Abstract
We report here on the characterization of a vancomycin-resistant Enterococcus faecalis (VREF) isolated from a dog with mastitis. The isolate was positive for the vanA, ermB, and tet(M) genes, with vanA and ermB carried on the same transferable plasmid. Comparison of this isolate with VREF from poultry and human sources in New Zealand demonstrated identical SmaI macrorestriction patterns and Tn1546-like elements. This is further evidence of a clonal lineage of VREF in New Zealand.
Vancomycin-resistant enterococci (VRE) have become an important source of nosocomial infection since their emergence in the late 1980s (23). Although VRE were first isolated in humans, the discovery of their carriage in food animals in 1993 (2) led to the hypothesis that animals could be a potential reservoir of resistance genes. A possible factor in the development of vancomycin resistance outside of the hospital environment was the widespread use of the glycopeptide growth promotant avoparcin in Europe.
In New Zealand, avoparcin was used in poultry animals from 1977 to June 2000 as a prophylactic for necrotic enteritis caused by Clostridium perfringens. VRE have been isolated from poultry sources in New Zealand (18); however, VRE causing infection in the clinical situation are rare (17). Chromosomal macrorestriction analysis of VRE isolates from poultry and humans in New Zealand has demonstrated that a dominant clonal lineage of vancomycin-resistant Enterococcus faecalis (VREF) exists (18). In this report, we describe the first VREF isolate (AR01/DG) recovered from a dog in New Zealand and show that it belongs to the same clonal lineage described in poultry and humans.
Antimicrobial susceptibility testing was carried out with E-test strips (AB BIODISK, Solna, Sweden) for vancomycin, gentamicin, ampicillin, erythromycin, and tetracycline in accordance with the manufacturer's instructions.
Species identification of the isolate was carried out by automated gram-positive identification (GPI Vitek card; bioMérieux, Hazelwood, Mo.). To corroborate its identification to the species level, the isolate was tested for the presence of the gene encoding E. faecalis antigen A (efaA) (22) as described previously (18). Detection of the vanA, ermB, tet(M), and vanX genes was determined by PCR as described by Manson et al. (18).
Cells for preparation of genomic DNA in agarose were grown to an optical density at 650 nm of 0.6 in 10 ml of brain heart infusion broth (Becton Dickinson & Co., Sparks, Md.). DNA embedded in agarose plugs was prepared, digested, and resolved by pulsed-field gel electrophoresis (PFGE) (18).
The Tn1546-like element was amplified with an Expand long-template PCR system (Roche Molecular Biochemicals, Mannheim, Germany) and primers described by Descheemaeker et al. (7), followed by ClaI digestion as described previously (18). Enterococcus faecium BM4147 was used as a positive control in the long-template PCR amplification and digestion of Tn1546.
Radiolabeled PCR products were prepared by incorporation of [α-32P]dCTP-labeled deoxynucleotides (Amersham) with an RTS Radprime DNA labeling kit (Gibco BRL Life Technologies, Gaithersburg, Md.). Southern transfer and hybridization were performed as described previously (18).
Transfer experiments were performed with broth as described by Christie et al. (6), with E. faecalis JH2-2 (13) and E. faecium GE-1 (10) as recipients. Transconjugants were selected on brain heart infusion agar (Becton Dickinson & Co.) containing vancomycin (32 μg/ml), rifampin (50 μg/ml), and fusidic acid (25 μg/ml).
Isolate AR01/DG was recovered from a swab of a 9-year-old dog with mastitis. The dog was first seen on 25 May 2001 and treated with a 6-day course of a cephalosporin antibiotic (cephalexin). Five days later, this was changed to a 5-day course of doxycycline. On 5 June, a swab was taken from the infection site and the dog was treated with amoxicillin-clavulanic acid (Augmentin), which subsequently cleared up the infection. The dog was put down on 15 June because of the presence of an underlying aggressive mammary tumor.
The isolate AR01/DG was identified, with biochemical testing and a species-specific probe, as E. faecalis and found to be susceptible to ampicillin (MIC, 0.25 μg/ml) and gentamicin (MIC, 2 μg/ml) but resistant to vancomycin, erythromycin, and tetracycline (MICs of ≥256, ≥256, and 64 μg/ml, respectively). The presence of the antibiotic resistance genes vanA, ermB, and tet(M) was confirmed by PCR.
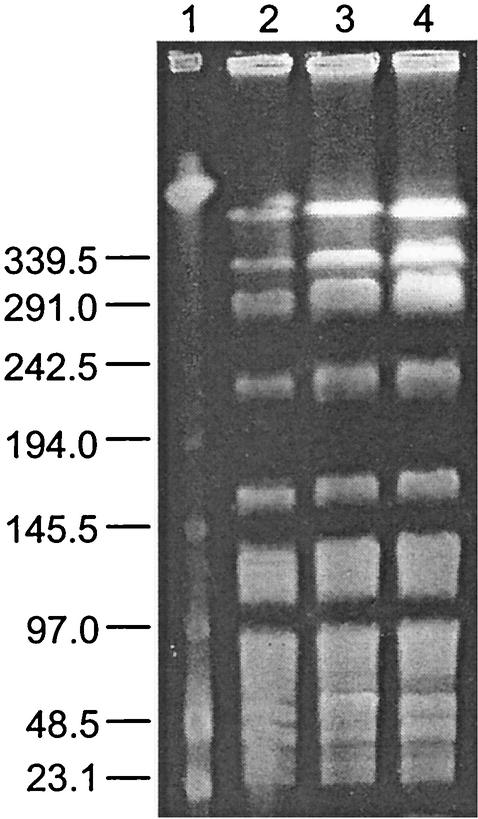
The genomic DNA fingerprint of AR01/DG, when digested with SmaI, was found to be genetically identical to the predominant poultry and human VREF clone found in New Zealand (18) (Fig. 1). Because of the genetic similarity of isolate AR01/DG and New Zealand poultry VREF, the vanX gene was sequenced, and this revealed a guanine base pair variation at position 8234, as previously reported for poultry isolates (14). ClaI restriction fragment length polymorphism analysis of the Tn1546-like element from isolate AR01/DG showed a pattern identical to those of both New Zealand poultry and human VREF isolates with the same PFGE pattern (pattern 1) (data not shown). The presence of identical Tn1546 types among these isolates implies horizontal gene transfer from a common resistance gene pool.
FIG. 1.
PFGE of SmaI macrorestriction patterns of VREF. Lanes: 1, lambda DNA ladder standard; 2, dog VREF isolate (AR01/DG); 3, poultry VREF isolate (5A-13); 4, human VREF isolate (AR96/36). Sizes are indicated in kilobases on the left.
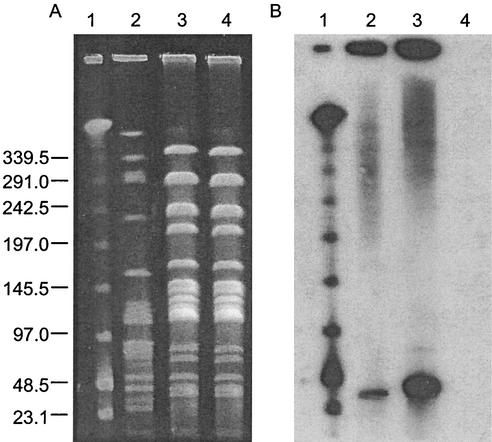
Resistance to erythromycin and vancomycin was transferred via broth mating with a frequency of conjugation of 1.23 × 10−10 per recipient to E. faecalis JH2-2 but not to E. faecium GE-1 (<1.0−11 per recipient). The transfer of antibiotic resistance genes was confirmed by hybridization of the vanA gene (Fig. 2B) and the ermB gene (data not shown) to a 36-kb SmaI fragment in AR01/DG and the transconjugant JH2-DG. Furthermore, I-CeuI digestion of the genomic DNA from isolate AR01/DG and transconjugant JH2-DG showed a band of the same size in both the digested and nondigested lanes, which hybridized to both the vanA and ermB genes (data not shown). Since I-CeuI cuts only chromosomal DNA, cleaving at a site in the rRNA operon, this implies that these genes are located together on a plasmid of approximately 36 kb. In our previous study, no transfer of the vanA and ermB genes, located on a 61-kb plasmid, was seen in human or poultry VREF isolates with the same PFGE pattern (pattern 1) (18). The plasmid containing the resistance genes is considerably smaller than that of the poultry and human VREF clone, and this may account for the differences in plasmid transferability between AR01/DG and other isolates of the same genotype. Southern blotting of the I-CeuI-digested DNA with a tet(M) gene probe showed hybridization, in both the digested and nondigested lanes, to a larger band of approximately 72 kb (data not shown).
FIG. 2.
Conjugational transfer of vancomycin resistance. PFGE of SmaI macrorestriction patterns (A) and corresponding Southern blot hybridized with a vanA gene probe (B). Lanes: 1, lambda DNA ladder standard; 2, donor dog VREF isolate AR01/DG; 3, transconjugant JH2-DG; 4, recipient E. faecalis JH2-2. Sizes are indicated in kilobases on the left.
To our knowledge, this is the first report of a VRE isolate recovered from a companion animal in New Zealand. Enterococcal infections are rare in animals, although it has been reported in Canada that enterococci causing urinary tract infections in dogs have increased significantly over the last 15 years (19). A VRE isolate has recently been documented as the causative agent of a canine urinary tract infection in the United States (21).
Studies on the colonization of VRE in companion animals are scarce, although VRE have been recorded in the intestinal tracts of dogs (3, 8, 24). The first documented canine VRE was isolated in Britain (3). It was later established that VRE colonization of dogs is not uncommon. Van Belkum et al. (24) found that 26% of the dogs tested were colonized with VanA-type E. faecium, and Devriese et al. (8) isolated a total of four VanA-type E. faecium strains from 49 canine fecal samples (8%). This is consistent with other European data, which show that VanA-type E. faecium dominates in VRE from animal, human, and environmental sources (1, 4, 8, 11, 12, 15, 20, 25). In these cases, it was suggested that colonization could be due to the ingestion of contaminated raw meat. VRE has been found previously in raw meat products (5, 15, 16) and also in dry dog food (9).
In a recent American study (21), a comparison of 13 canine enterococcal isolates with a database of 300 human VRE isolates revealed no PFGE pattern similarity. This suggests that there is little transfer of Enterococcus strains between these two sources. The fact that the same VREF clone has been found associated with canine and human infections in New Zealand raises the possibility of a common source of VREF and/or transfer of this bacterium between companion animals and humans.
Acknowledgments
We thank Alistair Newbold and Southern Community Laboratories, Dunedin, New Zealand, for supplying the AR01/DG isolate.
REFERENCES
- 1.Aarestrup, F. M., P. Ahrens, M. Madsen, L. V. Pallesen, R. L. Poulsen, and H. Westh. 1996. Glycopeptide susceptibility among Danish Enterococcus faecium and Enterococcus faecalis isolates of animal and human origin and PCR identification of genes within the VanA cluster. Antimicrob. Agents Chemother. 40:1938-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates, J., J. Z. Jordens, and D. T. Griffiths. 1994. Farm animals as a putative reservoir for vancomycin-resistant enterococcal infection in man. J. Antimicrob. Chemother. 34:507-514. [DOI] [PubMed] [Google Scholar]
- 3.Bates, J., Z. Jordens, and J. B. Selkon. 1993. Evidence for an animal origin of vancomycin-resistant enterococci. Lancet 342:490-491. [DOI] [PubMed] [Google Scholar]
- 4.Borgen, K., G. S. Simonsen, A. Sundsfjord, Y. Wasteson, O. Olsvik, and H. Kruse. 2000. Continuing high prevalence of VanA-type vancomycin-resistant enterococci on Norwegian poultry farms three years after avoparcin was banned. J. Appl. Microbiol. 89:478-485. [DOI] [PubMed] [Google Scholar]
- 5.Borgen, K., M. Sorum, Y. Wasteson, and H. Kruse. 2001. VanA-type vancomycin-resistant enterococci (VRE) remain prevalent in poultry carcasses 3 years after avoparcin was banned. Int. J. Food Microbiol. 64:89-94. [DOI] [PubMed] [Google Scholar]
- 6.Christie, P. J., R. Z. Korman, S. A. Zahler, J. C. Adsit, and G. M. Dunny. 1987. Two conjugation systems associated with Streptococcus faecalis plasmid pCF10: identification of a conjugative transposon that transfers between S. faecalis and Bacillus subtilis. J. Bacteriol. 169:2529-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Descheemaeker, P. R., S. Chapelle, L. A. Devriese, P. Butaye, P. Vandamme, and H. Goossens. 1999. Comparison of glycopeptide-resistant Enterococcus faecium isolates and glycopeptide resistance genes of human and animal origins. Antimicrob. Agents Chemother. 43:2032-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devriese, L. A., M. Ieven, H. Goossens, P. Vandamme, B. Pot, J. Hommez, and F. Haesebrouck. 1996. Presence of vancomycin-resistant enterococci in farm and pet animals. Antimicrob. Agents Chemother. 40:2285-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunne, M. B., B. S. Dunne, and D. Smith. 1996. Watch out where the huskies go. ASM News 62:283. [Google Scholar]
- 10.Eliopoulos, G. M., C. Wennersten, S. Zighelboim-Daum, E. Reiszner, D. Goldmann, and R. C. Moellering, Jr. 1988. High-level resistance to gentamicin in clinical isolates of Streptococcus (Enterococcus) faecium. Antimicrob. Agents Chemother. 32:1528-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gambarotto, K., M. C. Ploy, F. Dupron, M. Giangiobbe, and F. Denis. 2001. Occurrence of vancomycin-resistant enterococci in pork and poultry products from a cattle-rearing area of France. J. Clin. Microbiol. 39:2354-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambarotto, K., M. C. Ploy, P. Turlure, C. Grelaud, C. Martin, D. Bordessoule, and F. Denis. 2000. Prevalence of vancomycin-resistant enterococci in fecal samples from hospitalized patients and nonhospitalized controls in a cattle-rearing area of France. J. Clin. Microbiol. 38:620-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen, L. B. 1998. Differences in the occurrence of two base pair variants of Tn1546 from vancomycin-resistant enterococci from humans, pigs, and poultry. Antimicrob. Agents Chemother. 42:2463-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klare, I., H. Heier, H. Claus, G. Bohme, S. Marin, G. Seltmann, R. Hakenbeck, V. Antanassova, and W. Witte. 1995. Enterococcus faecium strains with vanA-mediated high-level glycopeptide resistance isolated from animal foodstuffs and fecal samples of humans in the community. Microb. Drug Resist. 1:265-272. [DOI] [PubMed] [Google Scholar]
- 16.Klein, G., A. Pack, and G. Reuter. 1998. Antibiotic resistance patterns of enterococci and occurrence of vancomycin-resistant enterococci in raw minced beef and pork in Germany. Appl. Environ. Microbiol. 64:1825-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi, K., M. Rao, S. Keis, F. A. Rainey, J. M. Smith, and G. M. Cook. 2000. Enterococci with reduced susceptibility to vancomycin in New Zealand. J. Antimicrob. Chemother. 46:405-410. [DOI] [PubMed] [Google Scholar]
- 18.Manson, J. M., S. Keis, J. M. B. Smith, and G. M. Cook. 2003. A clonal lineage of VanA-type Enterococcus faecalis predominates in vancomycin-resistant enterococci isolated in New Zealand. Antimicrob. Agents Chemother. 47:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prescott, J. F., W. J. Hanna, R. Reid-Smith, and K. Drost. 2002. Antimicrobial drug use and resistance in dogs. Can. Vet. J. 43:107-116. [PMC free article] [PubMed] [Google Scholar]
- 20.Schouten, M. A., J. A. Hoogkamp-Korstanje, J. F. Meis, and A. Voss. 2000. Prevalence of vancomycin-resistant enterococci in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 19:816-822. [DOI] [PubMed] [Google Scholar]
- 21.Simjee, S., D. G. White, P. F. McDermott, D. D. Wagner, M. J. Zervos, S. M. Donabedian, L. L. English, J. R. Hayes, and R. D. Walker. 2002. Characterization of Tn1546 in vancomycin-resistant Enterococcus faecium isolated from canine urinary tract infections: evidence of gene exchange between human and animal enterococci. J. Clin. Microbiol. 40:4659-4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh, K. V., T. M. Coque, G. M. Weinstock, and B. E. Murray. 1998. In vivo testing of an Enterococcus faecalis efaA mutant and use of efaA homologs for species identification. FEMS Immunol. Med. Microbiol. 21:323-331. [DOI] [PubMed] [Google Scholar]
- 23.Uttley, A. H., C. H. Collins, J. Naidoo, and R. C. George. 1988. Vancomycin-resistant enterococci. Lancet i:57-58. [DOI] [PubMed] [Google Scholar]
- 24.van Belkum, A., N. van den Braak, R. Thomassen, H. Verbrugh, and H. Endtz. 1996. Vancomycin-resistant enterococci in cats and dogs. Lancet 348:1038-1039. [DOI] [PubMed] [Google Scholar]
- 25.van den Braak, N., A. van Belkum, M. van Keulen, J. Vliegenthart, H. A. Verbrugh, and H. P. Endtz. 1998. Molecular characterization of vancomycin-resistant enterococci from hospitalized patients and poultry products in The Netherlands. J. Clin. Microbiol. 36:1927-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]