(B11) |
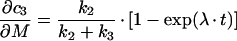 |
(B12) |
while the derivations for the BC compartment are:
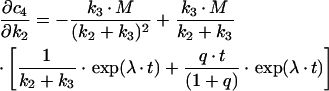 |
(B13) |
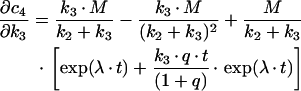 |
(B14) |
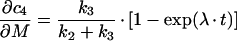 |
(B15) |
with
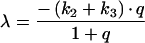 |
(B16) |
References
- 1.Ihrke G., Martin G. V., Shanks M. R., Schrader M., Schroer T. A., Hubbard A. L. Apical plasma membrane proteins and endolyn-78 travel through a subapical compartment in polarized WIF-B hepatocytes. J. Cell Biol. 1998;141:115–133. doi: 10.1083/jcb.141.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sormunen R., Eskelinen S., Lehto V. P. Bile canaliculus formation in cultured HEPG2 cells. Lab. Invest. 1993;68:652–662. [PubMed] [Google Scholar]
- 3.van Ijzendoorn S. C., Hoekstra D. (Glyco)sphingolipids are sorted in sub-apical compartments in HepG2 cells: a role for non-Golgi related intracellular sites in the polarized distribution of (Glyco)sphingolipids. J. Cell Biol. 1998;142:683–696. doi: 10.1083/jcb.142.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wüstner D., Mondal M., Huang A., Maxfield F. R. Different transport routes for high density lipoprotein and its associated free sterol in polarized hepatic cells. J. Lipid Res. 2004;45:427–437. doi: 10.1194/jlr.M300440-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Wüstner D., Herrmann A., Hao M., Maxfield F. R. Rapid nonvesicular transport of sterol between the plasma membrane domains of polarized hepatic cells. J. Biol. Chem. 2002;277:30325–30336. doi: 10.1074/jbc.M202626200. [DOI] [PubMed] [Google Scholar]
- 6.Wüstner D., Mukherjee S., Maxfield F. R., Müller P., Hermann A. Vesicular and nonvesicular transport of phosphatidylcholine in polarized HepG2 cells. Traffic. 2001;2:277–296. doi: 10.1034/j.1600-0854.2001.9o135.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamashiro D. J., Tycko B., Fluss S. R., Maxfield F. R. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- 8.Stoorvogel W., Strous G. J., Geuze H. J., Oorschot V., Schwartz A. L. Late endosomes derive from early endosomes by maturation. Cell. 1991;65:417–427. doi: 10.1016/0092-8674(91)90459-c. [DOI] [PubMed] [Google Scholar]
- 9.Stoorvogel W., Geuze H. J., Strous G. J. Sorting of endocytosed transferrin and asialoglycoprotein occurs immediately after internalization in HepG2 cells. J. Cell Biol. 1987;104:1261–1268. doi: 10.1083/jcb.104.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciechanover A., Schwartz A. L., Lodish H. F. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J. Biol. Chem. 1983;258:9681–9689. [PubMed] [Google Scholar]
- 11.Maxfield F. R., McGraw T. E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 12.Hao M., Maxfield F. R. Characterization of rapid membrane internalization and recycling. J. Biol. Chem. 2000;275:15279–15286. doi: 10.1074/jbc.275.20.15279. [DOI] [PubMed] [Google Scholar]
- 13.Sheff D., Pelletier L., O'Connell C. B., Warren G., Mellman I. Transferrin receptor recycling in the absence of perinuclear recycling endosomes. J. Cell Biol. 2002;156:797–804. doi: 10.1083/jcb.20111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheff D. R., Daro E. A., Hull M., Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verges M., Havel R. J., Mostov K. A tubular endosomal fraction from rat liver: biochemical evidence of receptor sorting by default. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10146–10151. doi: 10.1073/pnas.96.18.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang E., Brown P. S., Aroeti B., Chapin S. J., Mostov K. E., Dunn K. W. Apical and basolateral endocytic pathways of MDCK cells meet in acidic common endosomes distinct from a nearly-neutral apical recycling endosome. Traffic. 2000;1:480–493. doi: 10.1034/j.1600-0854.2000.010606.x. [DOI] [PubMed] [Google Scholar]
- 17.Brown P. S., Wang E., Aroeti B., Chapin S. J., Mostov K. E., Dunn K. W. Definition of distinct compartments in polarized Madin–Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. doi: 10.1034/j.1600-0854.2000.010205.x. [DOI] [PubMed] [Google Scholar]
- 18.Wüstner D. Mathematical analysis of hepatic high density lipoprotein transport based on quantitative imaging data. J. Biol. Chem. 2005;280:6766–6779. doi: 10.1074/jbc.M413238200. [DOI] [PubMed] [Google Scholar]
- 19.Wüstner D. Improved visualization and quantitative analysis of fluorescent membrane sterol in polarized hepatic cells. J. Microsc. 2005;220:47–64. doi: 10.1111/j.1365-2818.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- 20.van IJzendoorn S. C., Hoekstra D. Polarized sphingolipid transport from the subapical compartment changes during cell polarity development. Mol. Biol. Cell. 2000;11:1093–1101. doi: 10.1091/mbc.11.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayor S., Presley J. F., Maxfield F. R. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J. Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee S., Soe T. T., Maxfield F. R. Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J. Cell Biol. 1999;144:1271–1284. doi: 10.1083/jcb.144.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh R. N., Gelman D. L., Maxfield F. R. Quantification of low density lipoprotein and transferrin endocytic sorting in HEp2 cells using confocal microscopy. J. Cell Sci. 1994;107:2177–2189. doi: 10.1242/jcs.107.8.2177. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh R. N., Maxfield F. R. Evidence for nonvectorial, retrograde transferrin trafficking in the early endosomes of HEp2 cells. J. Cell Biol. 1995;128:549–561. doi: 10.1083/jcb.128.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn K. W., McGraw T. E., Maxfield F. R. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J. Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn K. W., Maxfield F. R. Delivery of ligands from sorting endosomes to late endosomes occurs by maturation of sorting endosomes. J. Cell Biol. 1992;117:301–310. doi: 10.1083/jcb.117.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellenberger S. L. PhD Thesis. The Netherlands: Technical University Delft; 2000. Influence of defocus on measurements in microscope images. (ASCI Dissertation Series 51, 20 June 2000, pp. 1–161, http://www.qi.tnw.tudelft.nl) [Google Scholar]
- 28.Wüstner D., Mondal M., Tabas I., Maxfield F. R. Direct observation of rapid internalization and intracellular transport of sterol by macrophage foam cells. Traffic. 2005;6:396–412. doi: 10.1111/j.1600-0854.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 29.Tycko B., Keith C. H., Maxfield F. R. Rapid acidification of endocytic vesicles containing asialoglycoprotein in cells of a human hepatoma line. J. Cell Biol. 1983;97:1762–1776. doi: 10.1083/jcb.97.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reference deleted.
- 31.Tyteca D., van IJzendoorn S. C., Hoekstra D. Calmodulin modulates hepatic membrane polarity by protein kinase C-sensitive steps in the basolateral endocytic pathway. Exp. Cell Res. 2005;310:293–302. doi: 10.1016/j.yexcr.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 32.Hemery I., Durand-Schneider A-M., Feldmann G., Vaerman J-P., Maurice M. The transcytotic pathway of an apical plasma membrane protein (B10) in hepatocytes is similar to that of IgA and occurs via a tubular pericentriolar compartment. J. Cell Sci. 1996;109:1215–1227. doi: 10.1242/jcs.109.6.1215. [DOI] [PubMed] [Google Scholar]
- 33.Soroka C. J., Pate M., Boyer J. L. Canalicular export pumps traffic with polymeric immunoglobulin A receptor on the same microtubule-associated vesicle in rat liver. J. Biol. Chem. 1999;274:26416–26424. doi: 10.1074/jbc.274.37.26416. [DOI] [PubMed] [Google Scholar]
- 34.Apodaca G., Katz L. A., Mostov K. E. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J. Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Futter C. E., Gibson A., Allchin E. H., Maxwell S., Ruddock L. J., Odorizzi G., Domingo D., Trowbridge I. S., Hopkins C. R. In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J. Cell Biol. 1998;141:611–623. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gagescu R., Demaurex N., Parton R. G., Hunziker W., Huber L. A., Gruenberg J. The recycling endosome of Madin–Darby canine kidney cells is a mildly acidic compartment rich in raft components. Mol. Biol. Cell. 2000;11:2775–2791. doi: 10.1091/mbc.11.8.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van IJzendoorn S. C., Zegers M. M., Kok J. W., Hoekstra D. Segregation of glucosylceramide and sphingomyelin occurs in the apical to basolateral transcytotic route in HepG2 cells. J. Cell Biol. 1997;137:347–357. doi: 10.1083/jcb.137.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widera A., Norouziyan F., Shen W. C. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv. Drug Deliv. Rev. 2003;55:1439–1466. doi: 10.1016/j.addr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Heinrich R., Schuster S. New York: Chapman and Hall; 1996. The Regulation of Cellular Processes; pp. 123–134. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.