Abstract
The assessment of immunogenicity of a diluted vaccinia vaccine for possible widespread use of a diluted vaccine in the event of a bioterrorist attack prompted us to focus on the development of a sensitive and specific plaque reduction neutralization (PRN) assay to assess the antibody response of volunteers to a vaccinia (Dryvax) vaccine. Two incubation times, 1 h or overnight (approximately 15 h), were explored for the neutralization step of the assay. In addition, serum samples were evaluated using both sonicated and nonsonicated virus in PRN assays with 1 and 15 h of incubation. The use of the overnight incubation method resulted in the detection of antibody in two vaccinated individuals who exhibited a take, i.e., a major reaction indicative of successive vaccination as defined by the Centers for Disease Control and Prevention, but did not have a fourfold increase in antibody to vaccinia virus by the 1-h-incubation method and increased the sensitivity from 94 to 100%. In addition to the increased sensitivity of the assay, we noted a significant increase (approximately 40-fold) in the PRN titer of serum samples tested with the 15-h-incubation method. The use of sonicated virus increased the reproducibility of the virus titers and PRN titers. Forty-two percent of the samples tested using sonicated virus had a PRN titer that was fourfold higher or greater than that of nonsonicated virus in the assay. A PRN titer that was threefold higher or greater was observed in more than half (58%) of the samples using sonicated virus. Therefore, the more sensitive, specific, and reproducible plaque neutralization assay for the detection of antibody to vaccinia virus is the method using a 15-h-incubation time and freshly sonicated vaccinia virus.
Recently, there has been renewed interest in the use of the smallpox vaccine (vaccinia vaccine) as a defense against the intentional release of smallpox as an act of bioterrorism (5). The last vaccinia vaccine manufactured in the United States was Dryvax, which was produced in the mid-1980s by Wyeth-Ayerst (now Wyeth), Marietta, Pa. There is a limited supply of Dryvax vaccine, and several studies have been performed to determine whether the remaining vaccine can be diluted to increase the number of available doses (2, 3). The objectives of these studies included determining the effect of diluting the Dryvax vaccine on the take rate, i.e., the rate of producing a major reaction indicative of successive vaccination as defined by the Centers for Disease Control and Prevention, as well as assessing the serum neutralizing antibody titers of volunteers before and after vaccination (10). Previous studies of the serological response of piglets to vaccinia vaccine used assays such as the hemagglutination inhibition assay and the indirect immunofluorescence assay (6). A variety of serum neutralizing assays have also been used to assess the antibody responses of humans and animals to vaccinia virus. These assays include the use of pock formation on scarified rabbit skin (8), inoculation of the chorioallantoic membranes of embryonated chicken eggs (9), or determination of the cytopathic effect of serum-virus mixtures on primary rhesus monkey kidney tissue culture cells (1). Each of the aforementioned techniques is a variation of the plaque reduction neutralization (PRN) assay. This report focuses on the development of an appropriate PRN assay to assess the antibody responses of volunteers to various doses of Dryvax. Two critical parameters of the PRN assay were measured, the effect of the length of incubation during the neutralization step of the assay and the effect of sonicating vaccinia virus prior to the neutralization step.
Previous reports on these two aspects of the PRN methodology used in the vaccinia virus plaque reduction serum neutralization assay contain differing procedures with relationship to the length of neutralization step in the assay and the use of sonicated reference virus in the assay (1, 4, 7, 11). There are conflicting reports on the length of the neutralization step of the PRN assay, and none of the reports address this aspect simultaneously with the use of sonicated or nonsonicated reference virus in the assay. In view of the differences found in the literature, we sought to determine the independent and combined effects of these two parameters on the neutralizing antibody titers of volunteers vaccinated with vaccinia virus.
MATERIALS AND METHODS
Samples.
Serum samples collected prior to vaccination with Dryvax and at various intervals following vaccination were stored at or below −20°C prior to testing. All serum samples were heat inactivated at 56°C for 30 min before being assayed.
Reference virus.
The reference virus used in the assay was prepared using previously frozen stock of Dryvax vaccine as the inoculum. The virus was propagated in BSC-40 cells (continuous African green monkey kidney cell line), frozen, thawed twice to release virus, and clarified by low-speed centrifugation prior to use in the assays.
Serum controls.
The negative-control serum sample was serum from a human who had not been vaccinated previously. The positive-control serum sample was a postvaccination serum sample from a human who had been vaccinated twice previously and was recently revaccinated with vaccinia (Dryvax) vaccine. Each of these serum samples was evaluated in PRN assays using both 1-h and 15-h-incubation times.
PRN antibody assays.
Serum samples were evaluated using the PRN assay and 1-h and 15-h incubation periods with and without freshly sonicated virus to determine the effect of each of the parameters on the serum neutralization titers. Sera tested in these comparison assays were collected from volunteers enrolled in vaccinia (Dryvax) vaccine studies that had been approved by the Saint Louis University Institutional Review Board. The data from the 1-h-incubation PRN assay have been previously published (2). Serial twofold or fourfold dilutions of heat-inactivated study sera and control sera were prepared in serum-free minimum essential medium (MEM) supplemented with antibiotics. Each dilution of serum was mixed with an equal volume of vaccinia virus that was not sonicated or had been freshly sonicated and then diluted to contain approximately 40 to 60 PFU of virus. Serum-virus mixtures were incubated for 1 h or overnight (approximately 15 h) at 37°C with 5% CO2. A reference virus control for each assay was prepared by mixing an equal volume of the reference vaccinia virus diluted to 40 to 60 PFU with an equal volume of MEM.
Previously prepared monolayers of BSC-40 cells in 24-well tissue culture plates were washed once with MEM supplemented with antibiotics. Serum-virus mixtures that had been incubated for the 1-h or overnight neutralization period were inoculated onto the washed BSC-40 cell monolayers in duplicate and allowed to adsorb for 1 h in a 37°C incubator with 5% CO2. After adsorption, the monolayers of BSC-40 cells were overlaid with MEM supplemented with 2% fetal bovine serum and antibiotics and returned to the 37°C incubator for 2 days. Following the 2-day incubation period, the tissue culture monolayers were fixed and stained in one step using a mixture of crystal violet and formalin. After the cells were stained, the plates were air dried, and the plaques were counted using a dissecting microscope.
The endpoint serum neutralizing antibody titer of each serum sample tested by the 1-h-incubation method was defined as the reciprocal of the highest dilution of the serum with a mean plaque count less than or equal to the 60% plaque reduction cutoff value for the assay. A modified linear regression analysis method that interpolated the endpoint titer was used in the 15-h-incubation assays. Briefly, the plaque counts for each serum dilution from 1:4 to 1:16,384 were determined manually as described above. Plaque counts above and below the 60% plaque reduction endpoint (assay cutoff) were entered into an Excel file where the endpoint titer was interpolated by linear regression.
Sonication.
The sonicated reference Dryvax vaccine used in the assays was treated as follows. The sealed vial containing Dryvax was immersed in cold water in the cuphorn attachment of a sonicator (model W220; Heat Systems Ultrasonics, Inc., Farmingdale, N.Y.) for three 20-s bursts of 75 W of output with each treatment.
Statistics.
Descriptive statistics are given for PRN titers. Sensitivity is defined as the proportion of subjects who had a fourfold increase in PRN titer among the subjects exhibiting takes. Specificity is defined as the proportion of subjects who did not have a fourfold increase in PRN titer among subjects who did not exhibit takes.
RESULTS
The negative-control serum sample had a PRN antibody titer of <4 (tested 16 times) using the 1-h-incubation method for neutralization and a mean titer of 4.7 (range, <4 to 13, tested 27 times) using the 15-h-incubation method. The positive-control serum sample had a mean neutralization titer of 23 (range, 8 to 64) or 811 (range, 406 to 1,447) using the 1-h or 15-h neutralization incubation period for the serum-virus mixtures, respectively (data not shown).
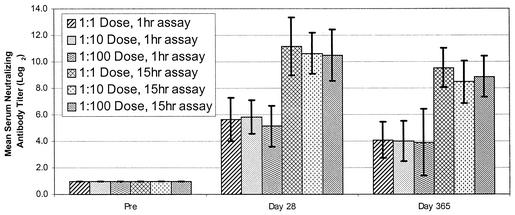
The results of the serum neutralization antibody assay using1-h versus overnight (15-h) incubation and nonsonicated vaccinia virus are shown in Fig. 1. Volunteers in this study received either undiluted Dryvax or Dryvax diluted 1:10 or 1:100. The overnight-incubation assay increased PRN antibody titers by 28- to 46-fold at day 28 and 22- to 44-fold at day 365. On the basis of the results from the 1-h-incubation method, two subjects who exhibited takes developed titers of 1:4 after vaccination, but these titers were not scored as fourfold antibody increases. However, both of these subjects had fourfold antibody increases (Table 1) based on data from the 15-h-incubation PRN method that increased the sensitivity from 94 to 100%.
FIG. 1.
Comparison of pre- and postvaccination PRN antibody titers among all volunteers with takes in a vaccinia vaccine pilot study (vaccinia- naive individuals) using 1-h and 15-h incubations of serum-virus mixtures. (Vaccinia virus used in the assays was not freshly sonicated.)
TABLE 1.
Comparison of the antibody titers from PRN assays of two subjects who had takes but did not exhibit a fourfold increase in antibody titera
| Subject | Incubation time (h) | PRN titer
|
||
|---|---|---|---|---|
| Prevaccination (day 0) | Postvaccination
|
|||
| Day 28 | Day 365 | |||
| 029 | 1 | <4 | 4 | <4 |
| 15 | <4 | 3,131 | 166 | |
| 038 | 1 | <4 | 4 | <4 |
| 15 | <4 | 652 | 138 | |
The antibody titers from 1-h and 15-h-inoculation PRN assays of two subjects who had takes but did not exhibit a fourfold increase in antibody titer with the 1-h-incubation PRN method. The vaccine was not diluted.
The samples shown in Fig. 1 were also evaluated with freshly sonicated virus using the 1-h-incubation PRN method to determine whether sonication of the reference vaccinia virus alone would increase the PRN titer of the samples. Serum neutralizing antibody titers of these samples (Table 2) using sonicated vaccinia virus actually showed a slight decrease in titer when evaluated in the 1-h-incubation PRN assay.
TABLE 2.
Serum neutralizing antibody titers among vaccinia naive individuals using nonsonicated and freshly sonicated reference vaccinia virus in the 1-h-incubation PRN assay
| Dilution of Dryvax vaccine | No. of subjects | Mean serum neutralization titer
|
|||||
|---|---|---|---|---|---|---|---|
| Nonsonicated virus
|
Sonicated virus
|
||||||
| Pre- vaccination (day 0) | Postvaccination
|
Pre- vaccination (day 0) | Postvaccination
|
||||
| Day 28 | Day 365 | Day 28 | Day 365 | ||||
| 1:1 | 19 | <4 | 49 | 17 | <4 | 29 | 14 |
| 1:10 | 14 | <4 | 57 | 16 | <4 | 29 | 5 |
| 1:100 | 3 | <4 | 35 | 15 | <4 | 16 | 3 |
The effect of sonication on the PRN titer of serum samples was also assessed in the 15-h-incubation PRN assay. PRN assays using nonsonicated vaccinia virus and freshly sonicated virus were performed on 193 serum samples collected from volunteers following vaccination with Dryvax. Mean PRN titers of samples tested without sonicated virus (Table 3) ranged from 423 to 778 at day 28 and 300 to 372 at day 56 postvaccination. In contrast to the decrease in titer that we observed when sonicated virus was used in the 1-h-incubation assay, the use of sonicated virus in the 15-h-incubation assay increased the mean titers approximately three- to fourfold overall with ranges of 1,755 to 2,511 (day 28) and 870 to 1,484 (day 56) compared with titers found in the assay without sonicated virus. Eighty-two (42%) of the samples tested exhibited a significant increase in PRN titer, i.e., an increase of fourfold or greater, when tested with sonicated virus versus nonsonicated virus in the assay, and more than half (58%) of the 193 samples tested exhibited a PRN titer that was threefold higher or greater using sonicated virus in the overnight-incubation assay. The majority of all samples tested showed some increase in titer when the sonicated virus was used in the PRN assay.
TABLE 3.
Serum neutralizing antibody titers among vaccinia naive individuals using nonsonicated and freshly sonicated reference vaccinia virus in the 15-h-incubation PRN assay
| Dilution of Dryvax vaccine | No. of subjects | Mean serum neutralization titer
|
|||||
|---|---|---|---|---|---|---|---|
| Nonsonicated virus
|
Sonicated virus
|
||||||
| Pre- vaccination (day 0) | Postvaccination
|
Pre- vaccination (day 0) | Postvaccination
|
||||
| Day 28 | Day 365 | Day 28 | Day 365 | ||||
| 1:1 | 34 | 4 | 423 | 300 | 4 | 1755 | 870 |
| 1:5 | 69 | 5 | 778 | 372 | 8 | 2511 | 1484 |
| 1:10 | 90 | 10 | 640 | 348 | 6 | 2016 | 1182 |
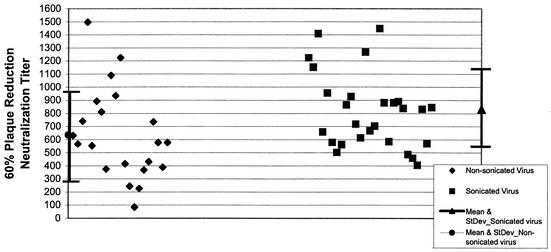
Prior to the use of sonicated virus in the assay, we observed a considerable difference in the reproducibility of the PRN titer of the positive-control serum sample and other serum samples tested by the 15-h-incubation PRN assay. Figure 2 illustrates the reproducibility of the titer of the positive-control serum used for the PRN assays when it was tested using nonsonicated or sonicated reference vaccinia virus in the assay. We observed substantial variation in the PRN titer of the positive-control serum sample in assays using nonsonicated vaccinia virus (range, 84 to 1,497) compared with the titer observed in assays using sonicated virus (range, 406 to 1,446). In addition, we observed a marginally significant (P = 0.06) increase in the mean PRN titer of the positive-control serum sample, 872 (standard deviation, 282) versus 637 (standard deviation, 349), using freshly sonicated or nonsonicated vaccinia virus, respectively.
FIG. 2.
PRN antibody titer of a positive-control serum sample using nonsonicated and sonicated reference vaccinia virus in the 15-h-incubation assay. StDev, standard deviation.
DISCUSSION
The PRN assay for vaccinia virus has evolved from early methods using the scarification of rabbit skin to chorioallantoic membrane inoculation and then to the use of a variety of tissue culture systems. During its evolution, the PRN antibody test has been dissected and reassembled numerous times using a variety of diluents, cell substrates, media, and incubation times and temperatures. One of the more recent reports compared the incubation of serum-virus mixtures for 2 h with an overnight incubation and showed that the 2-h and overnight-incubations gave equivalent results (7). The procedure used in that study compared a 2-h incubation at 37°C with a method using a 2-h incubation at 37°C followed by an overnight incubation at 4°C. We compared a 2-h versus 1-h incubation at 37°C and found them to give equivalent results (data not shown), so we tested sera from 60 volunteers in a pilot study evaluating undiluted and diluted Dryvax vaccine using a 1-h-incubation PRN method. Two individuals in the study exhibited takes but did not have a fourfold increase in antibody to vaccinia virus. We decided to try overnight (15-h) incubation to see whether the increased incubation time would result in a more sensitive assay. Based on the 15-h PRN method, the two volunteers with takes but without a fourfold increase in antibody exhibited pronounced increases on days 28 and 365 following vaccination, an increase that was not apparent using the shorter incubation method. As a result of this finding, the sensitivity of the PRN assay increased from 94 to 100% using the 15-h-incubation method. In addition to the increased sensitivity of the assay, we noted a significant (approximately 40-fold) increase in the PRN titer of serum samples tested with the 15-h-incubation method. The use of sonicated virus alone did not increase the PRN titers of samples when the 1-h-incubation method was used. However, using the 15-h-incubation method with sonicated vaccinia virus increased the mean PRN titers in most samples compared with the titers for samples in tests using the 15-h-incubation method with nonsonicated virus.
We also found that the reproducibility of the PRN assay was affected by whether or not the vaccinia virus was sonicated. Aggregates of virus and cell debris are believed to occur due to the membrane-associated nature of the poxviruses. Sonication of the virus immediately prior to the neutralization step in the 15-h-incubation PRN assay increased the serum neutralizing titer in most samples and gave us more reproducible results than the PRN assay using nonsonicated virus did. The 15-h-incubation PRN assay using freshly sonicated vaccinia virus is highly sensitive, specific, and reproducible for the detection of fourfold increases in serum neutralizing antibody to vaccinia virus.
Acknowledgments
This work was supported in part by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract number N01-AI-45250.
We are grateful to Walla Dempsey, Holli Hamilton, Steve Heyse, Wendy Fanaroff-Ravik, and Catherine Laughlin of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Cutchins, E., J. Warren, and W. P. Jones. 1960. The antibody response to smallpox vaccination as measured by a tissue-culture plaque method. J. Immunol. 85:275-283. [PubMed] [Google Scholar]
- 2.Frey, S. E., F. K. Newman, J. Cruz, W. B. Shelton, J. M. Tennant, T. Polach, A. L. Rothman, J. S. Kennedy, M. Wolff, R. B. Belshe, and F. A. Ennis. 2002. Dose-related effects of smallpox vaccine. N. Engl. J. Med. 346:1275-1280. [DOI] [PubMed] [Google Scholar]
- 3.Frey, S. E., R. B. Couch, C. O. Tacket, J. J. Treanor, M. Wolff, F. K. Newman, R. Atmar, R. Edelman, C. M. Nolan, and R. B. Belshe for the NIAID Smallpox Vaccine Study Group. 2002. Clinical responses to undiluted and diluted smallpox vaccine. N. Engl. J. Med. 346:1265-1274. [DOI] [PubMed] [Google Scholar]
- 4.Graham, B. S., R. B. Belshe, M. L. Clements, R. Dolin, L. Corey, P. F. Wright, G. J. Gorse, K. Midthun, M. C. Keefer, N. J. Roberts, Jr., D. H. Schwartz, J. M. Agosti, B. F. Fernie, D. M. Stablein, D. C. Montefiori, J. S. Lambert, S. L. Hu, J. R. Esterlitz, D. N. Lawrence, W. C. Koff, and the AIDS Vaccine Clinical Trials Network. 1992. Vaccination of vaccinia-naive adults with human immunodeficiency virus type 1 gp160 recombinant vaccinia virus in a blinded, controlled, randomized clinical trial. J. Infect. Dis. 166:244-252. [DOI] [PubMed] [Google Scholar]
- 5.Henderson, D. A. 1999. The looming threat of bioterrorism. Science 283:1279-1282. [DOI] [PubMed] [Google Scholar]
- 6.Imada, T., H. Kawamura, T. Nishimori, H. Murata, M. Narita, Y. Honda, and K. Ishigaki. 1989. Evaluation of the pathogenicity and immunogenicity of vaccinia virus to piglets. Jpn. J. Vet. Sci. 51:96-104. [DOI] [PubMed] [Google Scholar]
- 7.Katz, J. B. 1987. The effect of the virus-serum incubation period upon vaccinia virus serum neutralization titers. J. Biol. Stand. 15:389-392. [DOI] [PubMed] [Google Scholar]
- 8.Loutit, J. F., and D. McClean. 1945. The virus-neutralizing power of serum from recently vaccinated persons. J. Pathol. Bacteriol. 57:485-488. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy, K., and A. W. Downie. 1958. The antibody response in man following infection with viruses of the pox group. I. An evaluation of the pock counting method for measuring neutralizing antibody. J. Hyg. 56:84-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morbidity and Mortality Weekly Report. 2001. Vaccinia (smallpox) vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). Recommendations and Reports. Morb. Mortal. Wkly. Rep. 50:RR-10. [PubMed]
- 11.O'Brien, T. C., M. G. Myers, and N. M. Tauraso. 1971. Kinetics of the vaccinia virus plaque neutralization test. Appl. Microbiol. 21:968-970. [DOI] [PMC free article] [PubMed] [Google Scholar]