Abstract
We describe a patient with a history of asthma and remote use of steroids the development of necrotizing fascitis due to Vibrio alginolyticus after an injury from a coral reef during bathing in the Caribbean Sea off Colombia. The patient recovered with aggressive surgical debridement and antibiotics.
CASE REPORT
A 48-year-old female nurse suffered a blunt and penetrating trauma to the lower shin after contact with a coral reef while bathing in the Colombian coastal waters of the Caribbean Sea. A “V”-shaped wound was produced. The patient sought medical attention at the local hospital, where the wound was stitched and two doses of intramuscular clindamycin (600 mg) were prescribed, followed by three additional doses of oral dicloxacillin (500 mg). On evidence of increasing wound erythema, the patient was flown to a tertiary-care hospital, where she was examined in the emergency department. The patient's medical history included asthma diagnosed 27 years ago, for which she was taking inhaled albuterol (Salbutamol). She had received a 6-week tapering course of steroids 6 months before the current admission due to exacerbation of her asthma. On arrival, her temperature was 38°C, heart rate was 92 beats/min, respiratory rate was 16 breaths/min, and blood pressure was 120/70 mm Hg. Examination of the patient's left leg revealed a stitched “V”-shaped wound (3 by 2.5 cm) with mild cellulitis that extended from the wound toward the lateral region of the knee and with borders that were poorly defined. Moderate pain was present in cellulitic tissues, and no other wounds were present. The rest of the physical examination was unremarkable. Laboratory testing performed at admission included a peripheral white blood cell count of 12.0 cells/μl with 86% segmented neutrophils, 11% lymphocyes, and 2.4% monocytes. Creatinine, blood glucose, hemoglobin, plasma sodium, and plasma potassium levels were within normal limits. Ampicillin-sulbactam (3 g four times a day intravenously) was started, the wound was debrided and left open, and samples of wound discharge were taken in the emergency department. The patient was hospitalized.
During the first 2 days of hospitalization, the patient underwent several wound debridements, and culture biopsy samples of the lesion were taken. On day 3 of hospitalization, wound cultures were found positive for a gram-negative, oxidase-positive organism. Initial identification with the VITEK automated system (Biomerieux) yielded Vibrio alginolyticus. The microorganism was grown on thiosulfate-citrate-bile salts-sucrose agar, and a string test was positive. The fermentative phenotype was confirmed by inoculation in triple sugar iron medium and on Kligler iron agar. Further identification was confirmed manually with the following biochemical tests: growth at high concentrations of NaCl (up to 8%); reactions to urea, indole, lysine, arginine, ornithine, glucose, sucrose, trehalose, arabinose, gelatin, and acetate; and the Voges-Proskauer test. The isolate was sent to the National Reference Laboratory, where identification was further confirmed. Antibiotics were switched to clindamycin and ciprofloxacin. MICs were determined by using the agar dilution method according to NCCLS recommendations (14). The isolate appeared to be susceptible to tetracycline (MIC, 0.5 μg/ml), trimethoprim-sulfamethoxazole (TMP-SMX) (MIC, 0.12 μg/ml) (susceptible to ≤2/38 μg/ml), and chloramphenicol (MIC, 8.0 μg/ml) (susceptible to ≤8 μg/ml). The isolate was also susceptible by disk diffusion to ciprofloxacin (zone diameter, ≥16 mm).
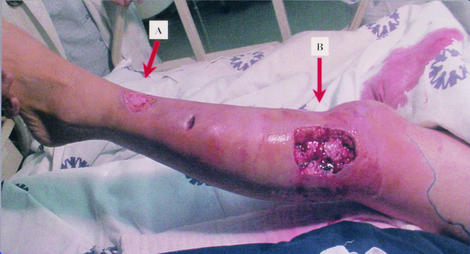
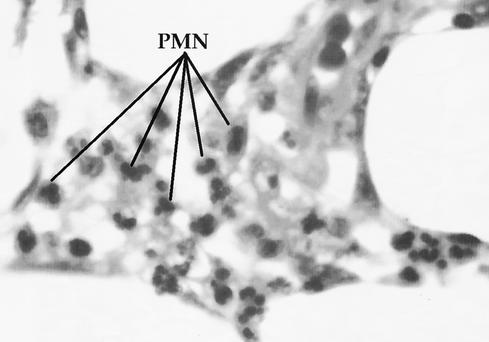
On day 4 of hospitalization, the patient was afebrile, with clinical improvement of the wound infection and no signs of systemic deterioration. However, a purplish rounded area displaying decreased sensation (3 cm in diameter) was noticed on the lateral aspect of the leg below the knee. Under clinical suspicion of developing necrotizing fasciitis (NF) and panniculitis, the patient was taken to surgery, and the wound was opened and debrided. Figure 1 shows the surgical defect after the necrotic tissues were excised. A biopsy sample of the fascia was taken during the procedure and reported as NF. The finding was confirmed in a later pathological examination and described as skin with marked subepidermal edema and dermal vesicle formation with infiltration of polymorphonuclear cells. Severe septal panniculitis was found, with thrombosis of small- and middle-caliber vessels and an important inflammatory response (Fig. 2). The area was extensively debrided, and a circular defect (diameter of 6 cm) was left open. After the procedure, signs of inflammation in the surrounding skin and tissues improved substantially within the following 5 days, with loss of erythema. Clindamycin was withdrawn, and ciprofloxacin was continued (14 days in total). The patient was discharged on day 11 for further dressings and outpatient care.
FIG. 1.
Primary and secondary skin involvement after surgical debridement. (A) Site of the original wound. (B) Debrided lesion that developed on day 4 of hospitalization. The lesion was distant from the original site of inoculation. Note the severe edema and necrosis of subdermal tissues.
FIG. 2.
Histopathological study of a biopsy sample taken from the patient's lesion. This representative section shows severe septal panniculitis. The fascia contains severe extensive necrosis and acute inflammatory changes marked by lines indicating polymorphonuclear cells (PMN).
Several marine organisms are found when soft tissues are injured in seawater or during contact with marine animals. In addition to vibrios, Aeromonas spp., Erysipelothrix spp., Bacteriodes spp., Chromobacterium violaceum, Clostridium perfringens, Pseudomonas spp., and Salmonella spp. have been reported (1). V. cholerae and several members of the family Vibrionaceae have been associated with human diseases. The halophilic vibrios are common inhabitants of seawater and have been isolated from brackish waters and seafood (4, 7, 12). Several of these vibrios have been associated with deep skin infections. The most common is V. vulnificus, which is primarily associated with severe soft tissue infections and bacteremia (rather than diarrheal disease) in immunocompromised and cirrhotic patients (2).
NF is a severe infection that involves the skin and subdermal tissues but spares the muscular fascia and muscle (16). Halophilic vibrios have been implicated as the cause of NF. In a series of 18 cases reported by Howard et al. (8), V. vulnificus and V. parahaemolyticus accounted for 50 and 16% of the cases, respectively. V. alginolyticus has been reported and documented only in two cases of NF: in a patient following a stingray injury in the Pacific Ocean near Hong Kong (6) and in another with an injured hand along the Sea of Cortez in Mexico (18). V. alginolyticus cellulitis without NF development has also been recorded in a patient following a coral reef injury in the Caribbean Sea (17). We describe the third case of NF due to V. alginolyticus. Other diseases associated with this species include otitis externa and conjuctivitis acquired by swimming in seawater (11, 13), gastroenteritis (including chronic diarrhea in an immunocompromised patient) (3, 20), intracranial infection following an injury in saltwater (15), and bacteremia (9). This microorganism is the most halophilic of the pathogenic Vibrio species, with nearly 70% of strains growing in the presence of NaCl at concentrations as high as 10% (5). Virulence is related to its ability to produce hemolysis, hemagglutination, and protease (21).
The majority of patients reported to have developed NF have had an associated medical condition known to increase susceptibility to Vibrio infections. Predisposing conditions included alcoholism and cirrhosis, oral steroid therapy, polycystic kidney disease and leukopenia, hemochromatosis, and multiple myeloma (8). In the two previous cases of NF caused by V. alginolyticus, one patient had cirrhosis and the other was apparently healthy (6, 8). Our patient had asthma, which was being controlled at the time of disease presentation. She had received a tapering course of steroids in the past but had stopped them at least 6 months before this admission. It is unlikely that any of these situations influenced the disease course. Factors that might have contributed to the development of the patient's clinical condition included the initial management in the local hospital, with stitching and the lack of appropriate antimicrobial coverage for Vibrio species (and other likely organisms) after an injury in seawater.
Prompt diagnosis and treatment of NF are crucial to improving the chances of patient survival. A high index of suspicion for the development of NF in this patient was the emergence of a new area of purplish discoloration and lack of sensation distant from the original site of inoculation (Fig. 1), despite the fact that the patient's clinical condition was improving. A biopsy sample taken during the debridement procedure revealed severe and extensive necrosis in the superficial fascia and surrounding soft tissues, with arterial and vein thrombosis and polymorphonuclear infiltration of the dermis, findings typical of NF (Fig. 2).
Treatment of NF relies on prompt surgical intervention and antibiotics. Aggressive debridement and resection of affected tissues are of paramount importance. When NF is caused by halophilic marine vibrios, prompt resuscitation with large volumes of fluids is required for patients presenting with hypotension (8). The vibrios are susceptible to a wide range of antimicrobial agents. However, V. parahaemolyticus and V. alginolyticus may have β-lactamase activity (10). The NCCLS (14) has interpretative standards for V. cholerae tested with tetracycline, TMP-SMX, and chloramphenicol. Standardized breakpoints for other Vibrio spp. have not been published. Using the V. cholerae breakpoints for the V. alginolyticus isolate from our patient, we found that it was susceptible to tetracycline, TMP-SMX, and chloramphenicol. The change to ciprofloxacin was based solely on clinical grounds due to the emergence of local signs of inflammation, and clindamycin was added on suspicion of synergistic anaerobic infection, which is common in patients with NF (19).
In summary, we describe a patient who had a history of asthma and who developed NF due to V. alginolyticus following a minor penetrating injury caused by coral. In any patient with a wound infection associated with coral and seawater, V. alginolyticus should be among the suspected causative organisms, and the physician should evaluate for the presence of NF. Surgical aggressive management should always be considered if signs and symptoms of NF develop in such a patient.
Acknowledgments
We are grateful to Monica Gutierrez and Jinnethe Reyes for technical assistance in microbiology techniques and susceptibility tests and to Elizabeth Castañeda (Instituto Nacional de Salud) for confirmation of microorganism identification. We are indebted to Rocio Lopez (Department of Pathology) for discussion of pathological findings and microscopic anatomy images.
REFERENCES
- 1.Auerbach, P. S., D. M. Yajko, P. S. Nassos, K. W. Kizer, J. E. McCosker, E. C. Geehr, and W. K. Hadley. 1987. Bacteriology of the marine enviroment: implications for clinical therapy. Ann. Emerg. Med. 16:643-649. [DOI] [PubMed] [Google Scholar]
- 2.Blake, P. A., M. H. Merson, R. E. Weaver, D. G. Hollis, and P. C. Heublein. 1979. Disease caused by a marine Vibrio. Clinical characteristics and epidemiology. N. Engl. J. Med. 300:1-5. [DOI] [PubMed] [Google Scholar]
- 3.Caccamese, S. M., and D. A. Rastegar. 1999. Chronic diarrhea associated with Vibrio alginolyticus in an immunocompromised patient. Clin. Infect. Dis. 29:946-947. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty, S., G. B. Nair, and S. Shinoda. 1997. Pathogenic vibrios in the natural aquatic environment. Rev. Environ. Health 12:63-80. [DOI] [PubMed] [Google Scholar]
- 5.Farmer, J. J., and F. Hickman-Brenner. 1992. The genera Vibrio and Photobacterium, p. 2952-3011. In A. Balows, H. G. Truper, K. H. Schleifer, et al. (ed.), The prokaryotes: a handbook of the biology of bacteria. Springer-Verlag, New York, N.Y.
- 6.Ho, P. L., W. M. Tang, K. S. Lo, and K. Y. Yuen. 1998. Necrotizing fasciitis due to Vibrio alginolyticus following an injury inflicted by a stingray. Scand. J. Infect. Dis. 30:192-193. [DOI] [PubMed] [Google Scholar]
- 7.Hoge, C. W., D. Watsky, R. N. Peeler, J. P. Libonati, E. Israel, and J. G. Morris, Jr. 1989. Epidemiology and spectrum of Vibrio infections in a Chesapeake Bay community. J. Infect. Dis. 160:985-993. [DOI] [PubMed] [Google Scholar]
- 8.Howard, R. J., M. E. Pessa, B. H. Brennaman, and R. Ramphal. 1985. Necrotizing soft-tissue infections caused by marine vibrios. Surgery 98:126-130. [PubMed] [Google Scholar]
- 9.Janda, J. M., R. Brenden, J. A. DeBenedetti, M. O. Constantino, and T. Robin. 1986. Vibrio alginolyticus bacteremia in an immunocompromised patient. Diagn. Microbiol. Infect. Dis. 5:337-340. [DOI] [PubMed] [Google Scholar]
- 10.Joseph, S. W., R. M. DeBell, and W. P. Brown. 1978. In vitro response to chloramphenicol, tetracycline, ampicillin, gentamicin, and beta-lactamase production by halophilic vibrios from human and environmental sources. Antimicrob. Agents Chemother. 13:244-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lessner, A. M., R. M. Webb, and B. Rabin. 1985. Vibrio alginolyticus conjunctivitis. First reported case. Arch. Ophthalmol. 103:229-230. [DOI] [PubMed] [Google Scholar]
- 12.Maugeri, T. L., D. Caccamo, and C. Gugliandolo. 2000. Potentially pathogenic vibrios in brackish waters and mussels. J. Appl. Microbiol. 89:261-266. [DOI] [PubMed] [Google Scholar]
- 13.Mukherji, A., S. Schroeder, C. Deyling, and G. W. Procop. 2000. An unusual source of Vibrio alginolyticus-associated otitis: prolonged colonization or freshwater exposure? Arch. Otolaryngol. Head Neck Surg. 126:790-791. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. NCCLS document M7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.Opal, S. M., and J. R. Saxon. 1986. Intracranial infection by Vibrio alginolyticus following injury in salt water. J. Clin. Microbiol. 23:373-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patiño, J. F., D. Castro, A. Valencia, and P. Morales. 1991. Necrotizing soft tissue lesions after a volcanic cataclysm. World J Surg. 15:240-247. [DOI] [PubMed] [Google Scholar]
- 17.Patterson, T. F., S. R. Bell, and F. J. Bia. 1988. Vibrio alginolyticus cellulitis following coral injury. Yale J. Biol. Med. 61:507-512. [PMC free article] [PubMed] [Google Scholar]
- 18.Spark, R. P., M. L. Fried, C. Perry, and C. Watkins. 1979. Vibrio alginolyticus wound infection: case report and review. Ann. Clin. Lab. Sci. 9:133-138. [PubMed] [Google Scholar]
- 19.Stevens, D. L., A. E. Bryant, and S. P. Hakett. 1995. Antibiotic effects on cell viability, toxin production, and host response. Clin. Infect. Dis. 2(Suppl. 2):S154-S157. [DOI] [PubMed] [Google Scholar]
- 20.Uh, Y., J. S. Park, G. Y. Hwang, I. H. Jang, K. J. Yoon, H. C. Park, and S. O. Hwang. 2001. Vibrio alginolyticus acute gastroenteritis: report of two cases. Clin. Microbiol. Infect. 7:104-106. [DOI] [PubMed] [Google Scholar]
- 21.Zanetti, S., A. Deriu, L. Volterra, M. P. Falci, P. Molicotti, G. Fadda, and L. Sechi. 2000. Virulence factors in Vibrio alginolyticus strains isolated from aquatic environments. Ann. Ig. 12:487-491. [PubMed] [Google Scholar]