Abstract
Simple and rapid diagnostic tests are needed to curtail human immunodeficiency virus (HIV) infection, especially in the developing and underdeveloped nations of the world. The visible-agglutination assay for the detection of HIV with the naked eye (NEVA HIV, which represents naked eye visible-agglutination assay for HIV) is a hemagglutination-based test for the detection of antibodies to HIV in whole blood. The NEVA HIV reagent is a cocktail of highly stable recombinant bifunctional antibody fusion proteins with HIV antigens which can be produced in large quantities with a high degree of purity. The test procedure involves mixing of one drop of the NEVA HIV reagent with one drop of blood sample on a glass slide. The presence of anti-HIV antibodies in the blood sample leads to clumping of erythrocytes (agglutination) that can be seen with the naked eye. Evaluation with commercially available panels of sera and clinical samples has shown that the performance of NEVA HIV is comparable to those of U.S. and European Food and Drug Administration-approved rapid as well as enzyme-linked immunosorbent assay kits. The test detects antibodies to both HIV type 1 (HIV-1) and HIV-2 in a single spot and gives results in less than 5 min. The test was developed by keeping in mind the practical constraints of testing in less developed countries and thus is completely instrument-free, requiring no infrastructure or even electricity. Because the test is extremely rapid, requires no sample preparation, and is simple enough to be performed by a semiskilled technician in any remote area, NEVA HIV is a test for the hard-to-reach populations of the world.
Present efforts to limit the spread of human immunodeficiency virus (HIV) rely on modification of sexual practices, public education, avoidance of HIV-contaminated needles and syringes, and blood screening. Behavioral patterns are difficult to change, and of all the measures mentioned above, screening of blood for HIV before transfusion followed by elimination of infected blood units has been the most successful effort in limiting infection in most developed countries. Unfortunately, due to inadequate blood screening measures, unscreened blood transfusions remain one of the major modes of HIV transmission in developing and underdeveloped countries.
The presence of antibodies to HIV in serum indicates viral infection. The immune response is primarily against viral proteins, particularly those comprising the gag and env regions. Most commercial diagnostic tests use regions of the envelope transmembrane protein, gp41 of HIV type 1 (HIV-1) and gp36 of HIV-2, for detection of anti-HIV antibodies. The strategy routinely used for the diagnosis of HIV infection consists of determination of seropositivity to HIV antigens by an enzyme-linked immunosorbent assay (ELISA), the result of which is confirmed by Western blotting. These assays require instrumentation (i.e., incubators, plate washers, and optical readers) and take a minimum of 2 to 4 h to a day to produce a result. Delay in the availability of test reports results in poor compliance in collection of the report and posttest counseling; consequently, large numbers of HIV-positive individuals remain unaware of their HIV infection status (2). Rapid detection can significantly decrease the rate of HIV transmission if these individuals are informed and counseled immediately (7, 9, 10). Rapid tests will also be useful in the immediate identification of HIV-infected pregnant mothers, who could be given antiviral therapy during labor to reduce the incidence of HIV transmission to the newborns. In addition, simple, rapid, economical tests for the diagnosis of HIV infections could improve the safety of the blood supply worldwide. Therefore, a variety of rapid assay formats are being developed and evaluated (12). These include particle agglutination assays, dot blot immunoassays, and passive hemagglutination assays (5, 14). However, these protocols require the isolation of serum or plasma. Test formats that use saliva or urine samples for testing have also been introduced. However, these tests need to be evaluated more thoroughly for their sensitivities in detecting anti-HIV antibodies (11). Recently, immunochromatography-based tests that can use EDTA-anticoagulated blood, serum, and plasma samples have been developed. These tests require special strips and take up to 30 min to produce a result (13), and most of the evaluation studies have been done with serum or plasma samples.
Kemp et al. (8) described a novel test for the detection of antibodies to HIV in a drop of blood. This test was based on a chemically prepared bifunctional molecule capable of cross-linking and agglutinating human red blood cells (RBCs) in the presence of anti-HIV antibodies. On the basis of this concept, we have recently described several recombinant bifunctional molecules for the detection of antibodies to HIV-1 and HIV-2 in whole blood (3, 4). These molecules are fusion proteins consisting of the monovalent antigen binding fragment (Fab) of anti-human RBC monoclonal antibody (MAb) B6 with 31-amino-acid immunodominant peptides derived from envelope glycoproteins gp41 and gp36 of HIV-1 and HIV-2, respectively (4). Since use of only one antigen derived from the envelope proteins may not be sufficient to detect HIV in samples from individuals at different stages of infection, a fusion protein consisting of Fab of another anti-human RBC MAb, A41, and a nonaggregating derivative of HIV-1 capsid protein p24 has been produced (3). This molecule detected anti-p24 antibodies in HIV-infected individuals by a hemagglutination assay. The present work describes the formulation of a cocktail of these antibody fusion proteins along with two more fusion proteins for the detection of antibodies to both HIV-1 and HIV-2 in a rapid, instrument-free hemagglutination assay named the visible-agglutination assay for the detection of HIV with the naked eye (NEVA HIV, which represents naked eye visible-agglutination assay for HIV). The evaluation of NEVA HIV highlights the importance of using a mixture of several fusion proteins in the detection assay and attests to the need for the inclusion of fusion proteins responsible for detection of anti-p24 antibodies. The evaluation data show that NEVA HIV is comparable to the presently available rapid tests for detection of HIV.
MATERIALS AND METHODS
A hybridoma for the production anti-RBC MAb 10F7 was obtained from the American Type Culture Collection, Manassas, Va. Mixed-titer serum panels and seroconversion panels were purchased from Boston Biomedica Inc. (Westbridgewater, Mass.) and NABI Biopharmaceuticals (Boca Raton, Fla.) and were used without dilution. The results of various enzyme immunoassays (EIAs), Western blotting, and immunofluorescence assay (IFA) were provided by the supplier. Clinical samples were supplied by Indian hospitals, namely, the Post Graduate Institute of Medical Education and Research, Chandigarh; the Christian Medical College, Vellore; the Institute of Pathology, New Delhi; the National AIDS Research Institute, Pune; and Safdarjang Hospital, New Delhi. These were previously collected serum or whole-blood samples that had been characterized for HIV infection status by immunoassays. For this study, they were provided as coded samples, and the identities of the donors were not disclosed.
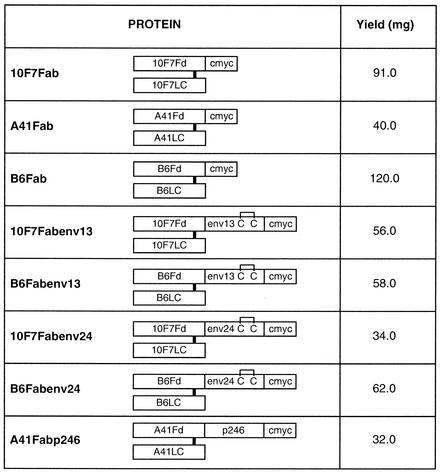
Eight recombinant Fab fusion proteins (Fig. 1) were prepared by previously described protocols (3, 4). These proteins result in agglutination of RBCs in the presence of anti-HIV antibodies.
FIG. 1.
Diagrammatic representation of various fusion proteins. Recombinant Fd of an MAb or its fusion protein makes an interchain disulfide bond with the corresponding light chain. These fusion proteins are obtained by assembling the light chain with Fd-Fd fusions containing HIV-derived peptide and by using a protocol of in vitro denaturation of inclusion bodies and subsequent renaturation under proper redox conditions to assemble the functional Fab. The monomeric Fab fusion proteins containing HIV-derived peptides are purified to a high degree and free of aggregates by multistep column chromatographic procedures. The intrachain disulfide bonds of the light chain and Fd are not shown. Fd, antibody fragment containing the variable domain and the first constant domain of the heavy chain; LC, light chain of antibody; env13, 31 amino acids (590 to 620) of HIV-1 envelope gp41 (AVERYLKDQQLLGIWGCSGKLICTTAVPWNA); env24, 31 amino acids (581 to 611) of HIV-2 envelope gp36 (AIEKYLQDQARLNSWGCAFRQVCHTTVPWVN); c-myc, decapeptide recognized by MAb 9E10; p246, amino acids 8 to 153 of HIV-1 capsid protein p24; C, cysteine. The yield corresponds to the amount of Fab obtained from 8 liters of renatured material containing 800 mg of total protein.
Stabilities of purified proteins.
The purified Fab fusion proteins were incubated at 4 and 37°C at a concentration of 100 μg/ml in a stabilizer solution consisting of 5 mM EDTA, 2% glycerol, 0.1% bovine serum albumin, 0.02% thimerosal, and sugars (0.5% each sucrose, sorbitol, and trehalose) in phosphate-buffered saline (20 mM phosphate buffer [pH 7.2] containing 145 mM NaCl). The agglutination activities of these proteins were tested at specific time intervals in a plate assay format by using anti-c-myc MAb 9E10 and by a protocol described earlier (4). Briefly, serial dilutions of Fab fusion proteins were incubated with RBCs, followed by the addition of MAb 9E10. The maximum dilutions of the fusion proteins that gave visible agglutination were recorded. For Western blot analysis, samples were prepared in nonreducing sample buffer and electrophoresed on a sodium dodecyl sulfate-10% polyacrylamide gel, transferred to a polyvinylidene difluoride membrane (Millipore), and probed with MAb 9E10, followed by probing with horseradish peroxidase-conjugated anti-mouse immunoglobulin G (IgG) (Fcγ-specific) antibody.
Formulation of cocktails of various fusion proteins.
Cocktails of various purified proteins were prepared in a stabilizer solution containing protease inhibitors (dilution solution) and were tested for their stabilities as described above for the purified fusion proteins.
To prepare the control reagent, 10F7Fab, A41Fab, and B6Fab, each at a final concentration of 7.5 μg/ml, were added to the dilution solution. The HIV reagent was prepared by mixing 10F7Fabenv13, B6Fabenv13, 10F7Fabenv24, and B6Fabenv24 at final concentrations of 7.5 μg/ml each and A41Fabp246 at a final concentration of 15 μg/ml in the dilution solution.
Slide agglutination assay to detect anti-HIV antibodies.
Whole-blood samples collected in different anticoagulants were used directly for testing. Human serum or plasma samples were mixed with washed RBCs from type O RhD-negative blood collected from an HIV-negative individual in a 1:1 ratio to make reconstituted blood and were then used for testing.
A drop of a reconstituted or whole-blood sample (approximately 20 μl) was dispensed on each spot on a glass slide (with two marked spots). One drop (20 μl) of the control reagent was added on the spot labeled “control,” and one drop of HIV reagent was added on the spot labeled “HIV.” The contents on each spot were mixed with a plastic stick. The slide was kept undisturbed for 3 min at room temperature (25 to 30°C), and agglutination (clumping of RBCs) was read with the naked eye while the slide was rotated. Agglutination was recorded on an arbitrary scale of 0 to 4, with 0 indicating no visible agglutination and 4 indicating strong agglutination with large clumps. Agglutination of 1 or more in the HIV spot in comparison to that on the control spot is interpreted as reactive.
RESULTS
NEVA HIV requires bifunctional molecules comprising monovalent antibody fragments against the human RBC surface fused to peptides and proteins derived from immunodominant regions of HIV-1 and HIV-2. Upon the addition of the molecules (monovalent anti-RBC-HIV peptide fusion proteins) to a drop of whole blood, the molecules coat the RBCs present in blood. If anti-HIV antibodies are present in the blood (as in the case of an HIV-infected individual), they bind to the HIV-derived peptide moiety of the fusion protein coating the RBCs and cause cross-linking of RBCs, resulting in visible agglutination. No agglutination occurs in the absence of anti-HIV antibodies in the blood sample, as in the case of a sample from an HIV-negative individual (6).
Various anti-RBC fusion proteins.
Three anti-RBC antibodies, namely, 10F7, A41, and B6, were selected to prepare Fab-based fusion proteins with HIV antigens. A41 and B6 have been described previously (3, 4). These antibodies bind to RBCs of all kinds of blood samples, irrespective of the major and minor blood groups. 10F7 binds to glycophorins of both the M and the N types (1). Competition experiments for RBC binding showed that these antibodies bind to different sites on the RBC surface (data not shown).
The Fab protein of each MAb comprises Fd (a fragment consisting of the variable domain and the first constant domain of the heavy chain of antibody) of an MAb carrying a 10-amino-acid tag, c-myc, and the corresponding light chain of antibody. To make Fab-based fusion proteins with HIV antigens, the DNA sequence encoding the HIV antigen was inserted between the Fd and the c-myc sequences to produce an Fd-HIV antigen-c-myc fusion protein that was then assembled with the corresponding light chain. B6Fab (4) and 10F7Fab were used as fusion partners with immunodominant fragments of gp41 of HIV-1 (env13) and gp36 of HIV-2 (env24); env13 and env24 consist of amino acids 591 to 620 of envelope glycoprotein gp41 and amino acids 581 to 610 of gp36, respectively (Fig. 1).
The third HIV antigen selected was a nonaggregating derivative of HIV-1 capsid protein p24, encompassing amino acids 8 to 153 of p24 and named p246. The Fd of MAb A41 was fused with p246 and renatured with A41LC to produce A41Fabp246 (3).
The Fab and Fab fusion proteins carrying the HIV antigen fused at the C terminus of Fd were assembled in vitro (renaturation) and purified by column chromatography procedures. MAb 10F7-derived fusion proteins, namely, 10F7Fab, 10F7Fabenv13, and 10F7Fabenv24, were purified by using the conditions described for the corresponding B6 Fab and B6Fab fusion proteins (4). The protocol was modified to obtain material sufficient for the formulation of reagents for more than 100,000 tests (Fig. 1).
Characterization of purified fusion proteins.
The purified proteins were individually tested for stability at 37 and 4°C in the presence of stabilizer. The plate agglutination assay, as described in Materials and Methods, was used to monitor the activities of the proteins, and Western immunoblotting of the proteins was performed to monitor any degradation of the proteins on prolonged incubation at a high temperature (37°C). All proteins were found to be stable for at least 30 days at 37°C and 6 months at 4°C, with no loss of agglutination activity during these periods.
These proteins were tested for their efficacies for the detection of anti-HIV antibodies in the sera of HIV-infected individuals in a microtiter plate-based agglutination assay. The B6 fusion proteins gave good reactivities with all samples (4). The 10F7 fusion proteins gave results similar to those obtained with the B6 fusion proteins (data not shown). A41Fabp246 also detected anti-p24 antibodies in these samples (3).
Formulation and characterization of cocktail of fusion proteins.
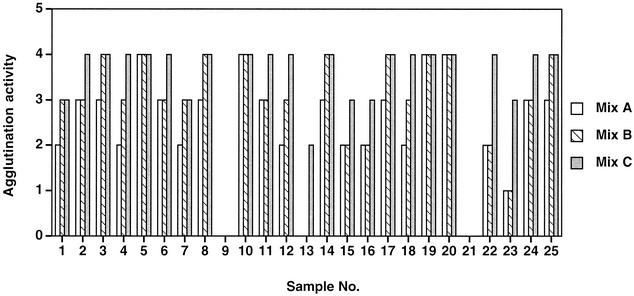
In order to ascertain if a cocktail of various Fab-HIV antigen fusion proteins was required for efficient detection of anti-HIV antibodies, three mixtures containing different Fab-antigen fusion proteins were prepared. Mix A contained B6Fabenv13 and B6Fabenv24 (10 μg/ml each); mix B contained, in addition to B6 fusion proteins, 10F7Fabenv13 and 10F7Fabenv24 (10 μg/ml each); and mix C was the same as mix B but also contained A41Fabp246 (15 μg/ml). All the three mixtures were tested with samples of a mixed-titer panel, panel PRB202 (Boston Biomedica Inc.), in a slide assay performed as described in Materials and Methods.
Two samples (samples 9 and 21) from panel PRB202 that were negative for anti-HIV antibodies by other tests (on the basis of the supplier's data) were negative with all three mixes (Fig. 2). With positive samples, mix B showed better reactivity than mix A, indicating that coating of RBCs with fusion proteins of two different antibodies makes more HIV antigen available for binding to anti-HIV antibodies, thereby resulting in increased cross-linking of RBCs, which is observed as better agglutination with bigger clumps.
FIG. 2.
Evaluation of various mixtures of Fab fusion proteins. Mix A contains B6Fabenv13 and B6Fabenv24 each at 10 μg/ml; mix B contains B6Fabenv13, B6Fabenv24, 10F7 Fabenv13 and 10F7Fabenv24 each at 10 μg/ml; and mix C contains A41Fabp246 (15 μg/ml) in addition to the components of mix B. All three mixtures were tested with the samples of panel PRB202 by a slide assay. Twenty microliters of a 1:1 mix of a serum sample from panel PRB202 and RBCs from an individual with type O RhD-negative blood was added to 20 μl of each reagent on a glass slide, and the agglutination reaction was read visually on an arbitrary scale of 0 to 4, with 0 indicating no visible agglutination and 4 indicating strong agglutination with large clumps.
A remarkable improvement in agglutination was observed with mix C, which contained another fusion protein, A41Fabp246, in addition to the components of mix B. As is clear from Fig. 2, mix C showed the strongest agglutination activity with all samples, with differences more pronounced for some samples (samples 15, 16, 22, and 23). Detailed analysis of the reactivity data from other assays indicated that on Western blotting, sample 23 had given weak reactivity to p24 and was nonreactive by many commercial EIAs and IFA. This sample was possibly obtained early in the seroconversion process and contained low levels of antibodies against envelope proteins, which are the major antigens detected by most kits. In our assay, this sample also showed very weak reactivities with mix A and mix B but showed a high level of reactivity with mix C, due to the detection of anti-p24 antibodies. Similar results were obtained for samples 15 and 16. One sample (sample 13), which gave bands corresponding to p24 and gp160 on Western blotting, was reactive only with mix C in our assay. This sample was also probably obtained early in the seroconversion process and had only anti-p24 antibodies. This analysis showed that inclusion of A41Fabp246 improved the sensitivity of the assay by detecting antibodies to p24 in HIV-infected samples. Also, it was clear that a cocktail of fusion proteins was required to detect anti-HIV antibodies with a high sensitivity. Therefore, all the fusion proteins of mix C were used to formulate the HIV reagent.
To determine the optimal ratio of the proteins in this reagent, we prepared six HIV reagent formulations that contained 5, 7.5, and 10 μg of 10F7 and B6env fusion proteins per ml and either 10 or 15 μg of the A41 fusion protein per ml. These were evaluated for their specificities and sensitivities in detecting anti-HIV antibodies by using a panel of 10 HIV-positive and 10 HIV-negative human serum samples. The cocktail containing the 10F7 and B6 fusion proteins each at 7.5 μg/ml and the A41 fusion protein at 15 μg/ml showed the best reactivity to all HIV-positive samples and no reactivity to HIV-negative samples and was therefore used as the HIV reagent for further studies. Accordingly, the control reagent was formulated to contain 10F7Fab, A41Fab, and B6Fab each at 7.5 μg/ml. These formulations were evaluated for stability by using the same protocol used for individual proteins. No appreciable change in the reactivity of any reagent was observed after incubation for 30 days at 37°C or for 6 months at 4°C (data not shown).
Evaluation of NEVA HIV.
The NEVA HIV reagents were evaluated for their immunoreactivities with commercially available mixed-titer panels and seroconversion panels from Boston Biomedica Inc. (see Tables 1, 2, and 3), seroconversion panels from NABI Diagnostics (see Table 3), and whole-blood or serum samples from several hospitals in India (see Table 4) by the slide agglutination assay format.
TABLE 1.
Evaluation of NEVA HIV with HIV-1 mixed-titer panels from Boston Biomedica Inc.a
| Panel | NEVAb HIV | Titer byc:
|
Result by:
|
||||
|---|---|---|---|---|---|---|---|
| GenSys HIV-1/2 | Abbott HIV-1/2 | Organon Teknika HIV-1 | Abbott HIV | Waldheim IFA RL1d | Ortho WBe | ||
| PRB106-01 | POS | 1.3 | 7.5 | 3.9 | 1.1 | R | POS |
| PRB106-02 | NEG | 0.2 | 1.8 | 0.4 | 3.3 | NR | NEG |
| PRB106-03 | POS | 1.6 | 2.0 | 2.4 | 2.7 | R | POS |
| PRB106-04 | POS | 3.9 | 8.9 | 4.2 | 0.5 | R | POS |
| PRB106-05 | POS | 2.5 | 17.6 | 5.8 | 0.4 | R | POS |
| PRB106-06 | NEG | 0.1 | 0.1 | 0.4 | 0.4 | NR | NEG |
| PRB106-07 | POS | 3.1 | 7.9 | 3.4 | 0.4 | R | POS |
| PRB106-08 | NEG | 0.9 | 15.7 | 0.9 | 11.5 | NR | IND |
| PRB106-09 | POS | 2.5 | 16.6 | 6.1 | 1.5 | R | POS |
| PRB106-10 | POS | 1.8 | 12.7 | 1.3 | 6.9 | R | POS |
| PRB106-11 | POS | 0.5 | 6.8 | 1.3 | 1.0 | NR | POS |
| PRB106-12 | POS | 0.6 | 2.4 | 3.1 | 10.6 | R | POS |
| PRB106-13 | POS | 2.5 | 10.5 | 3.8 | 0.4 | R | POS |
| PRB106-14 | POS | 1.7 | 6.0 | 2.2 | 2.0 | R | POS |
| PRB106-15 | POS | 1.9 | 5.5 | 4.2 | 1.6 | R | POS |
| PRB203-01 | POS | 4.6 | >16.3 | 6.4 | 0.9 | R | POS |
| PRB203-02 | POS | 6.8 | >16.3 | 6.6 | 1.0 | R | POS |
| PRB203-03 | NEG | 0.1 | 0.4 | 0.5 | 0.3 | NR | NEG |
| PRB203-04 | NEG | 0.3 | 13.1 | 0.6 | 23.3 | NR | IND |
| PRB203-05 | POS | 5.6 | >16.3 | 6.6 | 0.7 | R | POS |
| PRB203-06 | POS | 4.1 | 15.3 | 6.3 | 0.4 | R | POS |
| PRB203-07 | POS | 1.0 | 4.6 | 1.4 | 0.5 | R | POS |
| PRB203-08 | POS | 8.6 | >16.3 | 6.8 | 0.3 | R | POS |
| PRB203-09 | POS | 4.3 | >16.3 | 5.7 | 0.4 | R | POS |
| PRB203-10 | POS | 2.8 | 12.1 | 5.1 | 0.5 | R | POS |
| PRB203-11 | POS | 1.8 | 7.4 | 2.4 | 0.4 | R | POS |
| PRB203-12 | POS | 8.2 | >16.3 | 6.7 | 0.4 | R | POS |
| PRB203-13 | POS | 6.7 | >16.3 | 6.7 | 2.1 | R | POS |
| PRB203-14 | POS | 0.2 | 6.4 | 0.5 | 2.9 | NR | NEG |
| PRB203-15 | POS | 7.3 | >16.3 | 6.7 | 1.0 | R | POS |
| PRB203-16 | POS | 5.3 | 15.2 | 5.7 | 0.4 | R | POS |
| PRB203-17 | POS | 8.5 | >16.3 | 6.5 | 0.4 | R | POS |
| PRB203-18 | POS | 3.2 | 8.1 | 5.1 | 0.3 | R | POS |
| PRB203-19 | POS | 6.6 | >16.3 | 6.6 | 0.5 | R | POS |
| PRB203-20 | NEG | 0.1 | 0.3 | 0.4 | 0.4 | NR | NEG |
| PRB203-21 | POS | 7.8 | >16.3 | 6.4 | 0.3 | R | POS |
| PRB203-22 | POS | 0.8 | >16.3 | 1.8 | 11.0 | R | POS |
| PRB203-23 | POS | 7.6 | >16.3 | 6.7 | 1.1 | R | POS |
| PRB203-24 | POS | 1.0 | 6.5 | 3.2 | 0.4 | NR | POS |
| PRB203-25 | POS | 5.5 | >16.3 | 6.4 | 0.4 | R | POS |
The results obtained by a few EIAs, Western blotting, IFA, and HIV antigen detection are provided by panel supplier.
The agglutination reaction was recorded as negative (no visible agglutination) and positive (any agglutination more than that for the control reagent spot). POS, positive; NEG, negative.
In EIAs, values 1.0 or greater are considered reactive.
R, reactive; NR, nonreactive.
WB, Western blotting assay; POS, positive; NEG, negative; IND, indeterminate.
TABLE 2.
Evaluation of NEVA HIV with HIV-1/2 combination performance panel with HIV-1- and HIV-2-infected samples from Boston Biomedica Inc.
| Panel | HIV type | NEVA HIV resulta | Titer byb:
|
WB HIV1/2 Diagnostic Biotechnology Ltd.c | Dupont HIV antigen titer | |||
|---|---|---|---|---|---|---|---|---|
| Abbott HIV-1 | GenSys HIV-2 | GenSys HIV-1/2 | Abbott HIV 1/2 | |||||
| PRZ202-01 | 2 | POS | 2.8 | 9.8 | 5.3 | 12.7 | IND | 0.0 |
| PRZ202-02 | 1 | POS | >12.0 | 0.6 | 2.5 | 3.7 | POS | 0.2 |
| PRZ202-03 | 2 | POS | 1.3 | 12.3 | 7.5 | 11.5 | IND | 0.0 |
| PRZ202-04 | NEGd | NEG | 0.3 | 0.2 | 0.1 | 0.4 | NEG | 0.0 |
| PRZ202-05 | 2 | POS | 1.0 | 11.4 | 6.6 | 10.0 | IND | 0.1 |
| PRZ202-06 | 1 | POS | >12.0 | 1.0 | 3.5 | 17.6 | POS | 0.1 |
| PRZ202-07 | 1 | POS | >12.0 | 4.0 | 7.0 | 16.0 | POS | 0.0 |
| PRZ202-08 | 2 | POS | 3.6 | 11.0 | 6.3 | >17.9 | IND | 0.0 |
| PRZ202-09 | 2 | POS | 5.7 | 11.6 | 6.4 | 7.6 | POS | 0.0 |
| PRZ202-10 | 1 | POS | 0.5 | 0.1 | 0.2 | 7.6 | NEG | 14.4 |
| PRZ202-11 | 1 | POS | 5.9 | 0.4 | 1.6 | 0.5 | POS | 0.0 |
| PRZ202-12 | 2 | POS | 9.9 | 11.0 | 7.2 | 14.0 | POS | 0.0 |
| PRZ202-13 | 1 | POS | 7.0 | 0.7 | 1.7 | 3.5 | POS | 2.3 |
| PRZ202-14 | 2 | POS | >12.0 | 11.1 | 5.8 | >17.9 | IND | 0.0 |
| PRZ202-15 | 1 | POS | >12.0 | 12.0 | 8.4 | >17.9 | POS | 0.0 |
TABLE 3.
Evaluation of NEVA HIV using sero-conversion panels from Boston Biomedica Inc. and NABI Biopharmaceuticals
| Panel | Total no. of samples | No. of positive samples/total no. of samples
|
|||||
|---|---|---|---|---|---|---|---|
| NEVA HIVa | GenSys HIV | Abbott HIV | Organon Teknika HIV | Waldheim IFA RL1 | WB Orthob | ||
| BBIc | |||||||
| PRB923 | 13 | 4/13 | 4/13 | 4/13 | 4/13 | 4/13 | 2/13 |
| PRB932 | 9 | 5/9 | 5/9 | 2/9 | 3/9 | 5/9 | 5/9 |
| PRB940 | 8 | 5/8 | 1/8 | 4/8 | 4/8 | NAd | 0/8 |
| NABIe | |||||||
| SV-0321 | 5 | 2/5 | 1/5 | 3/5 | 3/5 | NA | 1/5 |
| SV-0341 | 6 | 3/6 | 0/6 | 3/6 | 3/6 | NA | 1/6 |
| SV-0361 | 6 | 2/6 | 0/6 | 2/6 | 2/6 | NA | 2/6 |
See footnote a of Table 1.
WB, Western blotting.
BBI, Boston Biomedica Inc.
NA, data not available.
NABI, NABI Biopharmaceuticals.
TABLE 4.
Evaluation of NEVA HIV with clinical samples from various hospitals in Indiaa
| Center | Nature of samples | No. of samples | Sensitivity | Specificity |
|---|---|---|---|---|
| Christian Medical College, Vellore | Serum and 26 whole-blood samplesb | 326 | 100c (174/174)d | 99.3c (151/152) |
| Institute of Pathology, New Delhi | Serum | 55 | 100 (5/5) | 100 (50/50) |
| Post Graduate Institute of Medical Research, Chandigarh | Serum | 132 | 100e (80/80) | 96.2e (50/52) |
| National AIDS Research Institute, Pune | Serum and 6 clotted blood samplesf | 269 | 100g (49/49) | 97.8g (215/220) |
| Safdarjang Hospital, New Delhi | Random whole-blood samples | 331 | 100h (1/1) | 98.8h (326/330) |
| Whole-blood samples from a sexually transmitted disease clinic | 164 | 100h (9/9) | 99.4h (155/156) | |
| All samples | 1,277 | 100 (318/318) | 98.6 (947/960) |
The serum samples were evaluated after they were mixed with type O negative RBCs (Table 1). Whole-blood samples were used directly (20 μl/spot). For clotted samples (clotted within 12 h of collection), the clot was broken with a plastic dropper, and cells from the clot mixed with plasma were used for the assay (20 μl/spot).
Including 5 serum samples from monkeys, 9 human T-cell leukemia type 1-positive samples, 21 samples from a sexually transmitted disease clinic, 13 samples from patients with typhoid fever, 3 samples from patients with tuberculosis, 8 samples from patients with syphilis, and 4 samples positive for HIV-2 only.
In comparison to center's data, which were obtained by two EIAs and Western blotting.
The values in parentheses indicate the number of samples positive/total number of samples tested.
In comparison to the center's data, which were obtained by two or three EIAs.
Including 116 samples from patients with tuberculosis, 34 samples from the Venereal Disease Research Laboratory, and 113 random serum samples.
In comparison to the center's data, which were obtained by EIA.
In comparison to data obtained by EIA.
Mixed-titer panels contain serum samples from different HIV-infected individuals with various titers of antibodies against different HIV proteins. The data on mixed-titer panels PRB203 and PRB106 in Table 1 show that the sensitivity and specificity of our reagent is comparable to those of other U.S. and European Food and Drug Administration (FDA)-approved kits. PRB106 is a panel of serum samples with low titers of anti-HIV antibodies. Two members of the panel (PRB106-02 and PRB106-06) that were negative with all commercial kits and by Western blotting were also negative with our reagent, indicating that the NEVA HIV reagent is highly specific. Sample PRB106-08 was negative with our reagent, was indeterminate by Western blotting, and was nonreactive by IFA and several EIAs. This sample had very high levels of p24 antigen and was probably collected early, before seroconversion. Another member, PRB106-11 was nonreactive by IFA and by EIAs from Genetic Systems and Organon Teknika and was indeterminate by Western blotting, giving a faint band corresponding to p24. This sample was positive with our reagent as well as by the Abbott HIV1/2 assay. Similarly, PRB106-12, which also had high levels of p24 antigen, was weakly positive by some EIAs and was missed by a few EIAs, including the GeneSys HIV1/2 EIA. This sample was clearly positive with the NEVA HIV reagent. Western blot analysis of the sample showed reactivity to only p24. PRB106-12 was from the same donor as PRB106-02 but was collected 2 days later. The positive reaction of PRB106-12 attests to the observation that antibodies to p24 are among the first to appear soon after seroconversion, and detection of anti-p24 antibodies can shorten the window period of diagnosis of HIV infection.
Most of the members of panel PRB203 had moderate to high titers of antibodies against several HIV proteins and gave good reactivities with our reagent. PRB203-03 and PRB203-20 were negative with all kits tested and also with our reagent. PRB203-04 was nonreactive with our reagent and with several ELISA kits. This sample contained very high levels of p24 antigen and gave weak reactivity to p24 on Western blotting. This sample was also nonreactive by IFA. Another sample, PRB203-14, was negative by several EIAs and IFA and failed to show any band on Western blotting. This sample had moderately high levels of p24 antigen. The sample was positive with the NEVA HIV reagent and also by the Abbott HIV1/2 assay. PRB203-24, collected from the same donor of sample PRB203-14 after 9 days, was positive by most EIAs, although it was still nonreactive by IFA and indeterminate by Western blotting. This indicates that PRB203-14 may have been collected very early after infection and had low titers of antibodies to HIV.
The results obtained on evaluation of another mixed-titer panel, PRB202, with our reagent corroborated well those obtained by other EIAs and Western blotting (data not shown). Again, a few ELISA kits, including the Abbott HIV1/2 assay, missed one member (PRB202-07), but the sample was clearly positive with our reagent (data not shown; the results of testing of this sample with mix C are shown in Fig. 2). On Western blotting, this sample showed weak reactivity to p24, indicating that it was probably collected early after infection. These results reinstate the importance of detection of anti-p24 antibodies in immunoassays.
Since NEVA HIV combines detection of both HIV-1 and HIV-2 in the same spot, we also evaluated a combination panel (PRZ202) that contains samples from patients infected with HIV-1 or HIV-2. As seen in Table 2, all HIV-2-positive samples were positive with the NEVA HIV reagent and with the other ELISA kits, although some samples were indeterminate by Western blotting. HIV-1-positive sample PRZ202-10, which contained high levels of p24 antigen, was negative by the GenSys HIV1/2 assay and Western blotting but was clearly positive with the NEVA HIV reagent as well as with the Abbott HIV1/2 assay. Similarly, PRZ202-11 was positive with the NEVA HIV reagent and by Western blotting but was missed by the Abbott HIV1/2 assay. All other HIV-1-positive samples were positive with our reagent and by the EIAs.
We also evaluated NEVA HIV using commercially available seroconversion panels. Seroconversion panels contain serum samples collected from the same HIV-infected individual over a period of few weeks. The consecutive samples show gradual changes in antibody profiles in terms of both titer and specificity. Evaluation with these panels reflects the sensitivity of detection of anti-HIV antibodies following seroconversion. The data obtained for three seroconversion panels from Boston Biomedica Inc. and three seroconversion panels from NABI Diagnostics are shown in Table 3. The samples in panels PRB923 and PRB932 were found to be positive with the NEVA HIV reagent during the same interval after infection as the interval of detection of positivity with most other kits. Members of panel PRB940 had low antibody titers. This was evident from the low readings obtained by all EIAs (data not shown). Western blotting gave indeterminate results with the last five samples, showing only bands for p24 and faint bands for gp120, which appeared in the last three samples. The last five samples were clearly positive with the NEVA HIV reagent, again highlighting the importance of detection of anti-p24 antibodies. Also, the NEVA HIV reagent can capture both IgG and IgM responses, while commercial EIAs that use anti-IgG conjugate miss samples with IgM antibodies.
With NABI Diagnostics panel SV0321 (Table 3), our reagent showed reactivity one sample later compared to the sample number found to be positive by several EIAs. The third member of the panel (which was not found to be positive with the NEVA HIV reagent) had very high p24 antigen levels and was also indeterminate by Western blotting. With panels SV0341 and SV0361, the results obtained with our reagent were concordant with those obtained by the commercial EIAs.
Evaluation of these two types of panels establishes that the NEVA HIV reagent compares well with several existing U.S. and European FDA-approved kits in terms of sensitivity and specificity. However, most of the panels contain samples infected with HIV subtype B. For more widespread use, a test needs to be evaluated in the hospital setting, where samples infected with other organisms and with different variants of HIV are found (11). We therefore evaluated our reagent in hospitals and reference centers in different geographical locations throughout India. Several samples were from patients infected with other pathogens such as Mycobacterium tuberculosis, Salmonella enterica serovar Typhimurium, and human T-cell leukemia virus type 1. The results of the evaluation are summarized in Table 4. Our reagent had 100% sensitivity and 98.6% specificity for the detection of HIV in all samples. No cross-reactivity was observed with samples carrying other infectious agents (Table 4).
One of the prime virtues of NEVA HIV is that it can be performed with whole blood and thus requires no processing of samples to prepare serum or plasma. For determination of the efficacy of the reagent for the detection of anti-HIV antibodies in whole blood, 500 blood samples were procured from a hospital in New Delhi, India, and tested with our reagent. The samples included blood collected from regular hospital patients representing low-risk group and samples collected from sexually transmitted disease clinic patients confirmed to be a population at high risk for HIV infection. The results obtained with the NEVA HIV reagent were compared with the results obtained with two commercially available ELISA kits. As shown in Table 4, the reagent was found to be 100% sensitive and 98.9% specific with these whole-blood samples.
NEVA HIV was also evaluated to determine its sensitivity by using serial dilutions of serum samples in a slide assay. Randomly collected samples from HIV-1- or HIV-2-infected patients could be diluted more than 100-fold, and agglutination could be seen (data not shown). Samples from seroconversion panels (SV0361D and SV0361E), which are expected to have low antibody titers, could be diluted two- to fourfold (data not shown). These results suggest that the reagent is able to detect antibodies over a wide range of titers. Nevertheless, this test is designed for use with whole-blood samples, and sample dilution is not required prior to testing.
DISCUSSION
We have developed a highly sensitive and specific rapid assay for the detection of antibodies to both HIV-1 and HIV-2 in a drop of blood. The test requires bifunctional molecules comprising monovalent antibody fragments against the RBC surface fused to peptides or proteins derived from immunodominant regions of HIV-1 and HIV-2. The original rapid whole-blood agglutination assay for HIV antibodies used multistep chemical conjugation procedures (8) to produce a bifunctional molecule. That assay was marketed as SimpliRED HIV-1/HIV-2 (AGEN Biomedical Limited, Brisbane, Australia). We have designed these bifunctional molecules by gene fusion techniques and optimized methods for bacterial expression and purification using column chromatography procedures to obtain these molecules in large quantities. The amount of reagent required per test is in submicrogram quantities (20 μl of concentrations of 7.5 to 15.0 μg/ml); therefore, a batch of purified proteins is sufficient for the formulation of reagent for more than 100,000 tests.
All the fusion proteins, individually and as the final reagent, are stable for at least 30 days at 37°C and for more than 6 months at 2 to 8°C. A batch of NEVA HIV reagents showed the same reactivity to a panel (PRB202) from Boston Biomedica Inc. over a period of 15 months of storage at 2 to 8°C (data not shown). The stabilities of the reagents at 37°C makes them suitable for use under relatively warm climatic conditions in the field and in testing places that do not have substantial refrigeration facilities.
The SimpliRED HIV-1/HIV-2 assay (AGEN Biomedical Limited) uses a reagent consisting of an epitope from gp41 of HIV-1 and an epitope from gp36 of HIV-2 linked to an Fab fragment of an anti-RBC antibody. However, as evident from the data presented in this paper, use of only these epitopes in immunoassays may not suffice for the detection of HIV in all types of samples. A large number of samples, especially from early seroconverters, have antibodies specific for the capsid protein, p24, of HIV. Our evaluation data show that the sensitivity of immunodetection was markedly improved by the inclusion of the p24 antigen fusion protein in the NEVA HIV reagent.
Furthermore, to avoid competition for binding to RBC surface antigen, we have attached HIV antigens to three different anti-RBC antibodies, which bind to different antigens on the RBC surface. While MAb 10F7 binds to glycophorin (1), the antigens of MAbs A41 and B6 are not known. However, competition studies have shown that they bind to different sites on the RBC surface. All three antibodies have been tested for their binding to more than 1,500 random blood samples to ascertain their universal reactivities. The presence of three antibody fusion proteins also ensures that no sample is missed due to a lack of an anti-RBC binding site on the RBC surface. This cocktail of three antibody fusion proteins (mix C) produces quicker and better agglutination compared to that of a mix with a single antibody, as seen from comparison of mixes A, B, and C (Fig. 2), presumably due to increased cross-linking of RBCs and better lattice formation.
Commercially available rapid tests and EIAs use conjugates to produce a visually readable color reaction. However, in most cases these conjugates are specific for human IgG and do not detect the IgM antibodies of the donor. Determine, a rapid test for the detection of HIV in whole blood, is capable of detecting only the IgG class of antibodies (13). NEVA HIV involves cross-linking of reagent-coated RBCs by the patient's anti-HIV antibodies and can therefore capture both the IgM and the IgG responses of the individual in the same spot.
Evaluations with commercially available mixed-titer panels, seroconversion panels, and clinical samples showed that NEVA HIV compares well with existing U.S. and European FDA-approved kits for the detection of anti-HIV antibodies. The samples tested were in different forms, i.e., serum, plasma, whole blood, and clotted blood. HIV could be detected in all types of samples with equal sensitivities, indicating the possible widespread use of these reagents.
The data presented here show that NEVA HIV can be used for the hemagglutination-based rapid detection of antibodies to HIV in whole blood with high sensitivity and specificity. Besides being extremely rapid (less than 5 min), the test is simple to learn and easy to perform, with results in concordance with Western blotting results. The high sensitivity of detection of HIV in samples of U.S. origin (samples from the panels) as well as Indian origin (clinical samples) indicates that the test can detect both HIV subtype B and HIV subtype C infections. Recently, we have evaluated a panel consisting of samples obtained from individuals infected with different subtypes of HIV-1 (M group) and found the test to have a high sensitivity (unpublished data). We have developed this test by keeping in mind the practical constraints involved in testing in developing and underdeveloped countries: the paucity of funds and even the lack of infrastructure for a proper supply of electricity and good-quality laboratory-grade water. This test is completely instrument-free, simple enough to be carried out by a semiskilled technician, based on whole blood, and extremely rapid and is primarily aimed to the hard-to-reach populations of the underdeveloped and developing nations of the world, but will also contribute toward better HIV detection compliance in developed nations.
Acknowledgments
We thank G. Sridharan (Christian Medical College), S. Sehgal (Postgraduate Institute of Medical Research), R. Paranjapye (National AIDS Research Institute), and K. Ray (Safdarjang Hospital) for samples.
This work was supported by the Department of Biotechnology, Government of India. A.G. is thankful for a research fellowship from the Council of Scientific and Industrial Research, Government of India.
REFERENCES
- 1.Bigbee, W. L., M. Vanderlaan, S. S. Fong, and R. H. Jensen. 1983. Monoclonal antibodies specific for the M- and N-forms of human glycophorin A. Mol. Immunol. 20:1353-1362. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1998. Update: HIV counseling and testing using rapid tests—United States, 1995. Morb. Mortal. Wkly. Rep. 47:211-215. [PubMed] [Google Scholar]
- 3.Gupta, A., and V. K. Chaudhary. 2002. Expression, purification and characterization of an anti-RBCFab-p24 fusion protein for haemagglutination based rapid detection of antibodies to HIV in whole blood. Protein Expr. Purif. 26:162-170. [DOI] [PubMed] [Google Scholar]
- 4.Gupta, A., S. Gupta, and V. K. Chaudhary. 2001. Recombinant fusion proteins for haemagglutination-based rapid detection of antibodies to HIV in whole blood. J. Immunol. Methods 256:121-140. [DOI] [PubMed] [Google Scholar]
- 5.Heberling, R. L., S. S. Kalter, P. A. Marx, J. K. Lowry, and A. R. Rodriguez. 1988. Dot immunobinding assay compared with enzyme-linked immunosorbent assay for rapid and specific detection of retrovirus antibody induced by human or simian acquired immunodeficiency syndrome. J. Clin. Microbiol. 26:765-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayaraman, K. S. 1995. India refining simple HIV test. Nat. Med. 1:984.. [DOI] [PubMed] [Google Scholar]
- 7.Kassler, W. J., B. A. Dillon, C. Haley, W. K. Jones, and A. Goldman. 1997. On-site, rapid HIV testing with same-day results and counseling. AIDS 11:1045-1051. [DOI] [PubMed] [Google Scholar]
- 8.Kemp, B. E., D. B. Rylatt, P. G. Bundesen, R. R. Doherty, D. A. McPhee, D. Stapleton, L. E. Cottis, K. Wilson, M. A. John, J. M. Khan, et al. 1988. Autologous red cell agglutination assay for HIV-1 antibodies: simplified test with whole blood. Science 241:1352-1354. [DOI] [PubMed] [Google Scholar]
- 9.Machado, A. A., R. Martinez, A. A. Haikal, and M. C. Rodrigues da Silva. 2001. Advantages of the rapid HIV-1 test in occupational accidents with potentially contaminated material among health workers. Rev. Inst. Med. Trop. Sao Paulo 43:199-201. [DOI] [PubMed] [Google Scholar]
- 10.McKenna, S. L., G. K. Muyinda, D. Roth, M. Mwali, N. Ng'andu, A. Myrick, C. Luo, F. H. Priddy, V. M. Hall, A. A. von Lieven, J. R. Sabatino, K. Mark, and S. A. Allen. 1997. Rapid HIV testing and counseling for voluntary testing centers in Africa. AIDS 11(Suppl. 1):S103-S110. [PubMed] [Google Scholar]
- 11.Phillips, S., T. C. Granade, C. P. Pau, D. Candal, D. J. Hu, and B. S. Parekh. 2000. Diagnosis of human immunodeficiency virus type 1 infection with different subtypes using rapid tests. Clin. Diagn. Lab. Immunol. 7:698-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spielberg, F., C. M. Kabeya, R. W. Ryder, N. K. Kifuani, J. Harris, T. R. Bender, W. L. Heyward, and T. C. Quinn. 1989. Field testing and comparative evaluation of rapid, visually read screening assays for antibody to human immunodeficiency virus. Lancet i:580-584. [DOI] [PubMed] [Google Scholar]
- 13.Vallari, A. S., R. K. Hickman, J. R. Hackett, Jr., C. A. Brennan, V. A. Varitek, Jr., and S. G. Devare. 1998. Rapid assay for simultaneous detection and differentiation of immunoglobulin G antibodies to human immunodeficiency virus type 1 (HIV- 1) group M, HIV-1 group O, and HIV-2. J. Clin. Microbiol. 36:3657-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasudevachari, M. B., K. W. Uffelman, T. C. Mast, R. L. Dewar, V. Natarajan, H. C. Lane, and N. P. Salzman. 1989. Passive hemagglutination test for detection of antibodies to human immunodeficiency virus type 1 and comparison of the test with enzyme-linked immunosorbent assay and Western blot (immunoblot) analysis. J. Clin. Microbiol. 27:179-181. [DOI] [PMC free article] [PubMed] [Google Scholar]