Abstract
Biological and geographic heterogeneity of anthropozoonosis caused by Anaplasma phagocytophilum is poorly understood. Seven North American A. phagocytophilum strains were compared by PFGE. The average genome size was 1.58 Mbp, and restriction patterns were identical. New World strains of A. phagocytophilum have a large genome and a high degree of genetic uniformity.
The recently renamed Anaplasma (Ehrlichia) phagocytophilum is an obligate intracellular bacterium that causes disease in humans, horses, dogs, and ruminants (5, 7). These organisms were originally classified as distinct species because of apparent diverse hosts and relatively remote geographic distributions. However, genetic and antigenic analyses showed only minor differences among Ehrlichia phagocytophila, Ehrlichia equi, and the human granulocytic ehrlichiosis (HGE) agent and revealed a close relationship to Anaplasma marginale, a ruminant erythrocyte pathogen (7). The new taxonomic assignment creates a single species but allows for biological and clinical heterogeneity, as is evident with the disparity between seroprevalence and incidence in humans and horses in North America and ruminants in Europe, in clinical severity between geographic locations in North America and Europe, and between clinical manifestations of infection in various mammalian hosts (1, 2, 4, 8-10, 11, 14, 19). The clinical and host tropism diversity suggest undiscovered differences among these bacteria. Thus, to determine whether A. phagocytophilum genomic variation might explain some heterogeneity, we analyzed genomic DNA of North American human, equid, and canid isolates by pulsed-field gel electrophoresis (PFGE).
(This work was presented in part at the Annual Meeting of the American Society for Tropical Medicine and Hygiene, Washington, D.C., November 1999.)
A. phagocytophilum (HGE agent and E. equi) strains (Table 1) were cultivated from peripheral blood in HL-60 cells and were passaged from 2 to 20 times prior to DNA preparation (3). The isolates were verified by PCR amplification and/or 16S rRNA gene sequencing and by reaction with A. phagocytophilum Msp2 (p44) monoclonal antibody. To preclude host cell DNA contamination, bacteria were purified from heavily infected HL-60 cells as previously described (3). Briefly, infected cells were harvested by centrifugation (500 × g at 4°C), resuspended in sucrose phosphate glutamine buffer (SPGn), and lysed by sonication on ice. Host cell debris was removed by centrifugation (500 × g), and the bacteria-enriched supernatant was treated with DNase I and RNase A (50 μg/ml) for 45 min at 37°C. Bacteria were centrifuged on discontinuous 45 and 30% meglumine diatrizoate density gradients for 1 h at 26,000 × g. The gradient interface was harvested and washed with SPGn. Protein concentration was determined, and suspensions were frozen at −80°C until they were used.
TABLE 1.
Isolates, strains, sources, and original locations of A. phagocytophilum used for PFGE
| Isolate or strain | Source | Location | Passage |
|---|---|---|---|
| NY8 (HGE agent) | Human | New York State | 5 |
| MDHGE (HGE agent) | Human | New York State | 3 |
| Webster (HGE agent) | Human | Wisconsin | 8 |
| Spooner (HGE agent) | Human | Wisconsin | 7 |
| 97HE97 (HGE agent) | Human | Wisconsin | 3 |
| 97E13 | Dog | Minnesota | 2 |
| E. equi MRK | Horse | California | 20 |
Thawed bacteria were embedded in 0.8% InCert agarose (FMC) in Bio-Rad PFGE plug molds by using sufficient bacteria to obtain 2.5 mg of protein/ml in a solution containing 10 mM Tris, 150 mM NaCl, 2 mM EDTA (pH 8.0). Genomic DNA was prepared by digestion in buffer containing 100 mM EDTA, 10 mM Tris, 1% sodium dodecyl sulfate (pH 8.0) with 1 mg of proteinase K/ml for two 24-h cycles at 37°C. The proteinase K was inactivated by three 1-h washes in a solution of 10 mM Tris (pH 8.0), 2 mM EDTA with 1 mM phenylmethylsulfonyl fluoride at room temperature and then three washes in 10 mM Tris (pH 8.0), and the plugs were finally stored in 200 mM EDTA. Genomic DNA in agarose plugs was equilibrated in restriction enzyme buffer for 1 h and was digested in 300 μl of 1× restriction enzyme with bovine serum albumin carrier, if required, overnight at an appropriate temperature. Each plug was equilibrated with 0.5× Tris-borate-EDTA (TBE) for 30 min and was applied to the gel. Various restriction enzymes were assessed for resolution and for a small number of bands, including AscI, EagI, PmeI, SwaI, SgfI, SmaI, BssHII, BclI, Eco47III, EcoRI, EcoRV, HindIII, HpaI, NsiI, SspI, XbaI, PmaCI, AgeI, PstI, ApaI, KpnI, NotI, NheI, SpeI, XhoI, SnaBI, PacI, RsrII, DraI, PvuI, and SfuI. The restriction enzyme-digested DNAs were separated by electrophoresis by using a Bio-Rad contour-clamped homogeneous electric field system and 1.6% Seakem Gold agarose (FMC) with the following empirically determined parameters: pulse times, initial 15 s and final 70 s; voltage, 6 V/cm; run time, 26 h; buffer, 0.5× TBE. Agarose gels were stained with ethidium bromide and were visualized. Molecular sizes of bands were recorded and compared by using the Bio-Rad Molecular Analyst and Fingerprinting Software, version 1.4.1.
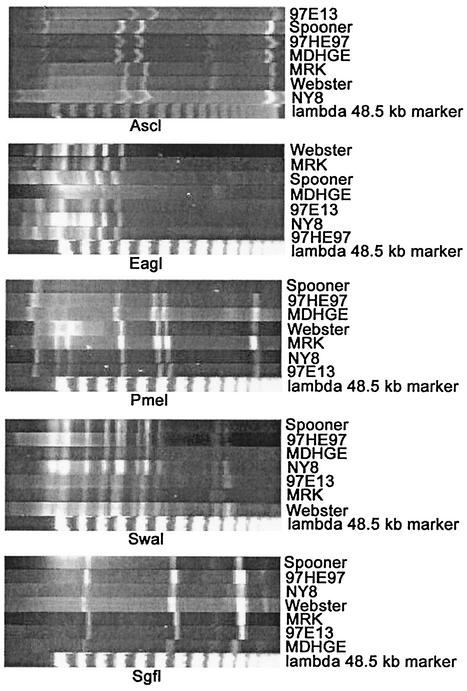
The restriction enzymes AscI, EagI, PmeI, SwaI, and SgfI reproducibly produced 3 to 11 bands. Each A. phagocytophilum isolate had identical PFGE patterns for any one enzyme (Fig. 1). Based on the sum of the molecular sizes of all bands, the average size of the A. phagocytophilum group genome was 1.58 Mbp, as previously documented for the Webster strain, and was much larger than the genome sizes of any other Anaplasmataceae or Rickettsiale (15).
FIG. 1.
Results of PFGE on seven isolates of A. phagocytophilum from different geographic regions in North America. The restriction enzyme used to digest the genomic DNA prepared from density gradient purified bacteria is labeled below each image. The isolate or strain of A. phagocytophilum is labeled on the right.
A high degree of diversity in clinical disease exists among strains of A. phagocytophilum that cause infections and disease worldwide (5, 9). The initial classification into European ruminant (E. phagocytophila), North American equine and canine (E. equi), and human or canine (HGE agent) strains belies such diversity. Although unification of these species into a single Anaplasma species is largely accepted, differences in infectivity and clinical disease are acknowledged (1, 4, 7, 9, 10, 13, 14, 17). Although the PFGE data presented here provide further support for the new taxonomic classification, no genetic attributes explain the biological and clinical differences.
HGE is more often described as a more severe infection in North America than in Europe (5, 9). Similarly, ruminant disease that is frequent in Europe is rare in North America (14, 16). Analyses of A. phagocytophilum genes show that North American strains differ more from European strains than they do from each other (6, 12, 18). In fact, analysis of groESL and ankA seem to confirm greater diversity among European strains, implying a longer interval of evolution that may correlate with the greater diversity of manifestations in European animals.
Complete genome sequences for many of the Anaplasmataceae will shortly become available, but considerable time may pass before genomic comparisons are able to provide information about diversity among organisms of a single species. The data here confirm determinations of the size of the A. phagocytophilum genome as well as the high degree of similarity among North American strains. Although PFGE interrogates only limited regions of the genome, it is interesting that differences in neither genome size nor restriction enzyme digestion pattern could be discerned, even among isolates from opposite sides of the continent. However, analysis by using other tools better designed to demonstrate differences, such as multilocus sequence typing, might improve detection of variations to correlate with biological behavior.
PFGE predicts that North American A. phagocytophilum has little heterogeneity, and potentially the reservoirs, infectious potential, and pathogenicity of this bacterium may be similar throughout North America (12, 18). A precise determination awaits further investigation of reservoirs and infections in humans and mammals with clinical signs.
Acknowledgments
This work was supported in part by grant RO1 AI-41213-01 from the National Institutes of Allergy and Infectious Diseases.
Special thanks are given to Susan Harrington for help with PFGE and to John E. Madigan, Maria Aguero-Rosenfeld, and Gary Wormser for help in obtaining isolates.
REFERENCES
- 1.Aguero-Rosenfeld, M. E., L. Donnarumma, L. Zentmaier, J. Jacob, M. Frey, R. Noto, C. A. Carbonaro, and G. P. Wormser. 2002. Seroprevalence of antibodies that react with Anaplasma phagocytophila, the agent of human granulocytic ehrlichiosis, in different populations in Westchester County, New York. J. Clin. Microbiol. 40:2612-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberdi, M. P., A. R. Walker, and K. A. Urquhart. 2000. Field evidence that roe deer (Capreolus capreolus) are a natural host for Ehrlichia phagocytophila. Epidemiol. Infect. 124:315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asanovich, K. M., J. S. Bakken, J. E. Madigan, M. Aguero-Rosenfeld, G. P. Wormser, and J. S. Dumler. 1997. Antigenic diversity of granulocytic Ehrlichia isolates from humans in Wisconsin and New York and a horse in California. J. Infect. Dis. 176:1029-1034. [DOI] [PubMed] [Google Scholar]
- 4.Bakken, J. S., P. Goellner, M. Van Etten, D. Z. Boyle, O. L. Swonger, S. Mattson, J. Krueth, R. L. Tilden, K. Asanovich, J. Walls, and J. S. Dumler. 1998. Seroprevalence of human granulocytic ehrlichiosis among permanent residents of northwestern Wisconsin. Clin. Infect. Dis. 27:1491-1496. [DOI] [PubMed] [Google Scholar]
- 5.Bakken, J. S., and J. S. Dumler. 2000. Human granulocytic ehrlichiosis. Clin. Infect. Dis. 31:554-560. [DOI] [PubMed] [Google Scholar]
- 6.Caturegli, P., K. M. Asanovich, J. J. Walls, J. S. Bakken, J. E. Madigan, V. L. Popov, and J. S. Dumler. 2000. ankA: an Ehrlichia phagocytophila group gene encoding a cytoplasmic protein antigen with ankyrin repeats. Infect. Immun. 68:5277-5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and 'HGE agent' as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. E vol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 8.Foley, J. E., J. E. Barlough, R. B. Kimsey, J. E. Madigan, E. DeRock, and A. Poland. 1998. Ehrlichia spp. in cervids from California. J. Wildl. Dis. 34:731-737. [DOI] [PubMed] [Google Scholar]
- 9.Lotric-Furlan, S., T. Avsic-Zupanc, M. Petrovec, W. L. Nicholson, J. W. Sumner, J. E. Childs, and F. Strle. 2001. Clinical and serological follow-up of patients with human granulocytic ehrlichiosis in Slovenia. Clin. Diagn. Lab. Immunol. 8:899-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madigan, J. E., S. Hietala, S. Chalmers, and E. DeRock. 1990. Seroepidemiologic survey of antibodies to Ehrlichia equi in horses of northern California. J. Am. Vet. Med. Assoc. 196:1962-1964. [PubMed] [Google Scholar]
- 11.Magnarelli, L. A., J. W. Ijdo, K. C. Stafford III, and E. Fikrig. 1999. Infections of granulocytic ehrlichiae and Borrelia burgdorferi in white-tailed deer in Connecticut. J. Wildl. Dis. 35:266-274. [DOI] [PubMed] [Google Scholar]
- 12.Massung, R. F., J. H. Owens, D. Ross, K. D. Reed, M. Petrovec, A. Bjoersdorff, R. T. Coughlin, G. A. Beltz, and C. I. Murphy. 2000. Sequence analysis of the ank gene of granulocytic ehrlichiae. J. Clin. Microbiol. 38:2917-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrovec, M., A. Bidovec, J. W. Sumner, W. L. Nicholson, J. E. Childs, and T. Avsic-Zupanc. 2002. Infection with Anaplasma phagocytophila in cervids from Slovenia: evidence of two genotypic lineages. Wien. Klein. Wochenschr. 114:641-647. [PubMed] [Google Scholar]
- 14.Pusterla, N., J. B. Pusterla, U. Braun, and H. Lutz. 1999. Experimental cross-infections with Ehrlichia phagocytophila and human granulocytic ehrlichia-like agent in cows and horses. Vet. Rec. Sep. 145:311-314. [DOI] [PubMed] [Google Scholar]
- 15.Rydkina, E., V. Roux, and D. Raoult. 1999. Determination of the genome size of Ehrlichia spp., using pulsed field gel electrophoresis. FEMS Microbiol. Lett. 176:73-78. [DOI] [PubMed] [Google Scholar]
- 16.Stuen, S., and K. Bergstrom. 2001. Serological investigation of granulocytic Ehrlichia infection in sheep in Norway. Acta Vet. Scand. 42:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuen, S., I. Van De Pol, K. Bergstrom, and L. M. Schouls. 2002. Identification of Anaplasma phagocytophila (formerly Ehrlichia phagocytophila) variants in blood from sheep in Norway. J. Clin. Microbiol. 40:3192-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumner, J. W., W. L. Nicholson, and R. F. Massung. 1997. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J. Clin. Microbiol. 35:2087-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walls, J. J., K. M. Asanovich, J. S. Bakken, and J. S. Dumler. 1998. Serologic evidence of a natural infection of white-tailed deer with the agent of human granulocytic ehrlichiosis in Wisconsin and Maryland. Clin. Diagn. Lab. Immunol. 5:762-765. [DOI] [PMC free article] [PubMed] [Google Scholar]