Abstract
Three different spotted-fever group rickettsiae—Rickettsia conorii, R. massiliae, and R. rhipicephali—were detected and identified by PCR-restriction fragment length polymorphism analysis in Rhipicephalus ticks collected from domestic animals in the Fokida region of Greece, where a high seroprevalence of antibodies to R. conorii was previously demonstrated. The infection rate of ticks was 1.6%. Moreover, R. conorii was isolated from one Rhipicephalus sanguineus tick.
Rickettsia conorii, the causative agent of Mediterranean spotted fever, is an obligate intracellular, gram-negative bacterium that infects humans. It is transmitted by the bite of infected arthropods (1, 9). The presence of the spotted-fever group (SFG) rickettsiae in ticks has been reported in several countries (2, 6-11, 13). Many rickettsiae remain poorly characterized due to the inability to be maintained in mammalian cell culture, embryonated chicken eggs, or small rodents, which have traditionally been the standard maintenance hosts used in rickettsiology. The advents of new culture techniques, such as the shell vial-centrifugation technique, and the detection of rickettsial DNA have increased the number of rickettsial species identified (6).
A seroepidemiological survey, using an immunofluorescence assay and a Western blot method, was conducted in three villages (Leukaditi, Vounichora, and Makrini) of the Greek province of Fokida in 1991. This study reported a high seroprevalence of antibodies to R. conorii in humans (46%) (1). In a culture survey of ticks from the same area, strain GS (for “Greek strain”) was isolated. This strain is genetically similar to Rickettsia massiliae; moreover, R. conorii was not found (2). As a follow-up to previous studies, we focused our efforts on determining the presence and infection rate of R. conorii in field-collected ticks from the three study villages of the Fokida (Fig. 1).
FIG. 1.
Geographic localization of collection sites in the Greek province of Fokida.
In the summer of 1998 (May to August), a total of 439 ticks collected from goats, sheep, and dogs were examined for the presence of SFG rickettsiae. According to standard taxonomic keys, 207 ticks were identified as Rhipicephalus sanguineus, 94 were identified as Rhipicephalus turanicus, and 138 were identified as Rhipicephalus bursa (8). Each tick was triturated in 500 μl of minimum essential medium supplemented with 4% fetal calf serum and 2 mM l-glutamine. Half of the above suspension was used for the detection of rickettsial DNA by PCR, and the rest was used for the isolation of rickettsiae by the shell vial technique (3, 4, 9, 11-13). The DNA extraction from the ticks was performed using the QIAamp tissue kit (Qiagen, Hilden, Germany), according to the instructions of the manufacturer. The primer set Rp.CS.877-Rp.CS.1258n was used to amplify a 381-bp sequence of the citrate synthase gene (9). As a negative control for each tick sample, pure PCR buffer treated in the same way as the tick samples was included. As a positive control, purified DNA from R. conorii (Moroccan strain) was used. The DNA from ticks that were positive for the citrate synthetase gene product was also amplified using the primer set Rr190.70p-Rr190.602n, which codes for a 532-bp sequence of the 190-kDa surface protein gene (2, 10, 12, 13).
Of the 439 ticks tested, 7 were positive by assays with both sets of primers (1.6%). The infection rate by rickettsiae was 2.4% (5 of 207) among R. sanguineus and 1.4% (2 of 138) among R. bursa.
Amplified products using the Rp.CS.877-Rp.CS.1258n primer pair were digested with AluI restriction endonuclease, while those using the Rr190.70p-Rr190.602n primer pair were digested with RsaI and PstI (2, 7). The identification of rickettsial species was made by comparison of the restriction fragment length polymorphism patterns with those of R. conorii and other references strains. The sizes of the generated fragments obtained from three positive ticks were identical to those of R. massiliae, two were identical to those of R. conorii, and two were identical to those of R. rhipicephali.
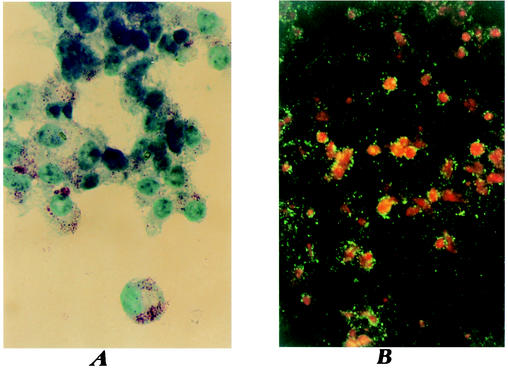
For each positive tick sample identified, we attempted to isolate and cultivate the strain using the shell-vial technique (6). One isolate of R. conorii was obtained. When a successful isolation was identified in the shell vials, the infected cells were passaged by trypsinization into a 25-cm2 tissue culture flask. Rickettsial infection was monitored by immunofluorescence assay and Gimenez staining of cells scraped from the bottom of the flasks (2, 5, 6) (Fig. 2). For characterization of the isolate, PCR followed by restriction fragment length polymorphism analysis was performed in the infected cells.
FIG. 2.
(A) Gimenez staining of rickettsiae in infected Vero cells. (B) Immunofluorescent staining of rickettsiae in infected Vero cells (magnification, × 400).
This is the first report of detection, isolation, and identification of R. conorii in Greece. Also, the presence of R. massiliae was confirmed. In this survey, R. rhipicephali, first isolated in the European region in France in 1992 (3), was also detected for the first time in ticks from Greece. The high seroprevalence of antibodies to R. conorii, reported in a previous survey (1), was confirmed by the detection of three SFG rickettsiae in ticks. Thus far, no isolations of rickettsiae from humans have been documented in this region. Because of the lack of specific clinical and laboratory criteria, many cases of rickettsioses may be underdiagnosed. Further studies are needed, as well as further education of the clinicians, in order to provide definite diagnoses and achieve isolation of SFG rickettsiae from human patients.
REFERENCES
- 1.Babalis, T., H. T. Dupont, Y. Tselentis, C. Chatzichristodoulou, and D. Raoult. 1993. Rickettsia conorii in Greece: comparison of a microimmunofluorescence assay and western blotting for seroepidemiology. Am. J. Trop. Med. Hyg. 48:784-792. [DOI] [PubMed] [Google Scholar]
- 2.Babalis, T., Y. Tselentis, V. Roux, A. Psaroulaki, and D. Raoult. 1994. Isolation and identification of a Rickettsial strain related to Rickettsia massiliae in Greek ticks. Am. J. Trop. Med. Hyg. 50:365-372. [DOI] [PubMed] [Google Scholar]
- 3.Drancourt, M., P. J. Kelly, R. Regnery, and D. Raoult. 1992. Identification of spotted fever group rickettsiae using polymerase chain reaction and restriction-endonuclease length polymorphism analysis. Acta Virol. 36:1-6. [PubMed] [Google Scholar]
- 4.Eremeeva, M., X. Yu, D. Raoult. 1994. Differentiation among spotted fever group rickettsiae species by analysis of restriction fragment length polymorphism of PCR-amplified DNA. J. Clin. Microbiol. 32:803-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gimenez, D. F. 1964. Staining rickettsiae in yolk-sac cultures. Stain Technol. 39:135-140. [DOI] [PubMed] [Google Scholar]
- 6.Kelly, P. J., D. Raoult, and P. R. Mason. 1991. Isolation of spotted fever group rickettsias from triturated ticks using a modification of the centrifugation-shell vial technique. Trans R. Soc. Trop. Med. Hyg. 85:397-398. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson, K., O. Lindquist, A. J. Liu, T. G. T. Jaenson, G. Friman, and C. Pahlson. 1999. Rickettsia helvetica in Ixodes ricinus ticks in Sweden. J. Clin. Microbiol. 37:400-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papadopoulos, B. 1990. Les Tiques des animaux domestiques et les hemoatozoaires qu'elles transmettent en Macedoine (Greece). Ph.D. thesis. University of Neuchatel, Neuchatel, Switzerland.
- 9.Psaroulaki, A., F. Loukaidis, C. Hadjichristodoulou, and Y. Tselentis. 1999. Detection and identification of the etiological agent of Mediterranean spotted fever (MSF) in two genera of ticks in Cyprus. Trans R. Soc. Trop. Med. Hyg. 93:597-598. [DOI] [PubMed] [Google Scholar]
- 10.Rydkina, E., V. Roux, N. Fetisova, N. Rudakov, M. Gafarova, I. Tarasevich, and D. Raoult. 1999. New rickettsiae in ticks collected in territories of the former Soviet Union. Emerg Infect. Dis. 6:811-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simser, J. A., A. T. Palmer, U. G. Munderloh, and T. J. Kurtti. 2001. Isolation of a spotted fever group rickettsia, Rickettsia peacockii, in a Rocky Mountain wood tick, Dermacentor andersoni, cell line. Appl. Environ. Microbiol. 67:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang, X. F., M. Y. Fan, J. Chen, and D. Z. Bi. 1994. Genotypic identification of seven Rickettsia conorii strains. Acta Virol. 38:35-37. [PubMed] [Google Scholar]
- 13.Zhang, J. Z., M. Y. Fan, Y. M. Wu, P. E. Fournier, V. Roux, and D. Raoult. 2000. Genetic classification of “Rickettsia heilongjiangii” and “Rickettsia hulinii,” two Chinese spotted fever group rickettsiae. J. Clin. Microbiol. 38:3498-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]