Abstract
A healthy vaginal ecosystem has been shown to be protective against the acquisition of human immunodeficiency virus and gonorrhea, and women who are colonized with H2O2-producing lactobacilli are more likely to maintain a normal vaginal flora than women with lactobacilli that do not produce H2O2. The purpose of this study was to formulate a testing medium that better supports the growth and detection of H2O2 by a broader range of lactobacilli than a published, widely used agar formulation (TMB). The new medium (TMB-Plus) consists of brucella agar base, 3,3′,5,5′-tetramethylbenzidine, horseradish peroxidase, starch, vitamin K, hemin, magnesium sulfate, manganese sulfate, and horse serum. To validate the new formula, 256 vaginal isolates and ATCC strains were inoculated onto TMB-Plus and, for comparison, onto TMB. Growth was enhanced for 69% of the isolates on TMB-Plus, and 48% had enhanced color production. The percentage of H2O2-positive isolates increased from 71% on TMB to 79% on TMB-Plus. Formulations using Rogosa or MRS agar base in combination with peroxidase and a chromogen did not support the growth of all of the strains of Lactobacillus, and fewer H2O2-producing strains were detected on these formulations than on TMB-Plus. This new medium better supports the growth of a wider range of Lactobacillus strains isolated from the vagina and enhances the color production of H2O2-producing strains.
While production of lactic acid has been widely considered to be the factor which allows Lactobacillus spp. to dominate the vaginal ecosystem, more-recent data suggest that H2O2 production by lactobacilli may be more relevant than lactic acid production. In a recent study of 101 women monitored over 8 months, 96% of women who maintained colonization by the same species of Lactobacillus were colonized by H2O2-producing strains of Lactobacillus crispatus or L. jensenii. In contrast, only 1 of 21 women who lacked H2O2-producing strains remained colonized by lactobacilli over the same period (19). Bacterial vaginosis, a condition characterized by reduced numbers of vaginal lactobacilli, has been associated with an increased risk of acquisition of human immunodeficiency virus (16), while the absence of vaginal Lactobacillus spp. is associated with increased acquisition of human immunodeficiency virus and gonorrhea (9). Women vaginally colonized by H2O2-producing strains of lactobacilli are only half as likely to acquire bacterial vaginosis as women colonized by lactobacilli that do not produce H2O2 (7).
Several methods have been used to detect production of H2O2 by lactic acid bacteria or macrophages. The assays are generally based on the oxidation of a chromogen that works as a hydrogen donor by a horseradish peroxidase-H2O2 complex, which results in a color or fluorescence change depending on the chromogen used. The methods used to quantitate H2O2 production require growth of the organism or cells in a broth culture, testing by addition of a chromogen and horseradish peroxidase, and measurement of the absorbance on a spectrophotometer or of the fluorescence on a spectrofluorometer (2, 5, 11, 13, 20, 21). The qualitative assays also use a chromogen and peroxidase but incorporate them into an agar base (3, 5, 10). For the qualitative assays, the organism is inoculated onto the agar, incubated anaerobically, and then exposed to air for a short time. The formation of color around the colonies indicates H2O2 production.
The most frequently cited qualitative assay relies on a brucella agar base plate supplemented with horseradish peroxidase and 3,3′,5,5′-tetramethylbenzidine dihydrochloride (TMB) (see Table 2) (3). The isolate to be tested is subcultured onto this medium and incubated at 37°C for 48 to 72 h in an anaerobic environment. After incubation, the plates are exposed to air for 30 min. The peroxidase oxidizes the TMB in the presence of H2O2, and a blue pigment appears on the colonies. Unfortunately, the medium does not uniformly support the growth of all isolates of Lactobacillus spp., and the color production can be weak or variable upon repeat testing. The goal of this study was to develop an improved formulation of the agar medium for detecting H2O2 production by lactobacilli.
TABLE 2.
Comparison of the new formulation (TMB-Plus) to the published formulation (TMB)
| Component | Amt in:
|
|
|---|---|---|
| TMB | TMB-Plus | |
| Brucella agar | 43 g | 43 g |
| Distilled water | 1 liter | 1 liter |
| TMB | 250 mg | 250 mg |
| Starch, soluble | 10 g | 20 g |
| Hemin solution | 10 ml | 10 ml |
| Vitamin K solution | 0.2 ml | 0.2 ml |
| Magnesium sulfate, anhydrous | 0 g | 0.57 g |
| Manganese sulfate monohydrate | 0 g | 0.12 g |
| Peroxidase solution (1 mg/ml) | 10 ml | 10 ml |
| Horse serum | 0 ml | 50 ml |
MATERIALS AND METHODS
Bacterial isolates used for testing.
The Lactobacillus isolates which were used for the comparison of media consisted of 143 well-characterized vaginal isolates identified to the species level by using whole chromosomal DNA probes based on 20 ATCC type strains, as previously described (1). They include L. crispatus (n = 57), L. jensenii (n = 42), L. gasseri (n = 10), and Lactobacillus strain 1086 (n = 34). The Lactobacillus strain 1086 group, which has not been given a species name, is found more frequently among women with bacterial vaginosis. The ATCC strains that were tested were L. plantarum ATCC 14917, L. casei subsp. casei ATCC 393, L. crispatus ATCC 33197 and 202225, L. jensenii ATCC 25256, L. gasseri ATCC 9857, and L. acidophilus ATCC 521. In addition, 109 recent vaginal isolates that had been identified only to the genus level based on gas chromatographic analysis of fermentation products were also evaluated. The Lactobacillus isolates were grown on Columbia agar with 5% sheep blood (Prepared Media Laboratory, Tulatin, Oreg.) for 48 h under 6% CO2 at 37°C.
Agar bases tested.
The different agar bases that were tested, brucella, brain heart infusion, tryptic soy, Columbia, and Wilkens-Chalgren (Becton Dickinson, Sparks, Md.), were selected because of their ability to support a wide range of organisms. Brucella, brain heart infusion, tryptic soy, and Columbia agar bases are similar in that they all contain peptones and one sugar, but they differ in the type and concentration of peptones and sugars. Wilkens-Chalgren agar base has, in addition, l-arginine, hemin, and vitamin K. Rogosa (Becton Dickinson) and MRS (Oxoid Ltd., Basingstoke, Hampshire, England) agar bases were tested because they are specifically designed for the growth of Lactobacillus spp., and other researchers have used these bases for testing for hydrogen peroxide production (5, 10). The concentration of each of the bases was determined by the manufacturer's specification.
Nutritional supplements tested.
Magnesium sulfate and manganese sulfate (Sigma Chemical Co., St. Louis, Mo.) are both components of MRS agar, and magnesium has been shown to enhance the production of H2O2 in Streptococcus gordonii (2). Sodium acetate, ammonium citrate, monopotassium phosphate, sorbitan monooleate (Sigma Chemical), dextrose, saccharose, and soluble starch (Becton Dickinson) are all components of Rogosa agar, which is known to enhance the growth of Lactobacillus spp. Horse serum (Gibco/Life Technologies, Rockville, Md.) was tested because blood products are useful for the support of fastidious organisms. Vitamin K and hemin (Sigma Chemical) were also included because their presence is beneficial for the growth of many organisms.
Reagents.
The two chromogens TMB and 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid), as well as horseradish peroxidase, were obtained from Sigma Chemical Co.
Testing method.
Each new formulation of agar medium was compared to the original TMB formulation by subculturing 1 to 2 Lactobacillus colonies to both media from the same plate on the same day. Both plates were incubated for 48 h in an anaerobic chamber at 37°C (Sheldon Manufacturing, Cornelius, Oreg.). After incubation, the plates were removed from the chamber and exposed to ambient air for 30 min to allow for color production. Growth and color production were compared by direct side-by-side observation. Growth on the new formulation was scored as less than, equivalent to, or greater than that observed on the original TMB agar formulation.
RESULTS
Initial testing of the agar bases showed that brain heart infusion, Columbia, and trypic soy agar bases did not support growth as well as Wilkens-Chalgrens or brucella agar base. Wilkens-Chalgren agar was equivalent to brucella agar in terms of growth and color production. The Rogosa and MRS agar bases produced larger colonies of L. crispatus and L. jensenii than brucella agar base, but none of the Lactobacillus strain 1086 isolates grew on either of these media. Of the 50 isolates that grew on Rogosa agar base, 16% failed to produce a blue color, compared to only 4% of isolates grown on brucella agar base. MRS agar base also yielded fewer H2O2-positive results than brucella agar base (4 of 15 [27%] negative versus 0%). Further, both Rogosa and MRS agar bases yielded colonies with a slight blue color, making the interpretation of blue color more subjective.
After assessment of the agar bases, Wilkens-Chalgren and brucella agars were chosen as the optimal bases and were used to test supplemental nutrients at the concentrations listed in Table 1. Addition of dextrose to a final concentration of 2% (brucella agar base contains 1%) enhanced growth slightly overall but did not increase color intensity. Addition of l-arabinose and saccharose had the same modest effect. Substitution of 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) for TMB as the chromogen did not result in superior color intensity. Of the 20 isolates tested simultaneously with both chromogens, 2 had equal color production on both media, 13 produced a darker color on TMB agar, and 5 were positive on TMB agar and negative when 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) was used. This suggested that TMB was a better chromogen for this application than 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid). Addition of sorbitan, which is found in Rogosa agar base, enhanced the growth of L. crispatus and L. jensenii but failed to support the growth of strain 1086 isolates. Thus, sorbitan was not selected as an additive for the modified agar formulation. Addition of ammonium citrate inhibited the growth of some of the H2O2-negative strains, whereas monopotassium phosphate, sodium acetate, ferrous sulfate, and XV factor, at the concentrations listed in Table 1, had no beneficial or negative effect on the growth of any of the isolates or on their H2O2 production (Table 1). Addition of manganese sulfate, magnesium sulfate, and horse serum, and increasing the concentration of starch, all improved growth and color intensity on both Wilkens-Chalgren and brucella agar bases. Because there was no significant difference between the brucella and Wilkens-Chalgren agar bases, we chose to use brucella agar for the remainder of the experiments based on its equivalent performance at a lower cost. Table 2 lists the additions that have been made to the revised formulation (TMB-Plus) compared to the published formulation (TMB).
TABLE 1.
Nutritional supplements tested
| Supplement addeda | Final concn (%) | Growth and color production |
|---|---|---|
| Dextrose* | 2.0 | Slight enhancement of growth |
| l-Arabinose* | 0.5 | Slight enhancement of growth |
| Saccharose* | 0.5 | Slight enhancement of growth |
| 2,2′-Azinobis(3-ethylbenzthiazoline-6-sulfonic acid) | 0.4 | Less color |
| XV factor | 0.5 | No benefit |
| Sorbitan* | 0.1 | Inhibition of H2O2-negative strains |
| Horse serum | 5.0 | Enhanced growth |
| Starch | 2.0 | Enhanced growth |
| Monopotassium phosphate* | 0.6 | No benefit |
| Ammonium citrate* | 0.2 | Inhibition of H2O2-negative strains |
| Sodium acetate* | 1.5 | No benefit |
| Manganese sulfate* | 0.057 | Enhanced color production and growth |
| Magnesium sulfate* | 0.012 | Enhanced color production and growth |
| Ferrous sulfate* | 0.003 | No benefit |
Supplements marked with asterisks are components of Rogosa agar.
In order to evaluate the performance of TMB-Plus in relation to that of TMB agar, 261 recent vaginal isolates and ATCC strains were inoculated onto both media. The growth and color production of 98% of the isolates were equivalent or better on TMB-Plus than on TMB agar (Table 3). Overall, 70% of the isolates tested grew better, and 47% had enhanced color production, on TMB-Plus versus TMB agar. With regard to growth enhancement, L. gasseri showed the most benefit, with 100% of the isolates growing better on TMB-Plus, followed by strain 1086 (88%), L. jensenii (72%), and L. crispatus (69%). Of all the isolates identified, only one, an L. crispatus isolate, grew better on TMB than on TMB-Plus, but that isolate had strong and comparable blue pigment production on both media.
TABLE 3.
Comparison of TMB-Plus to TMB for growth and color intensity of vaginal Lactobacillus isolates
| Organism(s) or strain (n) | No. (%) of isolates exhibiting the following characteristics on TMB-Plus vs TMB agar formulation:
|
|||||
|---|---|---|---|---|---|---|
| Growth
|
Color intensity
|
|||||
| Less | Equivalent | Greater | Less | Equivalenta | Greater | |
| All isolates (261) | 4 (2) | 74 (28) | 183 (70) | 2 (1) | 136 (52) | 122 (47) |
| Lactobacillusspeciesb (109) | 3 (3) | 41 (37) | 65 (60) | 2 (2) | 52 (48) | 55 (50) |
| L. crispatus (59) | 1 (2) | 17 (29) | 41 (69) | 0 | 27 (46) | 32 (54) |
| L. jensenii (43) | 0 | 12 (28) | 31 (72) | 0 | 18 (42) | 25 (58) |
| L. gasseri (11) | 0 | 0 | 11 (100) | 0 | 3 (27) | 8 (73) |
| Strain 1086 (34) | 0 | 4 (12) | 30 (88) | 0 | 32 (94) | 2 (6) |
| L. acidophilus (ATCC 521) | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) |
| L. plantarum (ATCC 14917) | 0 | 0 | 1 (100) | 0 | 1 (100) | 0 |
| L. casei subsp. casei (ATCC 393) | 0 | 0 | 1 (100) | 0 | 1 (100) | 0 |
| L. vaginalis (ATCC 49540) | 0 | 0 | 1 (100) | 0 | 1 (100) | 0 |
| L. fermentum (ATCC 23271) | 0 | 0 | 1 (100) | 0 | 1 (100) | 0 |
Blue color equally intense on both media or no color on both.
Organisms identified to the genus level only.
Detection of H2O2 production was also compared for the same 261 isolates on TMB versus TMB-Plus. The percentages of H2O2-positive isolates were 70% on TMB agar and 77% on TMB-Plus agar (Table 4). When isolates were categorized by group, H2O2 production was detected for 78 recent vaginal isolates (72%) on TMB and 89 (82%) on TMB-Plus. Of the isolates identified to the species level by DNA homology, L. gasseri showed the greatest increase in H2O2 production detection on TMB-Plus (from 64 to 100%), followed by L. jensenii (from 86 to 93%). While the proportion of H2O2-positive isolates did not increase greatly with the new medium formulation, the enhanced growth and color intensity on the TMB-Plus agar aided interpretation of the reaction (Fig. 1). Because of the enhanced growth on TMB-Plus, repeat testing was needed for only 1% of new clinical isolates, whereas 12% of isolates had to be retested when TMB agar was used.
TABLE 4.
Comparison of TMB-Plus to TMB for the detection of H2O2 produced by vaginal Lactobacillus spp.
| Organism(s) or strain (n) | No. (%) of strains for which H2O2 production was detected on:
|
|
|---|---|---|
| TMB | TMB-Plus | |
| All isolates (261) | 183 (70) | 202 (77) |
| Lactobacillus speciesa (109) | 78 (72) | 89 (82) |
| L. crispatus (59) | 58 (98) | 59 (100) |
| L. jensenii (43) | 37 (86) | 40 (93) |
| L. gasseri (11) | 7 (64) | 11 (100) |
| Strain 1086 (34) | 3 (9) | 3 (9) |
| L. acidophilus (ATCC 521) | 1 (100) | 1 (100) |
| L. plantarum (ATCC 14917) | 0 | 0 |
| L. casei subsp. casei (ATCC 393) | 0 | 0 |
| L. vaginalis (ATCC 49540) | 0 | 0 |
| L. fermentum (ATCC 23271) | 0 | 0 |
Organisms identified to the genus level only.
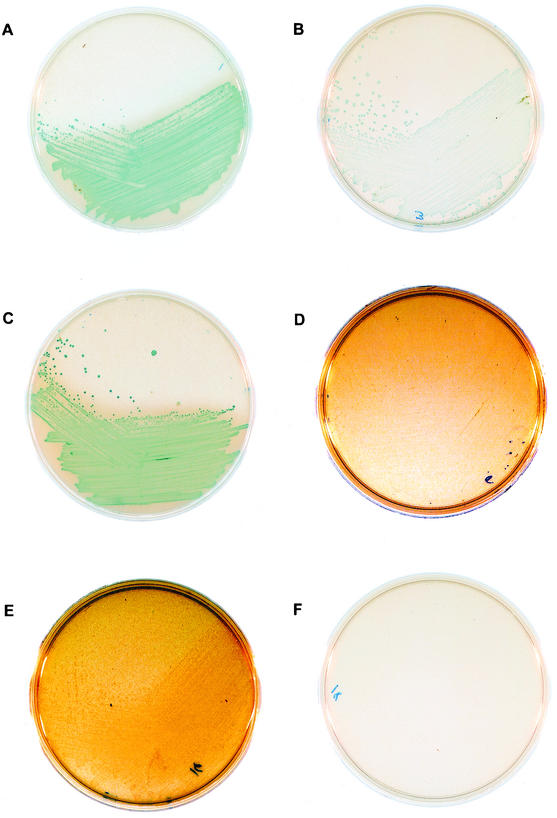
FIG. 1.
Examples of growth and color production of Lactobacillus on TMB (B, D, F) and TMB-Plus (A, C, E). L.crispatus, ATCC 202225 on TMB-Plus (Fig. 1A) vs. TMB (Fig. 1B). Note that both density of growth of the L.crispatus and intensity of blue pigment are enhanced on TMB-Plus. L. jensenii, ATCC 25258 is shown on TMB-Plus (Fig. 1C) and TMB (Fig. 1D). Note that the L. jensenii would be categorized as H2O2 negative on the TMB agar, whereas it was positive for H2O2 production on TMB-Plus. Lactobacillus 1086 grew well on TMB-Plus (Fig. 1E), whereas the TMB agar did not support the growth of this strain (Fig. 1F).
DISCUSSION
In this study we have shown that addition of horse serum, magnesium sulfate, manganese sulfate, and starch to the published TMB agar formulation increased the sensitivity for detection of H2O2 production. The new medium yielded larger colonies of all the species of Lactobacillus isolated from the vagina, thus eliminating repeat testing of isolates that either did not grow or grew very poorly. Further, there was enhanced color production resulting from the oxidation of TMB. Rogosa and MRS agars both contain magnesium, but when these two bases were compared to brucella agar with added magnesium, 7 of 21 isolates had greater color production on the brucella agar with added magnesium. Barnard and Stinson showed an increase in H2O2 production by S. gordonii when magnesium was added to the medium and further reported that the effect could be reversed by addition of potassium citrate, a chelator of magnesium (2). Rogosa and MRS agar bases both contain ammonium citrate, suggesting that one possible reason for the decreased detection of H2O2 on Rogosa and MRS agars was the chelation of magnesium by ammonium citrate in the medium.
In our study, the majority of the isolates were not affected by manganese, with the exception of L. casei and L. plantarum, which had enhanced H2O2 production in the presence of manganese. Both L. casei and L. plantarum are heterofermentative, and this group of lactobacilli has been reported to be stimulated by manganese (14). L. crispatus and L. jensenii, the most common vaginal species, are homofermentative, and their H2O2 production was not enhanced by addition of manganese. Because of the variability in nutritional requirements among the species of lactic acid bacteria, horse serum was also added as a nutritional supplement. Horse serum contains numerous trace elements including iron, zinc, magnesium, manganese, copper, chromium, cobalt, and selenium (17).
In the past, identification of Lactobacillus isolates to the species level has been unreliable because biochemical tests do not accurately reflect genotypic groups (4, 8). L. acidophilus has been divided into six DNA homology groups that include L. crispatus and L. gasseri. Giorgi et al. first showed by using DNA homology tests that L. crispatus, L. jensenii, and L. gasseri are the predominant species in the vagina (6). Antonio et al. and Vallor et al. have confirmed these results by DNA homology testing. They concluded that L. crispatus and L. jensenii, and not L. acidophilus, are the species most frequently isolated from the vagina (1, 19). In a study of 91 Japanese women with normal flora, 48 of 97 (53%) isolates were identified as L. crispatus by DNA-DNA homology. L. gasseri accounted for 21% of the isolates, followed by L. vaginalis (9%) and L. fermentans (6%) (15).
The results of the present study suggest that MRS and Rogosa agar bases fail to support the growth of more nutritionally fastidious strains of Lactobacillus. When Rogosa agar was originally developed in 1951 (12), it was shown to be superior to tomato juice agar in its ability to inhibit other organisms that are part of the normal oral, fecal, or vaginal flora but to still support the growth of Lactobacillus spp. In 1982 human bilayer Tween (HBT) agar was developed for the isolation of Gardnerella (Haemophilus) vaginalis (18). This selective medium, in addition to growing G. vaginalis, also supports the growth of the more fastidious strains of Lactobacillus, such as strain 1086, and can differentiate Lactobacillus spp. from G. vaginalis by the lack of hemolysis. For this study, Rogosa agar and HBT agar were used for initial isolation of Lactobacillus spp., and all of the Lactobacillus strain 1086 isolates were isolated from HBT agar only. Although Rogosa and MRS agars are excellent media for isolation of the majority of lactobacilli, they do not support the growth of all strains, and they appear to inhibit the detection of H2O2 when used in combination with a chromogen and horseradish peroxidase. For testing of pure strains it is not necessary to use a medium that has inhibitory components. With the additions that have been made to the TMB agar formulation (TMB-Plus), a broader range of lactobacilli can be tested for H2O2 production.
The importance of normal flora as a barrier to infection is increasingly recognized, and the role of H2O2 production by lactobacilli in maintaining a healthy vagina has been well documented. Further studies seeking to evaluate the role of lactobacilli in reproductive health should include the use of media that support the growth of a broad range of lactobacilli and should attempt detection of H2O2 production only when adequate growth is apparent on the agar plates.
Acknowledgments
This work was supported by grant 1UO1AI47785 from the U.S. Public Health Service.
We thank Colleen Bess for technical assistance.
REFERENCES
- 1.Antonio, M. A. D., S. E. Hawes, and S. L. Hillier. 1999. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J. Infect. Dis. 180:1950-1956. [DOI] [PubMed] [Google Scholar]
- 2.Barnard, J., and M. W. Stinson. 1999. Influence of environmental conditions on hydrogen peroxide formation by Streptococcus gordonii. Infect. Immun. 67:6558-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eschenbach, D. A., P. R. Davick, B. L. Williams, S. J. Klebanoff, K. Young-Smith, C. M. Critchlow, and K. K. Holmes. 1989. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J. Clin. Microbiol. 27:251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felten, A., C. Barreau, C. Bizet, P. H. Lagrange, and A. Philippon. 1999. Lactobacillus species identification, H2O2 production, and antibiotic resistance and correlation with human clinical status. J. Clin. Microbiol. 37:729-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontaine, E. A., and D. Taylor-Robinson. 1990. Comparison of quantitative and qualitative methods of detecting hydrogen peroxide produced by human vaginal strains of lactobacilli. J. Appl. Bacteriol. 69:326-331. [DOI] [PubMed] [Google Scholar]
- 6.Giorgi, A., S. Torriani, F. Dellaglio, G. Bo, E. Stola, and L. Bernuzzi. 1987. Identification of vaginal lactobacilli from asymptomatic women. Microbiologica 10:377-384. [PubMed] [Google Scholar]
- 7.Hawes, S. E., S. L. Hillier, J. Benedetti, C. E. Stevens, L. A. Koutsky, P. Wolner-Hanssen, and K. K. Holmes. 1996. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J. Infect. Dis. 174:1058-1063. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, J. L., C. F. Phelps, C. S. Cummins, J. London, and R. Gasser. 1980. Taxonomy of the Lactobacillus acidophilus group. Int. J. Syst. Bacteriol. 30:53-68. [Google Scholar]
- 9.Martin, H. L., B. A. Richardson, P. M. Nyange, L. Lavreys, S. L. Hillier, B. Chohan, K. Mandaliya, J. O. Ndinya-Achola, J. Bwayo, and J. Kreiss. 1999. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J. Infect. Dis. 180:1863-1868. [DOI] [PubMed] [Google Scholar]
- 10.McGroarty, J. A., L. Tomeczek, D. G. Pond, G. Reid, and A. W. Bruce. 1992. Hydrogen peroxide production by Lactobacillus species: correlation with susceptibility to the spermicidal compound nonoxynol-9. J. Infect. Dis. 165:1142-1144. [DOI] [PubMed] [Google Scholar]
- 11.Pick, E., and Y. Keisari. 1980. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 38:161-170. [DOI] [PubMed] [Google Scholar]
- 12.Rogosa, M., J. A. Mitchell, and R. F. Wiseman. 1951. A selective medium for the isolation and enumeration of oral and fecal lactobacilli. J. Bacteriol. 62:132-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Root, R. K., J. Metcalf, N. Oshino, and B. Chance. 1975. H2O2 release from human granulocytes during phagocytosis. 1. Documentation, quantitation and some regulating factors. J. Clin. Investig. 55:945-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharpe, M. E. 1960. Selective media for the isolation and enumeration of lactobacilli. Lab. Practice 9:223-227. [Google Scholar]
- 15.Song, Y.-L., N. Kato, Y. Matsumiya, C.-X. Liu, H. Kato, and K. Watanabe. 1999. Identification of and hydrogen peroxide production by fecal and vaginal lactobacilli isolated from Japanese women and newborn infants. J. Clin. Microbiol. 37:3062-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taha, T. E., R. H. Gray, N. I. Kumwenda, D. R. Hoover, L. A. R. Mitmavalye, G. N. Liomba, J. D. Chiphangwi, G. A. Dallabetta, and P. G. Miotti. 1999. HIV infection and disturbances of vaginal flora during pregnancy. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20:52-59. [DOI] [PubMed] [Google Scholar]
- 17.Tietz, N. W. (ed.). 1987. Fundamentals of clinical chemistry. W. B. Saunders Company, Philadelphia, Pa.
- 18.Totten, P. A., R. Amsel, J. Hale, P. Piot, and K. K. Holmes. 1982. Selective differential human blood bilayer media for isolation of Gardnerella (Haemophilus) vaginalis. J. Clin. Microbiol. 15:141-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallor, A. C., M. A. D. Antonio, S. E. Hawes, and S. L. Hillier. 2001. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J. Infect. Dis. 184:1431-1436. [DOI] [PubMed] [Google Scholar]
- 20.Yap, P. S., and S. E. Gilliland. 2000. Comparison of newly isolated strains of Lactobacillus delbrueckii subsp. lactis for hydrogen peroxide production at 5°C. J. Dairy Sci. 83:628-632. [DOI] [PubMed] [Google Scholar]
- 21.Zhou, M., D. Zhenjun, N. Panchuk-Voloshina, and R. P. Haugland. 1997. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 253:162-168. [DOI] [PubMed] [Google Scholar]