Abstract
An analysis of 691 French clinical Legionella isolates showed that the endemic L. pneumophila serogroup 1 strain Paris was responsible for 12.2% of all cases of legionellosis and had a specific pulsed-field gel electrophoresis pattern. We also demonstrated the presence of this endemic clone throughout Europe.
Legionella pneumophila is a common cause of hospital- and community-acquired pneumonia. About 90% cases of legionellosis are due to this species, and the predominant serogroup (sg) 1 of L. pneumophila accounts for 84% of cases (15). Legionella is present in naturally occurring and man-made water systems and is transmitted to humans by aerosol inhalation (2). The source of infection can be identified by comparing environmental and clinical L. pneumophila isolates with a variety of typing methods. Pulsed-field gel electrophoresis (PFGE) is one of the most widely used typing methods and is generally considered to be highly discriminatory (5, 10-12, 14). PFGE can identify unique strains of L. pneumophila with a specific PFGE profile; these strains are considered sporadic. Most reported cases of legionellosis in Europe are sporadic, as shown by the European Working Group on Legionella Infections (EWGLI) data set (http://www.ewgli.org/data/data_yeardatatables.asp). Confirmed epidemic cases of legionellosis are defined as at least two cases arising from the same source and due to the same strain. The recovery of PGFE-identical isolates over long periods in given countries or continents suggests that some L. pneumophila clones are endemic (13).
The presence of an endemic L. pneumophila sg 1 clone was suspected in 1997 when Lawrence et al. (9) reported that 33% of the cases of legionellosis identified in the Paris area between 1988 and 1997 were caused by a single L. pneumophila sg 1 strain. The cases had no apparent epidemiological links and were both hospital and community acquired. The same strain has since been repeatedly recovered throughout the Paris water distribution network. The distribution in France of this strain, designated L. pneumophila sg 1 strain Paris (CIP 107-629-T), is not known.
The French National Reference Center for Legionella (NRCL) collects all French clinical Legionella isolates and types them by conventional PFGE method by using the SfiI enzyme (9). We reviewed here the epidemiological data on 691 clinical Legionella strains isolated in 118 different towns in France between January 1998 and December 2002.
Legionella pneumophila constituted 98.6% of the 691 isolates (Table 1). L. pneumophila sg 1 was the predominant serogroup (90.0%), and sg 2 to 15 accounted for the remaining serogroups (10.0%). The most commonly isolated non-pneumophila species were L. longbeachae (four cases), L. anisa (four cases), and L. dumoffii and L. gormanii (one case each). This epidemiology is consistent with previous reports (8, 15).
TABLE 1.
Identification of species and subgroups of 691 clinical Legionella isolates received by the NRCL between January 1998 and December 2002
| Legionella sp. and serogroup | No. of isolates | % |
|---|---|---|
| L. pneumophila | 681 | 98.6 |
| 1 | 613 | 88.7 |
| 2 | 5 | 0.7 |
| 3 | 19 | 2.75 |
| 4 | 1 | 0.14 |
| 5 | 6 | 0.87 |
| 6 | 11 | 1.6 |
| 7 | 1 | 0.14 |
| 9 | 1 | 0.14 |
| 12 | 1 | 0.14 |
| 14 | 1 | 0.14 |
| Unknown | 22 | 3.2 |
| L. longbeachae | 4 | 0.6 |
| L. anisa | 4 | 0.6 |
| L. dumoffii | 1 | 0.14 |
| L. gormanii | 1 | 0.14 |
| Total | 691 | 100 |
A total of 559 different PFGE patterns were obtained with the 691 isolates. Most patterns (n = 543) were unique and corresponded to a single Legionella isolate; these were sporadic cases with no identified epidemiological link. Of the 691 isolates, 148 were associated with outbreaks or were linked to the Paris strain (84 isolates). Each of the 16 outbreaks was caused by a strain with a specific PFGE pattern and involved two to nine patients. We showed that the endemic Paris strain predominates in France, accounting for 12.2% (Table 2) of cases of legionellosis. This clone was associated with both hospital-acquired (52.4%) and community-acquired (40.5%) infections and caused both outbreaks and sporadic cases. It was mainly isolated in Paris (64.3%) but was also found in at least 15 other French towns located up to 900 km from Paris. The PFGE pattern of all of the Paris strains did not differ, even by a single band, in all French isolates, regardless of the site or time of isolation (Fig. 1).
TABLE 2.
Incidence and geographical distribution of L. pneumophila serogroup 1 strain Paris in France between 1998 and December 2002
| Yr | Total Legionella clinical strains (n) | Paris strain
|
Town(s) of clinical isolation | ||
|---|---|---|---|---|---|
| n (%) | Cases infected:
|
||||
| In the Paris area (n) | Outside the Paris area (n) | ||||
| 1998 | 126 | 11 (8.7) | 11 | 0 | Paris |
| 1999 | 106 | 8 (7.5) | 7 | 1 | Paris, Nice |
| 2000 | 135 | 29 (21.5) | 21 | 8 | Paris, Antibes, Frejus, Lyon, Marseille, Nice, Poitiers |
| 2001 | 160 | 17 (10.6) | 6 | 11 | Paris, Colmar, Grenoble, Limoges, Lyon, Marseille, Nice, Strasbourg |
| 2002 | 164 | 19 (11.6) | 9 | 10 | Paris, Annecy, Bordeaux, Compiegne, Frejus, Forbach, Lyon, Marseille, Nice, Reims |
| Total | 691 | 84 (12.2) | 54 | 30 | |
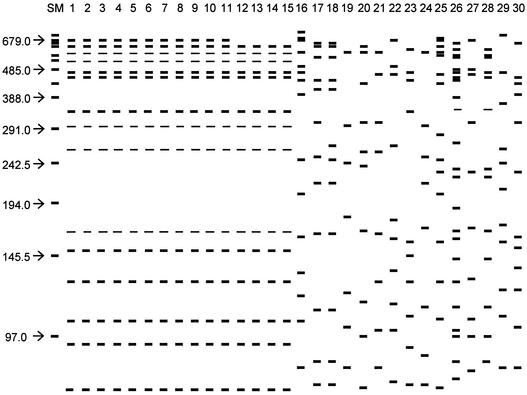
FIG. 1.
Schematic representation of PFGE profiles (using SfiI restriction enzyme) of the following clinical L. pneumophila strains: Paris strain isolated in different towns in France (lanes 1 to 11), strain EUL 1 Switzerland (lane 12), strain EUL 55 Spain (lane 13), strain EUL 104 Sweden (lane 14), strain EUL 37 Italy (lane 15), French epidemic strains (lanes 16 to 19), and French sporadic strains (lanes 20 to 30). Size markers (SM) are given in kilobases.
We also studied 79 unrelated L. pneumophila sg 1 clinical isolates from the European collection of L. pneumophila sg 1, provided by EWGLI, to detect the presence of strain Paris outside France (5). We demonstrated that the PFGE profile of the Paris strain was similar to that of strains EUL 1 and 3 (Switzerland); EUL 37, 38, and 43 (Italy); EUL 53 and 55 (Spain); and EUL 104 (Sweden). Figure 1 shows the pulsotypes of four of these strains; all of the European strains had the same profile, which was identical to the Paris strain except that it lacked a 680-kb fragment. This points to a widespread European distribution of an L. pneumophila sg 1 endemic clone. Endemic clones of L. pneumophila causing apparently unrelated cases of legionellosis have been identified by several authors (3, 4, 7, 10, 11, 13). For instance, Selander et al. observed endemic Legionella clones with a wide geographical distribution in a study of the genetic structure of L. pneumophila populations by multilocus enzyme electrophoresis (13).
The clinical predominance of this strain could be due to its greater abundance in water distribution systems, to its higher virulence, or to its greater facility to be recovered from clinical specimens, although we have no evidence to support either of these hypotheses. Indeed, the PFGE type distribution of environmental L. pneumophila sg 1 strains is not as well characterized in France as that of clinical strains (environmental isolates are not systematically typed by PFGE). The multiplication rate, which could reflect the virulence of the Paris clone for human cells (1, 6), is unknown. However, Lawrence et al. showed that patients infected by the Paris strain did not differ significantly from patients infected by other strains in terms of age, sex, risk factors, need for mechanical ventilation, or mortality (9).
The Paris strain is known to colonize the entire water distribution network in the Paris area since 1987 (9), suggesting that this clone is well adapted to environmental survival. A review of NRCL data show that isolates with the Paris profile have also been isolated from the water distribution systems of at least 10 of the French towns in which clinical strains have been isolated. Other researchers have suggested that widespread geographical diffusion of Legionella strains may occur through rain and wind transportation (13). The apparent spread of the Paris strain in France and Europe might simply reflect improved surveillance and recent routine application of molecular typing methods rather than being a recent phenomenon. The characteristics of the Paris strain appear to be very stable, since PFGE profiles remained stable among all French strains isolated between 1987 and 2002. The strict identity of all Paris isolates remains to be confirmed by multilocus sequence typing, a method based on DNA sequences that would allow us to compare the genetic background of the Paris strain to other clinical Legionella strains.
Acknowledgments
We thank David Young for editing the manuscript.
REFERENCES
- 1.Abu Kwaik, Y., L. Y. Gao, B. J. Stone, C. Venkataraman, and O. S. Harb. 1998. Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl. Environ. Microbiol. 64:3127-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atlas, R. M. 1999. Legionella: from environmental habitats to disease pathology, detection and control. Environ. Microbiol. 1:283-293. [DOI] [PubMed] [Google Scholar]
- 3.De Zoysa, A. S., and T. G. Harrison. 1999. Molecular typing of Legionella pneumophila serogroup 1 by pulsed-field gel electrophoresis with SfiI and comparison of this method with restriction fragment-length polymorphism analysis. J. Med. Microbiol. 48:269-278. [DOI] [PubMed] [Google Scholar]
- 4.Drenning, S. D., J. E. Stout, J. R. Joly, and V. L. Yu. 2001. Unexpected similarity of pulsed-field gel electrophoresis patterns of unrelated clinical isolates of Legionella pneumophila serogroup 1. J. Infect. Dis. 183:628-632. [DOI] [PubMed] [Google Scholar]
- 5.Fry, N. K., S. Alexiou-Daniel, J. M. Bangsborg, S. Bernander, M. Castellani Pastoris, J. Etienne, B. Forsblom, V. Gaia, J. H. Helbig, D. Lindsay, P. C. Lück, C. Pelaz, S. A. Uldum, and T. G. Harrison. 1999. A Multicenter evaluation of genotypic methods for the epidemiologic typing of Legionella pneumophila serogroup 1: results of a pan-European study. Clin. Microbiol. Infect. 5:462-477. [DOI] [PubMed] [Google Scholar]
- 6.Harb, O. S., L.-Y. Gao, and Y. A. Kwaik. 2000. From protozoa to mammalian cells: a new paradigm in the life cycle of intracellular bacterial pathogens. Environ. Microbiol. 2:251-265. [DOI] [PubMed] [Google Scholar]
- 7.Harrison, T. G., N. A. Saunders, A. Haththotuwa, G. Hallas, R. J. Birtles, and A. G. Taylor. 1990. Phenotypic variation amongst genotypically homogeneous Legionella pneumophila serogroup 1 isolates: implications for the investigation of outbreaks of Legionnaires' disease. Epidemiol. Infect. 104:171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helbig, J. H., S. Bernander, M. Castellani Pastoris, J. Etienne, V. Gaia, S. Lauwers, D. Lindsay, P. C. Luck, T. Marques, S. Mentula, M. F. Peeters, C. Pelaz, M. Struelens, S. A. Uldum, G. Wewalka, and T. G. Harrison. 2002. Pan-European study on culture-proven legionnaires' disease: distribution of Legionella pneumophila serogroups and monoclonal subgroups. Eur. J. Clin. Microbiol. Infect. Dis. 21:710-716. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence, C., M. Reyrolle, S. Dubrou, F. Forey, B. Decludt, C. Goulvestre, P. Matsiota-Bernard, J. Etienne, and C. Nauciel. 1999. Single clonal origin of a high proportion of Legionella pneumophila serogroup 1 isolates from patients and the environment in the area of Paris, France, over a 10-year period. J. Clin. Microbiol. 37:2652-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lück, P. C., J. H. Helbig, V. Günter, M. Assman, R. Blau, H. Koch, and M. Klepp. 1994. Epidemiologic investigation by macrorestriction analysis and by using monoclonal antibodies of nosocomial pneumonia caused by Legionella pneumophila serogroup 10. J. Clin. Microbiol. 32:2692-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pruckler, J. M., L. A. Mermel, R. F. Benson, C. Giorgio, P. K. Cassiday, R. F. Breiman, C. G. Whitney, and B. S. Fields. 1995. Comparison of Legionella pneumophila isolates by arbitrarily primed PCR and pulsed-field gel electrophoresis: analysis from seven epidemic investigations. J. Clin. Microbiol. 33:2872-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoonmaker, D., T. Heimberger, and G. Birkhead. 1992. Comparison of ribotyping and restriction enzyme analysis using pulsed-field gel electrophoresis for distinguishing Legionella pneumophila isolates obtained during a nosocomial outbreak. J. Clin. Microbiol. 30:1491-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selander, R. K., R. M. McKinney, T. S. Whittam, W. F. Bibb, D. J. Brenner, F. S. Nolte, and P. E. Pattison. 1985. Genetic structure of populations of Legionella pneumophila. J. Bacteriol. 163:1021-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Struelens, M. J. 1996. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin. Microbiol. Infect. 2:2-11. [DOI] [PubMed] [Google Scholar]
- 15.Yu, V. L., J. F. Plouffe, M. C. Pastoris, J. E. Stout, M. Schousboe, A. Widmer, J. Summersgill, T. File, C. M. Heath, D. L. Paterson, and A. Chereshsky. 2002. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J. Infect. Dis. 186:127-128. [DOI] [PubMed] [Google Scholar]