Abstract
To further evaluate recombinant Em18 antigen (rEm18) for immunodiagnosis of human alveolar echinococcosis, 208 serum samples were examined by enzyme-linked immunosorbent assay (ELISA). To comparatively assess the results of rEm18-ELISA, ELISA and immunoblot analysis with two affinity-purified native antigens were also performed with 45 selected serum samples. The results indicate that rEm18 is highly useful for serodiagnosis.
Alveolar echinococcosis (AE) caused by infection with the metacestode form of the fox tapeworm Echinococcus multilocularis is one of the most severe zoonoses. More than 98% of primary infections in human AE cases appear in the liver, with long asymptomatic periods (5 to 15 years) (1). By the time signs and symptoms become evident, the disease process may be so advanced that the disease is difficult to treat. Therefore, early diagnosis and treatment are crucial for the reduction of morbidity and mortality. Because imaging technology is not always available for local patients in areas of high endemicity, such as in China, because of poorly equipped medical facilities and high cost (7), serodiagnosis by ELISA or immunoblotting has been employed with specific and purified diagnostic antigens such as Em2plus (4) and Em18 (5). Also, crude antigen extracts of E. multilocularis have often been used for primary screening in an epidemiological survey (8).
Most recently, Sako et al. (10) reported the successful production of recombinant Em18 antigen (rEm18), and the usefulness of the rEm18 for identification of AE has been evaluated but only with a limited number of serum samples from patients with diseases other than echinococcosis (6, 10). In this study, we have undertaken a more extensive evaluation of the specificity and sensitivity of rEm18 using serum samples from patients with a variety of parasitic and hepatic diseases. Two affinity-purified native antigens prepared from E. multilocularis were also used for comparative purposes.
Preparation of antigens.
rEm18 was prepared as described previously (10). Antibody-affinity-purified native antigen was obtained as follows. Mono-specific polyclonal antibody against rEm18 was prepared by immunizing New Zealand White rabbits with rEm18 (365.8 μg of protein) on three occasions at 2-week intervals. Rabbits were bled 12 days after the third immunization, and the immunoglobulin G (IgG) antibody in serum was purified. IgG was then coupled to a column as described previously (6). To obtain affinity-purified native Em18 (aEm18), the crude antigen was extracted from E. multilocularis protoscolices (5) and purified with the use of the antibody-immobilized column (6). For comparison, another affinity-purified antigen (aEmII/3) was prepared with polyclonal antibody against rEmII/3 (2, 3).
Human serum samples.
A total of 208 serum samples were used for serodiagnosis. They included serum samples from 13 patients with parasitic diseases and from 2 patients with nonparasitic hepatic diseases. All diseases were confirmed serologically, pathologically, and/or clinically. First, all 208 serum samples were examined by rEm18-ELISA. Then, in order to evaluate the reliability of rEm18-ELISA, 45 of the 208 serum samples were selected on the basis of ELISA optical density (OD) results. These 45 samples were from patients with AE (n = 5), cystic echinococcosis (CE; n = 6), or other diseases (n = 34). All selected samples were tested by ELISA with two different affinity-purified antigens, aEm18 and aEmII/3, and the immunoblots with rEm18, aEm18, and aEmII/3 were probed with the tested serum samples.
Serodiagnosis.
ELISA was performed by a procedure described previously (6). ELISA plates were coated with 50 ng of rEm18 per well or 100 ng of either aEm18 or aEmII/3. Anti-human IgG antibody conjugated to horseradish peroxidase (Zymed Laboratories, Inc., South San Francisco, Calif.) was diluted 1:5,000 in rEm18-ELISA and 1:1,000 in ELISA with native antigens. Serum samples were recorded as positive if the OD at 405 nm (OD405) values were higher than three times the OD405 value of human sera pooled from 40 healthy Japanese adults.
For the performance of immunoblotting, sodium dodecyl sulfate-polyacrylamide gel electrophoresis was conducted. The gels were loaded with 350 ng of rEm18, aEm18, and aEmII/3. Immunoblotting was carried out using polyvinylidene difluoride membranes (Millipore). The membranes were probed with serum samples diluted 1:50 in the blocking solution and incubated with anti-human horseradish peroxidase-conjugated IgG diluted 1:1,000.
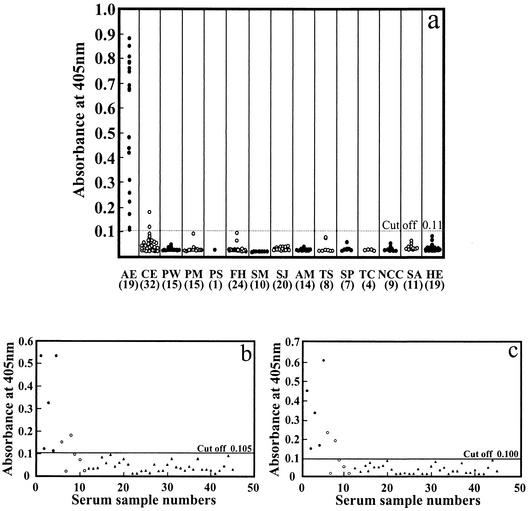
As shown in Fig. 1a, all AE cases gave positive reactions, whereas 2 of 32 CE serum samples displayed weakly positive reactions in rEm18-ELISA. According to clinical information, these two CE patients each had multiple cysts. No serum samples from patients with other diseases including amebiasis, sarcoidosis, and hepatoma were positive.
FIG. 1.
ELISA results for differentiation of AE from other diseases. (a) rEm18-ELISA; (b) aEm18-ELISA; (c) aEmII/3-ELISA. The cutoff was calculated as three times the OD value of negative control sera. The numbers in the parentheses indicate the numbers of serum samples examined. PW, paragonimiasis westermani; PM, paragonimiasis miyazakii; PS, paragonimiasis skriabini; FH, fascioliasis; SM, schistosomiasis mansoni; SJ, schistosomiasis japonica; AM, hepatic amebiasis; TS, trichinellosis; SP, sparganosis; TC, toxocariasis; NCC, neurocysticercosis; SA, sarcoidosis; HE, hepatoma.
Comparison of the results by ELISA with either aEm18 or aEmII/3 was made using 45 of the 208 serum samples. The results for aEm18 and aEmII/3 are illustrated in Fig. 1b and c. All AE and CE cases positive by rEm18-ELISA were also positive in these systems, and none of the serum samples from patients with other diseases was positive.
Analysis by immunoblotting revealed a pattern of reactivity similar to that by ELISA using rEm18, aEm18, and aEmII/3. All AE cases and two CE cases showed a positive antibody reaction. It was observed that AE cases with high antibody titers against Em18 by ELISA also showed strong clear bands in immunoblots. Correspondingly, AE and CE samples with low OD values but positive reactions in ELISA displayed relatively faint bands in immunoblots (data not shown).
Discussion.
Both ELISA and immunoblotting with rEm18 were able to detect all AE cases and gave no cross-reactions with sera from patients with nonechinococcal infections. Serum samples from only 2 of 32 CE cases, in which both patients had multiple cysts, reacted with these antigens.
The antibody response to Em18 may be a function of critical differences in antigen release or presentation of Em18 in patients with AE and CE because of different pathological features of metacestode-stage infection by these closely related cestodes. The E. granulosus metacestode grows within a double-walled cyst by endogenous budding, while the E. multilocularis metacestode grows by exogenous budding and the parasite tissue lacks a thick barrier from the adjacent host tissue (6, 9).
In this study, it has been confirmed that rEm18, aEm18, and aEmII/3 are highly reliable antigens for detection and differentiation of AE from other diseases including several hepatic diseases for which diagnosis is potentially confusing. However, the production of native aEm18 or aEmII/3 is limited in quantity and the quality varies among the different batches of the crude antigens. In contrast, as rEm18 is more easily produced in large amount, rEm18 has considerable advantage for serodiagnosis of AE.
Acknowledgments
We thank Marianna Wilson of the Centers for Disease Control and Prevention, Atlanta, Ga., for kindly providing several serum samples.
This study was supported by grants-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan and the Japan Society of Promotion of Science to A.I. (1255702414, 14256001), the National Health and Medical Research Council of Australia to M.W.L., and by the National Institute of Health (1 R01 TW01565-01; principal investigator, P. S. Craig).
REFERENCES
- 1.Craig, P. S., P. Giraudoux, D. Shi, B. Bartholomot, G. Barnish, P. Delattre, J. P. Quere, S. Harraga, G. Bao, Y. Wang, F. Lu, A. Ito, and D. A. Vuitton. 2000. An epidemiological and ecological study of human alveolar echinococcosis transmission in south Gansu, China. Acta Trop. 77:167-177. [DOI] [PubMed] [Google Scholar]
- 2.Felleisen, R., and B. Gottstein. 1993. Echinococcus multilocularis: molecular and immunochemical characterization of diagnostic antigen II/3-10. Parasitology 107:335-342. [DOI] [PubMed] [Google Scholar]
- 3.Felleisen, R., and B. Gottstein. 1994. Comparative analysis of full-length antigen II/3 from Echinococcus multilocularis and E. granulosus. Parasitology 109:223-232. [DOI] [PubMed] [Google Scholar]
- 4.Ito, A., L. Ma, M. Paul, J. Stefaniak, and Z. S. Pawlowski. 1998. Evaluation of Em18-, Em16-, antigen B-Western blots, Em2plus-ELISA and four other tests for differential serodiagnosis of alveolar and cystic echinococcosis patients in Poland. Parasitol. Int. 47:95-99. [Google Scholar]
- 5.Ito, A., L. Ma, M. Itoh, S. Y. Cho, Y. Kong, S. Y. Kang, T. Horii, X. L. Pang, M. Okamoto, T. Yamashita, M. W. Lightowlers, X. G. Wang, and Y. H. Liu. 1997. Immunodiagnosis of alveolar echinococcosis by enzyme-linked immunosorbent assay using a partially purified Em18/16 enriched fraction. Clin. Diagn. Lab. Immunol. 4:57-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito, A., N. Xiao, M. Liance, M. O. Sato, Y. Sako, W. Mamuti, Y. Ishikawa, M. Nakao, H. Yamasaki, K. Nakaya, K. Bardonnet, S. Bresson-Hadni, and D. A. Vuitton. 2002. Evaluation of an enzyme-linked immunosorbent assay (ELISA) with affinity-purified Em18 and an ELISA with recombinant Em18 for differential diagnosis of alveolar echinococcosis: results of a blind test. J. Clin. Microbiol. 40:4161-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito, A., C. Urbani, J. M. Qiu, D. A. Vuitton, D. C. Qiu, D. D. Heath, P. S. Craig, Z. Feng, and P. M. Schantz. 2003. Control of echinococcosis and cysticercosis: a public health challenge to international cooperation in China. Acta Trop. 86:3-17. [DOI] [PubMed] [Google Scholar]
- 8.Ito, A., and P. S. Craig. Immunodiagnostic and molecular approaches for the detection of taeniid cestode infections. Trends Parasitol., in press. [DOI] [PubMed]
- 9.Mamuti, W., H. Yamasaki, Y. Sako, K. Nakaya, M. Nakao, M. W. Lightowlers, and A. Ito. 2002. Usefulness of hydatid cyst fluid of Echinococcus granulosus developed in mice with secondary infection for serodiagnosis of cystic echinococcosis in humans. Clin. Diagn. Lab. Immunol. 9:573-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sako, Y., M. Nakao, K. Nakaya, H. Yamasaki, B. Gottstein, M. W. Lightowlers, P. M. Schantz, and A. Ito. 2002. Alveolar echinococcosis: characterization of diagnostic antigen Em18 and serological evaluation of recombinant Em18. J. Clin. Microbiol. 40:2760-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]