Abstract
We report a case of Lemierre's syndrome due to Fusobacterium nucleatum in a previously healthy 19-year-old male. This is the first case report of Lemierre's syndrome due to thrombophlebitis of the external jugular vein. The patient had a rapid clinical response to anticoagulation and antibiotics, as supported by anecdotal evidence.
CASE REPORT
A 19-year-old African-American male presented in the emergency room complaining of a 5-day history of fever, rigors, sore throat, pleuritic chest pain, and productive cough with blood-tinged sputum and a 2-day history of nausea and vomiting. One day prior to admission, the patient had presented in the emergency room with the same complaints. He was treated intravenously with ketorolac (60 mg) for his pain and discharged to his home with a course of amoxicillin for a presumptive case of pharyngitis. The patient had otherwise been healthy, and past medical history was remarkable for treatment of gonorrhea and chlamydia 1 month prior. Review of symptoms was negative for headache, photophobia, and dysuria, and the patient had no history of recent travel, tick exposure, or intravenous drug use.
On presentation, the patient had scleral icterus and was obviously uncomfortable. Vital signs included a temperature of 39.1°C, a regular pulse of 105 beats per minute, a respiratory rate of 20 breaths per minute, and supine blood pressure of 130 over 60. Oral examination demonstrated erythema and swelling of the posterior pharyngeal mucosa and tonsils, yet without exudates. The neck was diffusely tender to palpation. Multiple, nontender posterior cervical lymph nodes were palpable bilaterally, which the patient stated had been the case for several years. The lungs were clear to auscultation; however, the patient complained of diffuse chest tenderness to palpation. Cardiac exam results were significant for tachycardia, but no murmurs, rubs, or gallops were detected by auscultation. Abdominal exam results were negative for tenderness and for hepatosplenomegaly and other masses. Skin showed no rashes or petechiae, and the neurologic exam results were normal.
Laboratory data were significant for the following: leukocyte count, 12,600/mm3 (93% granulocytes, 2.8% lymphocytes); platelet count, 41,000/mm3; sodium, 127 mmol/liter; potassium, 3.1 mmol/liter; chloride, 87 mmol/liter; bicarbonate, 29 mmol/liter; blood urea nitrogen, 26 mg/dl; creatinine, 1.4 mg/dl; total bilirubin, 4.0 mg/dl; and lactate dehydrogenase, 260 U/liter. Hemoglobin level and hematocrit were normal at 14.0 g/dl and 41.7%, respectively. Specimens for a human immunodeficiency virus enzyme-linked immunoassay, a monospot, a hepatitis panel, and a drug screen and three sets of cardiac enzymes were drawn, and results were later returned as normal. Electrocardiogram results were significant for sinus tachycardia and high QRS voltage in chest leads V1 to V5. Results of a chest radiograph performed upon admission failed to demonstrate any acute process.
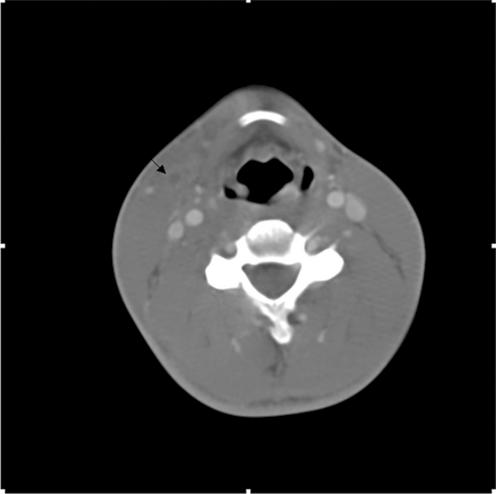
The patient was admitted to the Internal Medicine Hospitalist Service for further evaluation. Differential diagnoses included acute human immunodeficiency virus syndrome, acute hepatitis, mononucleosis, pneumonia, and tonsillitis. Intravenous levofloxacin treatment was initiated upon admission, and the patient was kept under close observation. The following day, two of four sets of cultures from blood samples inoculated into media at the time of admission showed anaerobic gram-negative rods. Ampicillin-sulbactam (1.5 g intravenously every 6 h) replaced levofloxacin. On hospital day 4, the organism in the blood culture was identified as Fusobacterium nucleatum. A presumptive diagnosis of Lemierre's syndrome was made, and antibiotic therapy was streamlined to ampicillin (2 g intravenously every 4 h). A contrast-enhanced computed tomogram (CT) scan of the neck revealed a hypodense filling defect suggestive of thrombus in the right external jugular vein (EJV), as well as parapharyngeal soft tissue edema without evidence of abscess (Fig. 1). A CT scan of the chest with contrast suggested septic emboli as well as bibasilar pneumonia. Results of abdominal and pelvic CT scans were normal. Despite appropriate antibiotic treatment, the patient remained febrile and complained of continuous chest pain through hospital day 4. After much discussion concerning the patient's failure to improve clinically, intravenous heparin treatment was initiated.
FIG. 1.
Results of a contrast-enhanced neck CT scan with arrow indicating a right EJV thrombus.
The patient improved clinically on hospital day 5, less than 24 h after the heparin treatment was started. Although the patient certainly could have improved without the heparin, the temporal relationship between the two might be considered more than coincidental. The platelet count returned to normal in conjunction with the resolution of symptoms (41,000/mm3 at admission to 160,000/mm3 on hospital day 5). Despite repeated attempts by the hospital staff to explain to the patient the severity of his condition and the necessity for intravenous antibiotic therapy, the patient left against medical advice on hospital day 7. A 4-week course of metronidazole (500 mg three times a day) was prescribed. The patient did not keep his follow-up appointment.
Lemierre's syndrome, or postanginal sepsis syndrome, begins as an oropharyngeal infection resulting in internal jugular vein (IJV) thrombophlebitis and subsequent metastatic infectious processes in previously healthy, young individuals. Classically, Lemierre's syndrome is caused by the pathogen Fusobacterium necrophorum, formerly known as Bacillus fundiliformis (11). Other organisms implicated as causes of Lemierre's syndrome include streptococcus species, staphylococci, Eikenella corrodens, Peptostreptococcus species, Bacteroides species, and other Fusobacterium species, including F. nucleatum as in this case (1, 11). The oropharyngeal infection caused by these pathogens spreads to the IJV via several anatomic routes, including propagation of thrombophlebitis from local oropharyngeal veins to the IJV, drainage of septic lymph leading to perivenous inflammation and ensuing IJV thrombophlebitis, and direct extension of infection through the neck tissue (11). Once IJV thrombophlebitis occurs, metastatic infections develop, and death may occur if a patient is left untreated, allowing the suppurative infection to consume the vessel wall, resulting in rupture into the ear, mediastinum, and cranial vault (3).
While one may be unable to differentiate clinically between infections produced by F. necrophorum and F. nucleatum, these organisms are distinguished from one another in the laboratory. F. necrophorum is described as a pleomorphic gram-negative anaerobe, with forms ranging from those of coccobacilli to long filaments, and is nonmotile and non-spore forming, in contrast to F. nucleatum, which is a more slender rod with sharply pointed ends (5, 11, 16, 18). F. necrophorum and F. nucleatum are discriminated from other species by their common abilities to grow in 20% bile, produce indole, display lipase activity, and form gas in glucose agar (5, 18). Furthermore, F. necrophorum is identified from among other Fusobacterium species by gas-liquid chromatography by its unique ability to ferment lactate to propionate and its hemolytic appearance in culture (5, 16). However, Hagelskjaer and Prag state that F. necrophorum is not regularly positive for alkaline phosphatase activity and a false negative test may fallaciously indicate the presence of F. nucleatum (5).
Among anaerobes of the normal oral, gastrointestinal, female genitourinary tract, and upper aerodigestive tract flora, F. necrophorum and F. nucleatum are among the most virulent species (4, 11, 12). Unique to anaerobes, F. necrophorum has the capability of invading as a primary pathogen in previously healthy individuals, most likely due to its toxins (4, 18). Similar to nonanaerobic gram-negative rods, F. necrophorum produces a lipopolysaccharide endotoxin. Exotoxins produced include hemolysin, heparinase, leucocidin, and other proteolytic enzymes (3, 5, 12). These exotoxins have been demonstrated in culture to depend on the leucocidin to produce an inflammatory response (18). It has been suggested that hemolysin may create an anaerobic environment by lysing erythrocytes and hence substantially limiting oxygen transport to the site of primary infection, making the site favorable for F. necrophorum growth (5). Creating an anaerobic environment allows heparinase and other proteolytic enzymes to aid in invasion of regional veins without destruction of tissue (3). In addition, in vitro studies have ascertained that the lipid A component of the lipopolysaccharide endotoxin produced by F. necrophorum can activate human Hageman factor and, hence, the intrinsic coagulation pathway and presumably disseminated intravascular coagulation (DIC) (15). Along the same lines, F. necrophorum is able to aggregate platelets on contact, not dependent on bacterial metabolism or even cell-cell adhesion (18). Whether or not this is the mechanism of the thrombocytopenia seen in some cases, including this case, is not known.
Similar to that of F. necrophorum, F. nucleatum's pathogenic mechanism relies on proteolytic enzymes to destroy oropharyngeal tissue (16). Common to both species, release of butyric acid prevents proliferation of fibroblasts and may result in the penetration of epithelium (16). Roberts further suggests that F. nucleatum may be able to activate human procollagenase, leading to the disintegration of surrounding connective tissue. As a result, adhesins on the surface of the anaerobe enable it to cohere to underlying neutrophils, provoking the release of the neutrophils' tissue-damaging lysosomal enzymes (16).
Findings of clinical examinations of patients with Lemierre's syndrome upon presentation are quite variable (5). Signs of exudative tonsillitis, pharyngitis, and thick gray pseudomembranes, as well as oral ulcers, have been reported (4, 17, 18). However, results of oropharyngeal examination may be normal since sepsis typically occurs 1 week after primary infection, allowing time for physical findings to resolve (4, 17). Other reported sources of primary infection include otitis media, mastoiditis complicated by Bezold's abscesses, parotitis, tooth infections, and sinusitis (5, 11). Neck pain, swelling, and trismus along the anterior border of the sternocleidomastoid muscle and at the angle of the mandible are the clinical manifestations of a septic thrombophlebitis of the ipsilateral IJV (1, 5, 10, 11, 14, 18). Signs and symptoms of pleuropulmonary embolization may be the presenting clinical finding, especially in cases in which oropharyngeal manifestations have resolved (1). Persistent fever and rigors despite antibiotics followed by complaint of pleuritic chest pain, hemoptysis, or dyspnea may be all that is required for presumptive diagnosis (10).
Opinions vary with regard to the best imaging modality for demonstration of IJV thrombophlebitis. It has been suggested that ultrasound should be utilized as an initial adjunct to CT scan to periodically follow the development and resolution of the thrombus and to diagnose suspected hepatosplenic abscesses (4, 11). However, Koay et al. argue that imaging for IJV thrombophlebitis should not be used as it does not alter therapy unless IJV ligation or use of anticoagulation is considered or unless it is used as an aid in the diagnosis of a suspected abscess (8). High-resolution chest CT scan or chest radiography can identify a pulmonary abscess or emboli (4).
If the correct diagnosis is missed upon initial presentation, particularly if the primary infection has resolved, distant metastases of infection may be the first clue to diagnosis (19). Sinave et al. report that in 6 of 38 cases (15.8%), patients developed metastases to the joints, manifesting as arthralgias or septic arthritis (18). Osteomyelitis, soft tissue abscesses, and meningeal involvement are less frequently reported complications (4, 5, 11, 18). Diffuse abdominal pain can be a chief complaint in Lemierre's syndrome. Although no definitive pathophysiologic explanation exists, it has been hypothesized that abdominal pain is secondary to either microabscesses or thrombophlebitis of the pelvic veins (5, 6). Fusibacter sepsis may involve the hepatobiliary system, manifesting as hepatosplenomegaly, scleral icterus, or “bacteremic jaundice” and detected as subclinical hyperbilirubinemia with slight elevation of liver function in up to 50% of cases (2, 4, 5, 6, 18). Liver biopsy may demonstrate intrahepatic cholestasis with little or no hepatocyte necrosis (18). Elevated blood urea nitrogen and creatinine levels, elevated erythrocyte sedimentation rate, and transient hematuria have also been noted (18).
As previously mentioned, hematologic abnormalities other than leukocytosis with a left shift can occur and include increased prothrombin conversion time with a normal fibrinogen and fibrin split products level, thrombocytopenia, and an isolated low level of antithrombin III (15). There is no mention in the literature of a correlation between resolution of thrombocytopenia and clinical improvement, as occurred in this patient. In addition, Hakelskjaer et al. (6) found that in 7 of 49 cases (14.3%), patients developed DIC. Nonetheless, DIC was mild in each case, did not require specific treatment, and was not associated with increased mortality (6).
At this time, proposed antibiotic regimens include beta-lactam agents (amoxicillin-clavulanate, ticarcillin-clavulanate, and cephalosporins) and metronidazole for several weeks (6), high-dose penicillin and metronidazole, and monotherapy with clindamycin for 2 to 6 weeks (5). Metronidazole is an attractive treatment choice for septic thrombi because it has good penetration of tissue (8). Since it is well absorbed into the gastrointestinal tract, it can be given orally once the patient becomes clinically stable (8). If the patient continues to suffer from refractory sepsis and repeated pulmonary emboli despite medical management, surgical ligation or excision of the IJV is required (1, 4, 5, 8, 9, 11, 12, 15, 18, 19).
Due to the increasing amount of anecdotal evidence, the use of anticoagulation as an integral part of the suggested therapeutic regimen is perhaps less controversial today than in years past. Some authors have suggested that anticoagulants should be added to an appropriate antibiotic regimen, once the diagnosis of Lemierre's syndrome has been established (18). Moore et al. state that in a review of 41 cases of Lemierre's syndrome, 11 patients were found to have improved following the addition of anticoagulation treatment, thus endorsing its use in cases of extensive thrombosis (13). Similarly, there has been a controlled study in the gynecologic literature suggesting the effectiveness of heparin therapy in treating septic pelvic thrombophlebitis. Josey and Staggers report that 42 of 46 patients treated with heparin and antibiotics for septic pelvic thrombophlebitis became afebrile in an average of 2.5 days, shortly after the therapeutic value for clotting time was achieved (7). This case supports previously described anecdotal evidence that anticoagulation treatment should be considered for patients with poor clinical responses despite 48 to 72 h of antibiotic therapy (3, 5, 11). Although controlled studies are needed to clarify optimal treatment, the rare incidence of this illness probably limits studies to anecdotal descriptions.
In conclusion, this is an interesting case of Lemierre's syndrome demonstrating the unique finding of an EJV septic thrombophlebitis rather than the classic IJV thrombophlebitis. No other case of Lemierre's syndrome due to EJV septic thrombophlebitis could be found in the literature. It also provides an opportunity to review F. necrophorum and F. nucleatum, two potentially virulent anaerobes of the normal oropharyngeal flora. In addition, this case highlights the hematologic abnormality of isolated thrombocytopenia and supports a correlation of normalizing platelet count with clinical improvement. The issue of utilizing anticoagulation in the absence of contraindication to hasten defervescence and clinical improvement as well as minimize embolic phenomenon remains unresolved.
REFERENCES
- 1.Bach, M. C., J. H. Roediger, and H. M. Rinder. 1988. Septic anaerobic jugular phlebitis with pulmonary embolism: problems in management. Rev. Infect. Dis. 10:424-427. [DOI] [PubMed] [Google Scholar]
- 2.DeSena, S., D. L. Rosenfeld, S. Santos, and I. Keller. 1996. Jugular thrombophlebitis complicating bacterial pharyngitis (Lemierre's syndrome). Pediatr. Radiol. 26:141-144. [DOI] [PubMed] [Google Scholar]
- 3.Goldenhagen, J., B. A. Alford, L. H. Prewitt, L. Thompson, and M. K. Hostetter. 1988. Suppurative thrombophlebitis of the internal jugular vein: report of three cases and review of the pediatric literature. Pediatr. Infect. Dis. J. 7:410-414. [DOI] [PubMed] [Google Scholar]
- 4.Golpe, R., B. Marin, and M. Alonso. 1999. Lemierre's syndrome (necrobacillosis). Postgrad. Med. J. 75:141-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagelskjaer, K. L., and J. Prag. 2000. Human necrobacillosis, with emphasis on Lemierre's syndrome. Clin. Infect. Dis. 31:524-532. [DOI] [PubMed] [Google Scholar]
- 6.Hagelskjaer, L. H., J. Prag, J. Malcynzski, and J. H. Kristensen. 1998. Incidence and clinical epidemiology of necrobacillosis, including Lemierre's syndrome, in Denmark 1990-1995. Eur. J. Clin. Microbiol. Infect. Dis. 17:561-565. [DOI] [PubMed] [Google Scholar]
- 7.Josey, W. E., and S. R. Staggers. 1974. Heparin therapy in septic pelvic thrombophlebitis: a study of 46 cases. Am. J. Obstet. Gynecol. 120:228-233. [DOI] [PubMed] [Google Scholar]
- 8.Koay, C. B., T. Heyworth, and P. Burden. 1995. Lemierre syndrome—a forgotten complication of acute tonsillitis. J. Laryngol. Otol. 109:657-661. [DOI] [PubMed] [Google Scholar]
- 9.Lee, B., F. Lopez, M. Genovese, and J. S. Loutit. 1997. Lemierre's syndrome. South. Med. J. 90:640-643. [DOI] [PubMed] [Google Scholar]
- 10.Lemierre, A. 1936. On certain septicemias due to anaerobic organisms. Lancet i:701-703.
- 11.Lustig, L. R., B. C. Cusick, S. W. Cheung, and K. C. Lee. 1995. Lemierre's syndrome: two cases of postanginal sepsis. Otolaryngol. Head Neck Surg. 112:767-772. [DOI] [PubMed] [Google Scholar]
- 12.Moller, K., and B. Dreijer. 1997. Post-anginal sepsis (Lemierre's disease): a persistent challenge. Presentation of 4 cases. Scand. J. Infect. Dis. 29:191-194. [DOI] [PubMed] [Google Scholar]
- 13.Moore, B., C. Dekle, and J. Werkhaven. 2002. Bilateral Lemierre's syndrome: a case report and literature review. Ear Nose Throat J. 81(4):234-245. [PubMed]
- 14.Moreno, S., J. G. Altozano, B. Pinilla, J. C. Lopez, B. de Quiros, and E. Bouza. 1989. Lemierre's disease: postanginal bacteremia and pulmonary involvement caused by Fusobacterium necrophorum. Rev. Infect. Dis. 11(2):319-324. [DOI] [PubMed] [Google Scholar]
- 15.Page, Y., C. Comtet, B. Tardy, F. Zeni, D. Thevenet, F. Lucht, and J. C. Bertrand. 1990. Disseminated intravascular coagulation in Fusobacterium necrophorum septicemia. Scand. J. Infect. Dis. 22:743-747. [DOI] [PubMed] [Google Scholar]
- 16.Roberts, G. L. 2000. Fusobacterial infections: an underestimated threat. Br. J. Biomed. Sci. 57(2):156-162. [PubMed] [Google Scholar]
- 17.Seidenfeld, S. M., W. L. Sutker, and J. P. Luby. 1982. Fusobacterium necrophorum septicemia following oropharyngeal infection. JAMA 248:1348-1350. [PubMed] [Google Scholar]
- 18.Sinave, C. P., G. J. Hardy, and P. W. Fardy. 1989. The Lemierre syndrome: suppurative thrombophlebitis of the internal jugular vein secondary to oropharyngeal infection. Medicine 68:85-94. [PubMed] [Google Scholar]
- 19.Weesner, C. L., and J. E. Ciseks. 1993. Lemierre syndrome: the forgotten disease. Ann. Emerg. Med. 22:256-258. [DOI] [PubMed] [Google Scholar]