Abstract
Severe illness caused by viridans streptococci rarely occurs in immunocompetent hosts. Between December 1990 and May 1991, thousands of patients in the YangZi River Delta area of Jiangsu Province, China, suffered from scarlet fever-like pharyngitis. Fewer cases occurred in subsequent years with the same seasonality. Approximately half of the cases developed complications characteristic of streptococcal toxic shock-like syndrome (TSLS). Throat cultures yielded predominant growth of alpha-hemolytic streptococci. All cases admitted to Haian People's Hospital were investigated. Clinical specimens were collected, medical records were reviewed, and bacterial isolates were identified phenotypically and analyzed by 16S rRNA gene sequencing and pulsed-field gel electrophoresis (PFGE). Proteins were purified from culture supernatants by extraction, ammonium sulfate precipitation, and fast-protein liquid chromatography. Biological activities of protein components were determined by subcutaneous inoculation into rabbits. A total of 178 cases of non-beta-hemolytic streptococcal scarlet fever-like pharyngitis were studied. In 88 (79.3%) of 111 patients, oropharyngeal swab cultures grew morphologically identical alpha-hemolytic streptococci. A protein in culture supernatants was pyrogenic in rabbits, was mitogenic for splenocytes, and enhanced rabbit susceptibility to endotoxin challenge. The N-terminal amino acid sequence of this 34-kDa protein showed no homology with known Streptococcus pyrogenic exotoxins. The organism was identified as Streptococcus mitis based on biochemical and 16S rRNA sequence analyses. Representative outbreak isolates from 1990 to 1995 displayed identical PFGE patterns. This TSLS outbreak in southeastern China was caused by a toxigenic clone of S. mitis. An apparently novel toxin may explain the unusual virulence of this organism.
Toxic shock-like syndrome (TSLS) caused by Streptococcus pyogenes exotoxin was first reported in 1983 (39). Numerous cases of TSLS have been reported since Stevens et al. (34) first described a 1989 outbreak of life-threatening streptococcal TSLS in 20 young individuals in the Rocky Mountains. The Working Group on Severe Streptococcal Infections created criteria in 1993 to define group A streptococcal (GAS) TSLS (3). The streptococcal pyrogenic exotoxins (SPEs) produced by GAS cause fever, erythematous skin rashes, and various immunopathological and cytotoxic effects. The SPEs range in size from 20 to 40 kDa for the cloned toxin gene products (5, 11, 28). The genes for three types of SPEs have been cloned and sequenced (31, 33). All are superantigens that bind to human and mouse major histocompatibility complex (MHC) proteins (7, 15, 24). The SPE type A (SPEA), SPEC, and several variants of the streptococcal mitogenic exotoxin Z are members of a superantigen family. Five additional superantigens (types G, H, J, K, and I) have been identified based on sequence homology (24, 28).
Human cases of TSLS due to non-GAS species have been reported. Schlievert et al. (30) described a 27-year-old woman with a TSLS illness consisting of fever, hypotension, and multiorgan system involvement. Group B beta-hemolytic streptococci were isolated from urine and vaginal cultures. These isolates produced a substance that behaved like pyrogenic exotoxins in experimental animals. Group C beta-hemolytic streptococci have also been reported to cause TSLS (19).
An outbreak of non-beta-hemolytic streptococcal scarlet fever-like pharyngitis began in the winter of 1990 in the YangZi River Delta area of Jiangshu Province in southeastern China (40). The first case was identified on 15 December 1990 in Haian County. All initial cases were residents of urban areas of Haian County, and the outbreak later involved surrounding areas. Cases peaked between February and March 1991, with the last case reported on 6 May 1991 for the 1990 to 1991 outbreak season. Males 15 to 34 years of age had the highest incidence rate. Approximately half of the patients presented with symptoms and/or signs of TSLS characterized by hypotension and multiorgan failure. Most patients responded to antimicrobial agents, glucocorticoids, and intravenous fluids and recovered within weeks. There were very few deaths (40). After numerous reports of apparent scarlet fever in the same area, mostly in primary and middle-school students, thousands of cases were reported during the winter and following spring seasons. Similar smaller outbreaks occurred each subsequent year.
During a 9-year period, all patients hospitalized with scarlet fever-like pharyngitis at one of the local county hospitals were further studied. The present study investigated the clinical manifestations of this scarlet fever-like pharyngitis, isolated and characterized the bacterial strains recovered from these patients, and characterized the TSLS-related toxin from these isolates.
(This work was presented in part at the 10th International Congress on Infectious Diseases, 11 to 14 March 2002, Singapore.)
MATERIALS AND METHODS
Participants.
Haian People's Hospital, a 300-bed facility in Haian County, serves a population of 950,000. From October 1990 to May 1998, all hospitalized patients who met the case definition of fever, erythematous rash, and scarlet fever-like pharyngitis were included in the study. Patients were excluded if diagnosed with GAS infection, Staphylococcus aureus infection, infectious mononucleosis, drug rash, or other viral infections. Oropharyngeal cultures were performed on patients suspected of having GAS infection based on purulent oropharyngeal exudate. Cases enrolled during 1990-1991 season were studied retrospectively. Those enrolled in subsequent years were identified prospectively by one of the authors (B.Z.). During the first outbreak season, physicians and nurses in the hospital were specifically trained to assess children and adults for this infection. Clinical specimens were collected, medical records were reviewed, and a questionnaire was completed that included clinical diagnosis, underlying illnesses, history of sick contacts, and social activities in the prior 2 weeks. Informed consent was obtained from all subjects. The Science and Research Bureau of Fudan University approved the study.
Bacteria isolation and phenotypic identification.
Oropharyngeal swab specimens for culture were immediately placed onto 5% sheep blood agar. Plates were incubated for 24 to 48 h in 5% carbon dioxide. Isolates were identified presumptively by colony appearance, pattern of hemolysis, and Gram stain. Isolates were further identified by an API 20 Strep System (bioMérieux, Inc., Hazelwood, Mo.) according to the manufacturer's instructions (14). Blood and urine cultures were routinely performed on patients with temperatures, obtained orally, of >38°C. Isolates were tested for susceptibility to penicillin G, ampicillin, ceftriaxone, cefazolin, ceftazidime, gentamicin, ciprofloxacin, norfloxacin and vancomycin. The MICs of antibiotics were determined by E-test according to the manufacturer's instructions. National Committee for Clinical Laboratory Standards breakpoints were used to interpret E-test results (26). Strains were stored at −70°C in Trypticase soy broth for further analysis.
Bacterial genotypic identification.
Bacterial genomic DNA was extracted by using a QIAamp DNA Mini kit (Qiagen, Inc., Valencia, Calif.) according to the manufacturer's instructions. A primer set spanning the region of the 16S rRNA gene corresponding to nucleotide positions 5 to 1,540 of Escherichia coli (8) was used to amplify the DNA fragment by PCR. The PCR-amplified products were sequenced by using two PCR primers and six additional internal primers as previously described (38). Double orientation sequences of the whole 16S rRNA gene were determined by using the OpenGene sequencing system (Visible Genetics, Inc., Toronto, Ontario, Canada). Sequence sample files were compared to more than 1,100 validated 16S rRNA gene sequences in the MicroSeq database library (Applied Biosystem, Foster City, Calif.) (37).
Toxin isolation and purification.
A bacterial isolate recovered during the 1991 spring outbreak season (Sm91) was arbitrarily chosen and cultured in a Bact API 20 culture medium (bioMérieux) at 35.5°C in 5% CO2 for 18 h. After centrifugation, the supernatant was precipitated with 20, 40, 60, and 80% ammonium sulfate successively at 4°C for 10 h. Precipitates were dissolved in phosphate-buffered saline (5 mM sodium phosphate, 150 mM NaCl; pH 7.2). Dialyzed protein was obtained after sequential anion exchange (DEAE-52), size exclusion on a fast-protein liquid chromatography system, and polymixin B affinity chromatography (29). Active toxin fractions of purified protein were studied by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 12% gel, followed by Coomassie brilliant blue staining. A control S. mitis strain was provided by the Shanghai Anti-Epidemic and Health Center. The isoelectric point (pI) was determined by agarose thin-layer isoelectric focusing (IEF) (36). The amino acid composition of the purified protein was analyzed with a Beckman 344 HPLC apparatus and then compared to other SPEs (31). The N-terminal amino acid sequencing of the purified protein was achieved by automated Edman degradation in gas-phase sequenator as described previously (18).
Rabbit inoculation.
Male New Zealand rabbits (average weight, 2 kg) were monitored daily for fever, diarrhea, and reddening of the ears, mucous membranes, and eyes after subcutaneous injection with 250 μg of purified protein in 0.2 ml of phosphate-buffered saline (20). Control rabbits received the same amount of protein extract from culture supernatants of a nonoutbreak S. mitis strain. Rectal temperature was monitored. Spleens were sampled histologically 4 days after inoculation. To assess enhanced susceptibility to endotoxin shock, 4 h after inoculation rabbits were injected intravenously with 10 μg (equivalent to 0.02 to 0.05 50% lethal dose) of E. coli endotoxin (O111:B4; Sigma)/kg as described previously (29).
Detection of spe gene.
Nested PCR was adapted to detect several spe genes, including speA, speB, and speC, according to procedures published previously (5).
Bacterial typing.
Genomic DNA was extracted from logarithmic-phase bacterial cultures, prepared in low-melting-point agarose plugs (pulsed-field certified agarose; Bio-Rad, Hercules, Calif.), and digested with SmaI (New England Biolabs, Beverly, Mass.) (12). The DNA size was determined by using a bacteriophage lambda ladder (Bio-Rad). Electrophoresis was performed with a CHEF DR-III apparatus (Bio-Rad). Run conditions were 240 V while switching from 10 to 50 s for 18.5 h at 14°C. Gels were stained with ethidium bromide, rinsed, and photographed under UV light.
Statistical analysis.
Statistical comparisons were performed with Epiinfo software (version 6; Centers for Disease Control and Prevention, Atlanta, Ga.). A P value of ≤0.05 was considered significant. Phylogenetic analysis by the neighbor-joining method was performed as described previously (1).
RESULTS
During the eight years beginning in December 1990, 178 patients with non-beta-hemolytic streptococcal scarlet fever-like pharyngitis were hospitalized in the Haian People's Hospital. Nearly 90% of the cases investigated occurred during the first two seasons (113 cases [63.5%] in 1990 and 1991 and 45 cases [25.3%] in 1991 and 1992). Sporadic cases occurred during the same season in subsequent years. The average age was 28.5 years (range, 5 to 46 years), and 133 patients (74.7%) were male. Of the 178 patients, 87 (48.9%) presented with characteristic findings of TSLS (3) as summarized in Table 1. All received empirical therapy with broad-spectrum antimicrobials, and there were no deaths.
TABLE 1.
Clinical features in 178 patients with S. mitis-related scarlet fever-like pharyngitis
| Clinical features | No. of patients (%) |
|---|---|
| Temp ≥38.9°C | 153 (86.0) |
| Hypotension (<90/60 mm Hg) | 87 (49.9) |
| Clinically diagnosed shock | 71 (39.9) |
| Gastrointestinal tract involvement | |
| Vomiting | 109 (61.2) |
| Diarrhea | 53 (29.8) |
| Mucous membrane involvement | |
| Injected pharynx | 107 (60.1) |
| Conjunctivitis | 21 (11.8) |
| Strawberry tongue | 11 (6.2) |
| Erythematous macular or maculopopular rash | 178 (100.0) |
| Desquamation | 178 (100.0) |
| Complications | |
| Acute respiratory distress syndrome | 2 (1.1) |
| Acute renal failure | 17 (9.6) |
| Laboratory findings | |
| White blood cell count at ≥15.0 × 103 cells/mm3 | 93 (52.2) |
| Platelets at ≤100 × 103 cells/mm3 | 30 (16.9) |
| Creatinine at ≥2 times upper limit of normal | 34 (19.1) |
| Transaminases at ≥2 times upper limit of normal | 39 (21.9) |
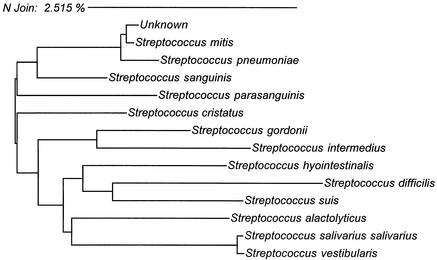
All blood cultures remained negative after 7 days of incubation, and admission urine samples were nondiagnostic. Admission oropharyngeal swab cultures were performed on 111 patients, from which 88 (79.3%) grew either pure culture or predominantly gram-positive alpha-hemolytic streptococci of identical colony morphology. All 88 isolates had identical biochemical reaction profiles and showed 92.2% similarity to Streptococcus mitis in the API 20 Strep System. The organisms were susceptible to all antimicrobials tested. Five representative isolates, each from a different season, were further analyzed by 16S rRNA gene sequencing. The sequence of each human oropharyngeal-swab specimen isolate was 100% identical and diverged from the reference S. mitis sequence at only 3.5 nucleotide positions (99.8% similarity) (Fig. 1).
FIG. 1.
Neighbor-joining analysis of DNA sequences from specimens found to have homology with the human isolates. Phylogenetic analysis was based on whole 16S rRNA gene sequences. Five clinical isolates arbitrarily selected from each year from 1991 to 1995 show identical 16S rRNA gene sequence information. The scale shows relative phylogenetic distance.
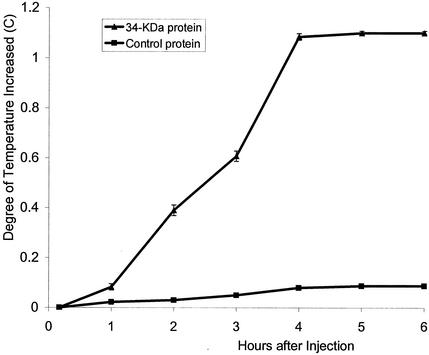
A 34-kDa protein precipitated from culture supernatant by 20% ammonium sulfate was pyrogenic in rabbits. The temperature increased steadily over 4 h after injection, with a mean temperature increase of 1.1°C (Fig. 2). Fever decreased over the next 6 to 8 h and returned to normal within several days. All animals injected with the 34-kDa protein also developed diarrhea, as well as redness of the ears and mucous membranes. Gross examination revealed that cardiac tissue was rigid, and histological examination showed punctate hemorrhages. At 4 days the spleens showed increased mitotic features with congestion of red pulp and proliferation of white pulp. Intravenous challenge of animals previously injected with the 34-kDa protein by injecting E. coli endotoxin caused death in all animals within 16 to 29 h, confirming increased susceptibility to lethal endotoxic shock. In contrast, culture supernatant from the control S. mitis strain displayed none of these effects, and animals survived after E. coli endotoxin challenge.
FIG. 2.
Fever in rabbits after injection of a secreted 34-kDa S. mitis protein. Mean rectal temperature change (in degrees centigrade ± the standard deviation) from four rabbits in each group is shown.
The pI of the 34-kDa protein was 6.2. Amino acid analysis showed a pattern distinct from SPEA, SPEB, and SPEC, with substantially lower molecular ratios in tryptophan, cysteine, methionine, and tyrosine and higher molecular ratios in glycine and alanine (Table 2). The N-terminal amino acid sequence of the 34-kDa protein was VSNLSRGMGA. This showed no homology to other pyrogenic toxins, including SPEA, SPEB, and SPEC. Nested PCR procedures targeting known SPEs genes, including speA, speB, and speC, were negative for these five representative 34-kDa protein-producing S. mitis strains. This suggests that the 34-kDa protein belongs to a novel group of the SPE exotoxins.
TABLE 2.
Comparison of amino acid composition of S. mitis 34-kDa protein to those reported for other streptococal exotoxins
| Amino acid | Molecular ratio (%)a
|
|||
|---|---|---|---|---|
| 34 kDa protein | SPEA | SPEB | SPEC | |
| Tryptophan | 0.0 | 0.5 | 1.6 | 0.0 |
| Lysine | 6.7 | 10.0 | 5.5 | 10.1 |
| Histidine | 1.6 | 2.8 | 2.8 | 2.9 |
| Arginine | 3.7 | 1.4 | 2.8 | 3.4 |
| Aspartic acid | 9.2 | 14.0 | 13.0 | 7.3 |
| Threonine | 5.0 | 6.5 | 3.6 | 5.7 |
| Serine | 5.0 | 6.9 | 8.7 | 7.7 |
| Glutamic acid | 13.6 | 12.7 | 9.1 | 8.1 |
| Proline | 5.6 | 4.2 | 4.7 | 1.9 |
| Glycine | 11.8 | 4.2 | 11.9 | 5.3 |
| Alanine | 10.6 | 1.9 | 7.1 | 2.4 |
| Cysteine | 0.3 | 1.4 | 0.4 | 0.5 |
| Valine | 8.1 | 6.4 | 7.5 | 3.4 |
| Methionine | 0.3 | 1.4 | 4.0 | 1.4 |
| Isoleucine | 5.4 | 5.9 | 4.3 | 0.5 |
| Leucine | 6.7 | 9.1 | 5.5 | 6.2 |
| Tyrosine | 2.1 | 7.7 | 5.9 | 7.7 |
| Phenylalanine | 4.3 | 4.1 | 1.6 | 5.3 |
The molecular ratios for SPEA, SPEB, and SPEC were reported elsewhere (31). Boldface type identifies differences in molecular ratios exceeding twofold.
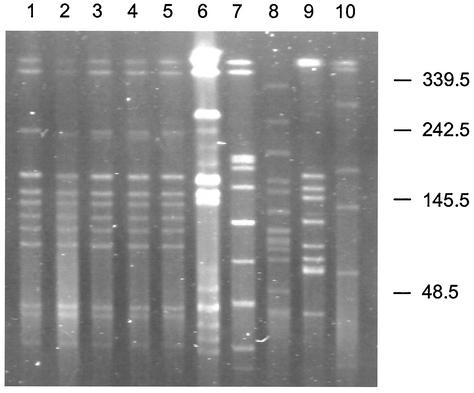
The epidemiologic relatedness of the five strains collected during different seasons was further analyzed by PFGE. For comparison, five S. mitis isolates from normal human throats collected in the same geographic region and season were included. The PFGE patterns of the outbreak isolates were identical to one another but completely different from the comparison isolates (Fig. 3). These data suggest that the isolates causing the large outbreak of human disease were epidemiologically related and clonal in origin.
FIG. 3.
PFGE patterns of SmaI-digested genomic DNA of clinical S. mitis isolates. Lanes 1 to 5 are S. mitis isolates recovered from 1991 to 1995, respectively. Lanes 6 to 10 were S. mitis isolates recovered from the throats of local healthy volunteer during the same period. Molecular sizes are in kilobases.
DISCUSSION
The present study investigated an S. mitis-related TSLS outbreak involving thousands of patients in Southeastern China from 1990 to 1998. A total of 178 patients who suffered from non-β-streptococcal scarlet fever-like pharyngitis admitted to a local hospital were investigated. S. mitis was recovered from throat swabs of most patients, based on phenotypic and genotypic characteristics, including biochemical profiles and 16S rRNA gene sequences. Representative outbreak strains had identical PFGE patterns, suggesting that a clonal strain of S. mitis caused this TSLS outbreak. This report heralds the emergence of a particularly virulent exotoxin-producing strain of viridans streptococci and should promote vigilance for further outbreaks.
S. mitis, one of the more common species of viridans streptococci, has generally been minimally pathogenic and relatively avirulent. Even when it causes endovascular infection such as subacute bacterial endocarditis, the affected patients are often not severely ill (35). When isolated from upper respiratory samples, it is often dismissed as a nonpathogenic commersal. Some aggressive strains have been reported to cause sepsis, acute respiratory distress syndrome, and shock in neutropenic hosts (9, 13, 27). It was reported to cause lower respiratory tract infection in two patients based on culture of transtracheal aspirate and pleural fluid. The first patient presented with empyema and lung abscess, and the second presented with uncomplicated pneumonia complicating lymphoma (27). Extracellular products of S. mitis isolates recovered from infants with Kawasaki syndrome behaved as superantigens in a rabbit model (23). Three apparent cases of acute community-acquired viridans streptococcal pneumonia in previously healthy adults have also been reported (9). Viridans streptococci are associated with the development of a rapidly fulminant shock syndrome in neutropenic patients, and these organisms stimulate a panel of cytokines, which may contribute to septic shock (16, 32). In our study, none of the patients with S. mitis-associated TSLS had evidence of systemic infection such as endocarditis or bacteremia.
Since 1980, there has been a resurgence of severe invasive diseases due to group A streptococci (S. pyogenes), including necrotizing fasciitis and myonecrosis with or without STLS (25, 33). Both group A streptococci and S. aureus produce potent exotoxins that belong to the pyrogenic toxin superantigen family. These simultaneously bind to both the class II MHC molecule and to the Vβ chain of the T-cell receptor (TCR) outside of the conventional peptide groove (22). Through this interaction, these potent toxins subvert normal immune response by forming a TCR-superantigen-MHC ternary complex that stimulates T cells bearing the appropriate Vβ region (2). In recent years, many bacterial exotoxins have been shown to act as superantigens and cause toxicity by causing excessive immune activation (7, 24). Whether the S. mitis exotoxin described here is truly a superantigen merits further investigation.
STLS is a severe, multisystem illness characterized by the rapid onset of fever and hypotension and is a subset of invasive streptococcal disease. The SPEs (also known as erythrogenic toxins or scarlet fever toxins) include the serologically distinct types A, B, C, D, F, G, and H, as well as streptococcal superantigen and streptococcal mitogenic exotoxin Z (6, 10, 17). The SPEs are responsible for the fever, rash, and severe clinical manifestations of TSLS. Other than some members of the Streptococcus milleri group, viridans streptococci, including S. mitis, are not considered to possess traditional virulence factors (4). We identified a novel 34-kDa protein from S. mitis that was highly pyrogenic and enhanced susceptibility to lethal endotoxin shock.
We previously described an Enterococcus faecium-related sepsis outbreak that involved both pigs and humans in 1998 that occurred in the same geographic region and that in some cases also shared clinical features of TSLS (21). We suspect that this region of China may be a reservoir for a novel bacterial exotoxin gene acquired by either E. faecium or S. mitis and that this may have played a role in both outbreaks. Efforts are being focused on isolating and characterizing a possibly novel spe gene.
Acknowledgments
We thank all patients, doctors, and nurses who were involved in the study. We thank Yi-Pin Chen, Xi-Nan Jiang, Zheng Mao, Mao-Yin Pang, Bao-Zheng Peng, Kai-Cheng Qian, Rao-Zhong Shi, Jian-Wu Tang, Xing-Hai Sun, Guang-He Wang, and Hua-Yu Wang for their excellent assistance. We also thank Zhi-Yin Dai, Zhu-Xun Gong, Xiao-Zhang Pan, Yu-Mei Wen, Zheng-Shi Yang, and Marie Griffin for thoughtful discussion and/or critical review of the manuscript.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, P. S., P. M. Lavoie, R. P. Sekaly, H. Churchill, D. M. Kranz, P. M. Schlievert, K. Karjalainen, and R. A. Mariuzza. 1999. Role of the T-cell receptor alpha chain in stabilizing TCR-superantigen-MHC class II complexes. Immunity 10:473-483. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 1993. Defining the group A streptococcal toxic shock syndrome: rationale and consensus definition. JAMA 269:390-391. [PubMed] [Google Scholar]
- 4.Arala-Chaves, M. P., T. B. Higerd, M. T. Porto, J. Munoz, J. M. Goust, H. H. Fudenberg, and C. B. Loadholt. 1979. Evidence for the synthesis and release of strongly immunosuppressive, noncytotoxic substances by Streptococcus intermedius. J. Clin. Investig. 64:871-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black, C. M., D. F. Talkington, T. O. Messmer, R. R. Facklam, E. Hornes, and O. Olsvik. 1993. Detection of streptococcal pyrogenic exotoxin genes by a nested polymerase chain reaction. Mol. Cell. Probes 7:255-259. [DOI] [PubMed] [Google Scholar]
- 6.Bohach, G. A., D. J. Fast, R. D. Nelson, and P. M. Schlievert. 1990. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit. Rev. Microbiol. 17:251-272. [DOI] [PubMed] [Google Scholar]
- 7.Braun, M. A., D. Gerlach, U. F. Hartwig, J. H. Ozegowski, F. Romagne, S. Carrel, W. Kohler, and B. Fleischer. 1993. Stimulation of human T cells by streptococcal “superantigen” erythrogenic toxins (scarlet fever toxins). J. Immunol. 150:2457-2466. [PubMed] [Google Scholar]
- 8.Brosius, J., M. L. Palmer, P. J. Kennedy, and H. F. Noller. 1978. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc. Natl. Acad. Sci. USA 75:4801-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catto, B. A., M. R. Jacobs, and D. M. Shlaes. 1987. Streptococcus mitis. A cause of serious infection in adults. Arch. Intern. Med. 147:885-888. [DOI] [PubMed] [Google Scholar]
- 10.Cleary, P. P., E. L. Kaplan, J. P. Handley, A. Wlazlo, M. H. Kim, A. R. Hauser, and P. M. Schlievert. 1992. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet 339:518-521. [DOI] [PubMed] [Google Scholar]
- 11.Cone, L. A., D. R. Woodard, P. M. Schlievert, and G. S. Tomory. 1987. Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N. Engl. J. Med. 317:146-149. [DOI] [PubMed] [Google Scholar]
- 12.D'Agata, E. M., H. J. Li, C. Gouldin, and Y. W. Tang. 2001. Clinical and molecular characterization of vancomycin-resistant Enterococcus faecium during endemicity. Clin. Infect. Dis. 33:511-516. [DOI] [PubMed] [Google Scholar]
- 13.Elting, L. S., G. P. Bodey, and B. H. Keefe. 1992. Septicemia and shock syndrome due to viridans streptococci: a case-control study of predisposing factors. Clin. Infect. Dis. 14:1201-1207. [DOI] [PubMed] [Google Scholar]
- 14.Fertally, S. S., and R. Facklam. 1987. Comparison of physiologic tests used to identify non-beta-hemolytic aerococci, enterococci, and streptococci. J. Clin. Microbiol. 25:1845-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerlach, D., K. H. Schmidt, and B. Fleischer. 2001. Basic streptococcal superantigens (SPEX/SMEZ or SPEC) are responsible for the mitogenic activity of the so-called mitogenic factor (MF). FEMS Immunol. Med. Microbiol. 30:209-216. [DOI] [PubMed] [Google Scholar]
- 16.Hanage, W. P., and J. Cohen. 2002. Stimulation of cytokine release and adhesion molecule expression by products of viridans streptococci. J. Infect. Dis. 185:357-367. [DOI] [PubMed] [Google Scholar]
- 17.Kamezawa, Y., T. Nakahara, S. Nakano, Y. Abe, J. Nozaki-Renard, and T. Isono. 1997. Streptococcal mitogenic exotoxin Z, a novel acidic superantigenic toxin produced by a T1 strain of Streptococcus pyogenes. Infect. Immun. 65:3828-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kehoe, M. A., V. Kapur, A. M. Whatmore, and J. M. Musser. 1996. Horizontal gene transfer among group A streptococci: implications for pathogenesis and epidemiology. Trends Microbiol. 4:436-443. [DOI] [PubMed] [Google Scholar]
- 19.Keiser, P., and W. Campbell. 1992. “Toxic strep syndrome” associated with group C streptococcus. Arch. Intern. Med. 152:882-884. [DOI] [PubMed] [Google Scholar]
- 20.Lottenberg, R., C. C. Broder, M. D. Boyle, S. J. Kain, B. L. Schroeder, and R. Curtiss III. 1992. Cloning, sequence analysis, and expression in Escherichia coli of a streptococcal plasmin receptor. J. Bacteriol. 174:5204-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu, H. Z., X. H. Weng, H. Li, Y. K. Yin, M. Y. Pang, and Y. W. Tang. 2002. Enterococcus faecium-related outbreak with molecular evidence of transmission from pigs to humans. J. Clin. Microbiol. 40:40.:913-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin, D. R., and L. A. Single. 1993. Molecular epidemiology of group A streptococcus M type 1 infections. J. Infect. Dis. 167:1112-1117. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita, K., W. Fujimaki, H. Kato, T. Uchiyama, H. Igarashi, H. Ohkuni, S. Nagaoka, M. Kawagoe, S. Kotani, and H. Takada. 1995. Immunopathological activities of extracellular products of Streptococcus mitis, particularly a superantigenic fraction. Infect. Immun. 63:785-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormick, J. K., A. A. Pragman, J. C. Stolpa, D. Y. Leung, and P. M. Schlievert. 2001. Functional characterization of streptococcal pyrogenic exotoxin J, a novel superantigen. Infect. Immun. 69:1381-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murono, K., K. Fujita, M. Saijo, Y. Hirano, J. Zhang, and T. Murai. 1999. Emergence and spread of a new clone of M type 1 group A Streptococcus coincident with the increase in invasive diseases in Japan. Pediatr. Infect. Dis. J. 18:254-257. [DOI] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M100-S10. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 27.Pratter, M. R., and R. S. Irwin. 1980. Viridans streptococcal pulmonary parenchymal infections. JAMA 243:2515-2517. [PubMed] [Google Scholar]
- 28.Proft, T., V. L. Arcus, V. Handley, E. N. Baker, and J. D. Fraser. 2001. Immunological and biochemical characterization of streptococcal pyrogenic exotoxins I and J (SPE-I and SPE-J) from Streptococcus pyogenes. J. Immunol. 166:6711-6719. [DOI] [PubMed] [Google Scholar]
- 29.Schlievert, P. M., K. M. Bettin, and D. W. Watson. 1978. Effect of antipyretics on group A streptococcal pyrogenic exotoxin fever production and ability to enhance lethal endotoxin shock. Proc. Soc. Exp. Biol. Med. 157:472-475. [DOI] [PubMed] [Google Scholar]
- 30.Schlievert, P. M., J. E. Gocke, and J. R. Deringer. 1993. Group B streptococcal toxic shock-like syndrome: report of a case and purification of an associated pyrogenic toxin. Clin. Infect. Dis. 17:26-31. [DOI] [PubMed] [Google Scholar]
- 31.Smith, G. P., and J. K. Scott. 1993. Libraries of peptides and proteins displayed on filamentous phage. Methods Enzymol. 217:228-257. [DOI] [PubMed] [Google Scholar]
- 32.Soto, A., P. H. McWhinney, C. C. Kibbler, and J. Cohen. 1998. Cytokine release and mitogenic activity in the viridans streptococcal shock syndrome. Cytokine 10:370-376. [DOI] [PubMed] [Google Scholar]
- 33.Sriskandan, S., M. Unnikrishnan, T. Krausz, and J. Cohen. 1999. Molecular analysis of the role of streptococcal pyrogenic exotoxin A (SPEA) in invasive soft-tissue infection resulting from Streptococcus pyogenes. Mol. Microbiol. 33:778-790. [DOI] [PubMed] [Google Scholar]
- 34.Stevens, D. L., M. H. Tanner, J. Winship, R. Swarts, K. M. Ries, P. M. Schlievert, and E. Kaplan. 1989. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N. Engl. J. Med. 321:1-7. [DOI] [PubMed] [Google Scholar]
- 35.Sussman, J. I., E. J. Baron, M. J. Tenenbaum, M. H. Kaplan, J. Greenspan, R. R. Facklam, M. B. Tyburski, M. A. Goldman, B. F. Kanzer, and R. A. Pizzarello. 1986. Viridans streptococcal endocarditis: clinical, microbiological, and echocardiographic correlations. J. Infect. Dis. 154:597-603. [DOI] [PubMed] [Google Scholar]
- 36.Talkington, D. F., B. Schwartz, C. M. Black, J. K. Todd, J. Elliott, R. F. Breiman, and R. R. Facklam. 1993. Association of phenotypic and genotypic characteristics of invasive Streptococcus pyogenes isolates with clinical components of streptococcal toxic shock syndrome. Infect. Immun. 61:3369-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang, Y. W., N. M. Ellis, M. K. Hopkins, D. H. Smith, D. E. Dodge, and D. H. Persing. 1998. Comparison of phenotypic and genotypic techniques for identification of unusual aerobic pathogenic gram-negative bacilli. J. Clin. Microbiol. 36:3674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang, Y. W., M. K. Hopkins, C. P. Kolbert, P. A. Hartley, P. J. Severance, and D. H. Persing. 1998. Bordetella holmesii-like organisms associated with septicemia, endocarditis, and respiratory failure. Clin. Infect. Dis. 26:389-392. [DOI] [PubMed] [Google Scholar]
- 39.Willoughby, R., and R. N. Greenberg. 1983. The toxic shock syndrome and streptococcal pyrogenic exotoxins. Ann. Intern. Med. 98:559.. [DOI] [PubMed] [Google Scholar]
- 40.Zhu, B., G. H. Wang, X. H. Sun, Z. G. Luu, Z. Mao, X. N. Jiang, Y. K. Yin, S. F. Guo, and F. X. Lin. 1993. Clinical and experimental studies on scarlet fever associated with Streptococcus mitis. Chinese J. Infect. Dis. 11:68-70. [Google Scholar]