Abstract
This report describes the first isolation of the ameba Balamuthia mandrillaris from an environmental soil sample associated with a fatal case of amebic encephalitis in a northern California child. Isolation of the ameba into culture from autopsied brain tissue confirmed the presence of Balamuthia. In trying to locate a possible source of infection, soil and water samples from the child's home and play areas were examined for the presence of Balamuthia. The environmental samples (plated onto nonnutrient agar with Escherichia coli as a food source) contained, in addition to the ameba, a variety of soil organisms, including other amebas, ciliates, fungi, and nematodes, as contaminants. Presumptive Balamuthia amebas were recognized only after cultures had been kept for several weeks, after they had burrowed into the agar. These were transferred through a succession of nonnutrient agar plates to eliminate fungal and other contaminants. In subsequent transfers, axenic Naegleria amebas and, later, tissue cultures (monkey kidney cells) served as the food source. Finally, the amebas were transferred to cell-free axenic medium. In vitro, the Balamuthia isolate is a slow-growing organism with a generation time of ∼30 h and produces populations of ∼2 × 105 amebas per ml. It was confirmed as Balamuthia by indirect immunofluorescence staining with rabbit anti-Balamuthia serum and human anti-Balamuthia antibody-containing serum from the amebic encephalitis patient. The environmental isolate is similar in its antimicrobial sensitivities and identical in its 16S ribosomal DNA sequences to the Balamuthia isolate from the deceased patient.
Over the last 3 to 4 decades, free-living amebas of the genera Acanthamoeba and Naegleria have come to be recognized as etiologic agents of amebic encephalitis in humans and other animals (8). These two amebas are found widely distributed in soil and water and can be readily recovered from both habitats (9). About a dozen years ago, a previously unknown ameba was isolated from the brain of a pregnant mandrill baboon that died at the San Diego Wildlife Park (15, 16). This organism, subsequently named Balamuthia mandrillaris, has since been recognized as the etiologic agent of approximately 90 cases of human amebic encephalitis globally, with about half of the cases diagnosed in the United States. Although the ameba was presumed to be free living, it has never been described or recovered from environmental samples. Attempts had been made to isolate the organism from soil samples related to previous encephalitis cases, but they were unsuccessful (10). Thus, the presumed free-living status of the ameba has been open to question.
We recently had an opportunity to screen environmental samples for Balamuthia following the death of a 3-year-old northern California resident from amebic encephalitis caused by Balamuthia (2). The child developed symptoms of encephalitis, and over a period of ∼30 days, her condition continued to deteriorate. Several days before she died, a serum sample was tested for Balamuthia antibodies by indirect immunofluorescence. Based on a reciprocal titer of 512 and a clinical picture consistent with amebic encephalitis, a preliminary diagnosis of Balamuthia encephalitis was made. Before any therapy could be initiated, however, the child was declared brain dead. Upon autopsy, unstained sections of necrotic brain tissue were immunostained with rabbit anti-Balamuthia antibody, and the presence of amebas was confirmed (2). Macerated samples of brain tissue were also introduced into tissue culture flasks containing monkey kidney cells, and after ∼12 days, amebas were seen actively feeding on the tissue culture cells. The ameba was isolated and established in culture, both on tissue culture cells and in an enriched cell-free culture medium, and its identity as Balamuthia was further confirmed by immunofluorescence with rabbit anti-Balamuthia serum.
It was thought worthwhile to collect samples for Balamuthia in the child's home and the outdoor play areas that she frequented. As a result of this survey, an ameba was isolated from the soil in a potted plant in the child's home. This ameba showed all the characteristics of Balamuthia and may have been the source of the child's infection.
(Portions of this work were presented at the May 2002 meeting of the Society of Protozoologists, Salt Lake City, Utah.)
MATERIALS AND METHODS
Environmental sampling.
Approximately 4 weeks after the child's death, 18 soil and water samples were collected from areas in the child's home and in outdoor locations where she had played. The samples from the home included water, filter sediment, and surface swabs from a fresh water aquarium; soil samples from several potted plants present in the house; swab samples from bathroom sink and tub drains; and droppings in a bird cage (cockatiel and parakeet). Outdoor samples included soil from an area in the immediate vicinity of the child's home, as well as water from a large duck pond in an adjacent community where the child spent time playing in the runoff from the pond. Sampling was done with sterile cotton swabs (aquarium and drains), taking soil samples (potted plants), or collecting water and/or soil (duck pond and outdoor play areas) in sterile glass scintillation vials. No specific amounts of soil or water were collected, nor were samples concentrated prior to processing.
Processing samples.
The basic procedure for surveying samples was to plate material onto nonnutrient agar prepared in Hanks' balanced salt solution in petri plates (100 mm) and coated with a suspension of Escherichia coli as previously described (11, 14). Soil samples were hydrated with sterile distilled water, and aliquots of the suspension were added to the agar surface. Water samples were applied directly to the agar surface, and cotton swabs were swiped across the agar surface to deposit material. Plates were incubated at room temperature (about 25°C) for the short term and at about 17°C for prolonged storage. The edges of the plates were sealed with Parafilm to retard drying of the agar.
Ameba isolation.
Surface scrapings or agar blocks with suspected Balamuthia amebas were removed from the primary plates and transferred to second or third, etc., nonnutrient plates spread with E. coli until the transferred material was free of fungal contamination and endogenous bacteria. After having moved away from contaminating organisms, suspect balamuthias were transferred in small blocks of agar to nonnutrient agar plates with axenic Naegleria spp. grown as previously described (11) on the surface as a food source. Ultimately, presumptive balamuthias were transferred into tissue culture flasks containing monolayers of E6 monkey kidney cells (MKC) growing in RPMI 1640 medium with 10% fetal calf serum and penicillin-streptomycin (200 U/ml) in tissue culture flasks (25 cm2). Cultures were incubated at 30 or 37°C to allow the amebas to feed on the tissue culture cells. In order to establish the amebas in culture without MKC, cell-free Balamuthia medium, BM-3 (12), was added to flasks containing large numbers of amebas that had cleared the MKC monolayer, after the RPMI 1640 medium had been aspirated. Flasks were incubated at 30 or 37°C.
Clonal isolations.
Amebas (∼103 amebas per ml) that had fed upon and cleared cultures of MKC were distributed in 1-μl aliquots into the center of each well of six 24-well tissue culture plates. A suspension of MKC (∼5,000 cells per ml) in 1 ml of tissue culture medium was added only to those wells confirmed by microscopic observation (×63 objective) to contain one trophozoite or one cyst, followed by incubation at 37°C in a 5% CO2-95% air atmosphere. Some 7 to 10 days later, a single focus representing the feeding of a single trophic ameba or single excysted trophic ameba was found in 50% of those isolated. Fluids from four to six wells containing numerous clonal populations of amebas were transferred to separate tissue culture flasks (25 cm2) containing MKC for culture expansion.
Immunofluorescence.
Cultures of the isolate grown in BM-3 medium were harvested for indirect immunofluorescence antibody (IIF) staining as previously described (15). Fluorescein-5-isothiocyanate-labeled rabbit anti-Balamuthia serum or goat anti-human serum (Cappel Research, ICN Biomedicals, Inc., Costa Mesa, Calif.) was used for IIF staining, and the results were evaluated under a fluorescence microscope according to a rating of +1 to +4 (15). For IIF, serum from the northern California patient who died from the Balamuthia infection and rabbit anti-Balamuthia serum were used.
Electron microscopy.
Amebas from cultures were harvested and fixed in Karnovsky's fixative, followed by dehydration through alcohols, embedding, sectioning, and viewing in a Philips CM 12 electron microscope.
Antimicrobial sensitivity testing.
The sensitivity of the environmental and the patient isolates to a variety of antimicrobial agents was tested by using 12-well tissue culture plates containing MKC, as previously described (13). The following antimicrobials were tested at final concentrations of 1, 5, and 10 μg/ml: amphotericin B (Fungizone; Sigma), azithromycin (Zithromax; Pfizer), fluconazole (Diflucan; Pfizer), flucytosine (Sigma), pentamidine isethionate (May & Baker, Ltd.), and sulfadiazine (Sigma). Control wells contained MKC without amebas and MKC plus amebas, but without an antimicrobial agent added. Drugs were not toxic for MKC at the concentrations used (data not included). After ∼40 h of incubation in a 5% CO2-95% air atmosphere, medium was aspirated, and the cell layer was fixed and stained to evaluate the degree of destruction of the monolayer (13). The degree of destruction of the cell layer was a function of the ability of the drug to inhibit ameba growth. The results were compared to those for a confirmed B. mandrillaris isolate obtained from humans (ATCC 50606), which has been in culture since ∼1990 (12, 15).
DNA extraction, purification, and amplification.
Cultures of the environmental isolate and the isolate from the dead child were grown in cell-free medium at 37°C until a large population of amebas was produced. Flasks were chilled and then agitated to release amebas from the growth surface. DNA was extracted from the pellets of amebas by adding lysis buffer (6), vortexing the suspension, and allowing it to remain at room temperature for 10 m. Following addition of isopropanol to precipitate nucleic acid, the tubes were again vortexed and then centrifuged for 10 min at 10,000 × g. The supernatant was aspirated, and the pellet was washed with 0.75 ml of 70% ethyl alcohol, vortexed, and centrifuged. Alcohol was removed, and the tubes were placed in a heating block at 65°C for 10 min to drive off residual alcohol, after which tubes were cooled to room temperature and stored frozen.
Following DNA extraction, PCR amplification was done with the primer set 5′Balspec16S and 3′Balspec16S (4), which amplifies an ∼1,075-bp portion of the mitochondrial 16S ribosomal DNA (rDNA) gene from B. mandrillaris. The PCR product (40 μl) was run on 1% agarose gel and purified with a Prep-A-Gene purification kit (Bio-Rad Laboratories, Hercules, Calif.). The concentration of gel-purified DNA was determined with the Low Mass DNA ladder (Invitrogen, Carlsbad, Calif.). The final elution volume was 50 μl.
DNA sequencing.
PCR products amplified with 5′Balspec16S and 3′Balspec16S were sequenced with the internal primer mt900 (5′-AGTATGTGGTTTAATTTG-3′), which determines the primary sequence of the phylogenetically informative 5′ region of this amplimer (7). Fluorescent sequencing was performed on an ABI 310 automated sequencing system (Applied Biosystems, Foster City, Calif.) using the ABI BigDye cycle sequencing kit (version 2.0) according to the manufacturer's protocols.
Phylogenetic analysis.
Mitochondrial 16S rDNA sequences obtained from fluorescent cycle sequencing were aligned to the mitochondrial rDNA sequence of B. mandrillaris and other mitochondrial rDNA sequences with the sequence alignment program ESEE (5).
Nucleotide sequence accession number.
The RP-5 and SAm mitochondrial 16S rDNA sequences determined in these analyses have been deposited in GenBank under accession no. AY 263351 (human isolate, SAm) and AY 263352 (environmental isolate, RP-5).
RESULTS
Primary cultures.
All samples, except the bird droppings, contained an abundant array of amebas, ciliated protozoa, and fungi. Some contained nematodes. The amebas present were part of the typical fauna that one might expect to recover from soil or water samples (9). These included Acanthamoeba spp. (probably the most abundant ameba present in soil) (9), hartmannellids, vahlkampfiids, and other genera of amebas. No attempt was made to identify these amebas at the species level, nor were attempts made to isolate any of these organisms. Ciliates proliferated on the agar surface of some samples when fluid was present. Nematode worms actively bored into the agar substrate of some platings. Fungi grew abundantly in most samples, often obscuring protozoa on the agar surface. It is noteworthy that replicate plates from the same soil suspension varied in the protozoal fauna appearing on the surface.
After several weeks, a plate containing soil from a flowerpot (one plate of six), showed amebas of moderate size (∼50 μm), looking not unlike small leptomyxid amebas. These amebas were seen in the agar as irregular profiles with projecting pseudopods. It is not clear whether they were able to burrow into the agar or whether they were using channels made by fungi or worms. Balamuthia amebas do not feed on bacteria (15) and appeared to be feeding on other amebas present on the agar.
Secondary and subsequent cultures.
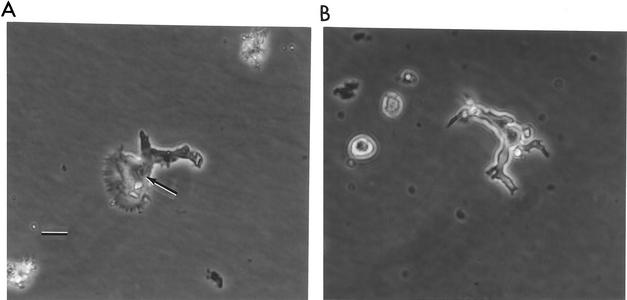
Bits of agar containing the presumed Balamuthia ameba (and clear of fungal growth) were transferred to plates of nonnutrient agar. Other amebas (Naegleria gruberi or Naegleria fowleri for plates placed at 37°C) or suspensions of MKC were added to the agar surface as a food source for the presumptive balamuthias (Fig. 1A). The balamuthias fed upon the Naegleria and MKC, and in so doing, they migrated across the agar, leaving contaminating bacteria behind.
FIG. 1.
(A) Phase-contrast micrograph of the RP-5 ameba, isolated from the environment, on an agar surface. The arrow points to the nucleus of the organism. Bar, 10 μm. Original magnification, ×600. (B) Phase-contrast micrograph of the trophic SAm ameba, from the deceased child, on an agar surface. In size and general appearance, SAm and RP-5, the environmental isolate, are similar. Original magnification, ×600.
After elimination of contaminating bacteria and fungi, amebas were transferred into monolayer cultures of MKC, where they cleared the feeder layer and produced large numbers of amebas. These amebas were transferred to Balamuthia medium (12) at 37°C, containing penicillin-streptomycin (200 U/ml) to inhibit contaminants. Initial growth was slow, but on subsequent transfers, amebas grew more rapidly, producing larger and larger populations of cells. Their appearance was similar to that of the Balamuthia strain isolated from the patient (Fig. 1B).
Cultures of the environmental isolate (RP-5) produced populations of ∼2 × 105 amebas per ml in BM-3 medium at 37°C, with a generation time of 30 h. At 37°C, the isolate from the patient (SAm), produced ∼5 × 105 amebas per ml, with a generation time of 29 h. Thus, with regard to growth rate, little or no difference was seen between the two isolates.
The ameba.
The trophic ameba in cell-free, axenic culture measured 15 to 60 μm, with an average size of ca. 35 μm. Morphology varied, with some amebas having extended pseudopods, and as noted previously (16), trophozoites showed a “walking,” crablike movement when seen in time-lapse videotapes taken on an inverted microscope of cells in tissue culture or in cell-free medium. Cysts had an appearance typical of other Balamuthia isolates, with a thick wall (16). In cell-free cultures, amebas tended to round up as the culture aged, but appeared not to develop into characteristic thick-walled cysts.
Immunofluorescence.
Immunofluorescence staining was done with serum from the northern California patient, who died of a Balamuthia infection, against the ameba isolated from the patient's brain (SAm) and the environmental isolate (RP-5). Both gave reciprocal titers of 512. When immunostained with rabbit anti-Balamuthia serum, RP-5 gave a reciprocal titer of 128, Acanthamoeba gave a titer of 32. When immunostained with rabbit anti-Acanthamoeba serum, RP-5 gave a reciprocal titer of 8, while the titer for Acanthamoeba was 256. Allowing for a degree of cross-reactivity, the RP-5 isolate checked out as Balamuthia and not Acanthamoeba.
Electron microscopy.
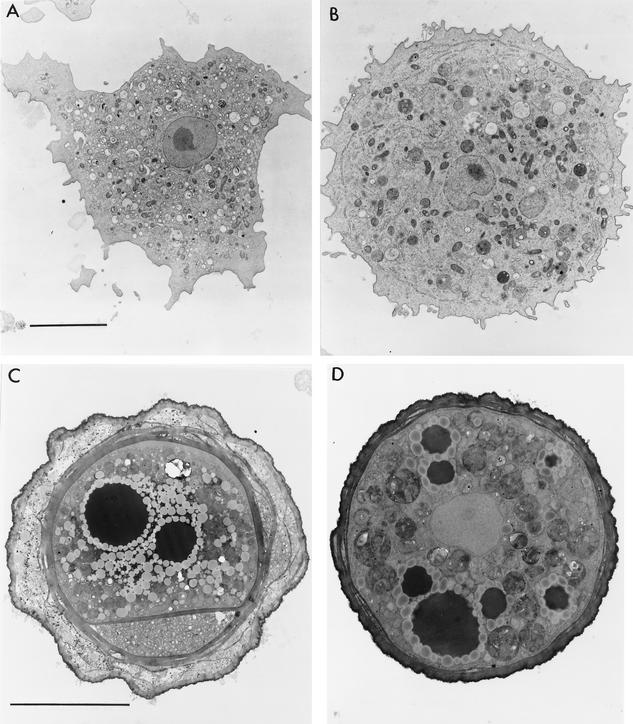
Electron micrographs of the RP-5 environmental isolate show an ameba that resembles clinical isolates of Balamuthia amebas as previously reported (15, 16). Features that are seen include an ectoplasmic rim surrounding the vesicle-filled endoplasm of the ameba, a large and irregularly shaped nucleus with a round, usually centrally located nucleolus (Fig. 2A and B), and similar types of mitochondria. The cysts of the environmental isolate are similar to those of clinical isolates (15, 16), with a trilayer wall lacking pores, and one or more electron-dense bodies surrounded by a ring of electron-translucent vesicles (Fig. 2C and D).
FIG. 2.
(A) Electron micrograph of clonal isolate of the trophic environmental ameba. A prominent nucleus with a centrally located nucleolus is seen. An ectoplasmic rim is seen about the periphery of the ameba. The endoplasm contains mitochondria and assorted vesicles, some of which contain particles. Bar, 5 μm. Original magnification, ×5,000. (B) Electron micrograph of clonal isolate of environmental Balamuthia from a single cyst. A large nucleus with an irregular outline is seen, as are numerous particles in the endoplasm. An ectoplasmic rim is seen, in which vesicles of endoplasmic reticulum can be recognized. (See the bar in panel A for scale.) Original magnification, ×5,000. (C) A mature cyst from the same clone as the trophozoite seen in panel A. A three-part wall is seen, consisting of a thick inner layer, an irregular outer layer, and an amorphous layer in between. Electron-dense vesicles surrounded by small electron-lucent vesicles are present in the cytoplasm. A portion of the inner cyst wall spans the cyst cytoplasm. Bar, 5 μm. Original magnification, ×8,000. (D) Cyst from a clonal population of amebas, as represented in panel B. A section through the nucleus, excluding the nucleolus, is seen in the center of the cyst, and numerous electron-dense and electron-lucent vesicles are seen in the cytoplasm. This is a developing cyst, as indicated by the wall, which has not fully formed into the typical three-layer structure. (Compare the wall with that in panel C, and see the bar in panel C for scale.) Original magnification, ×5,000.
Antimicrobial sensitivity testing.
Antimicrobials selected for sensitivity testing on the environmental and patient isolates of Balamuthia were chosen from several agents that had been previously tested and showed some efficacy in inhibiting the growth of the amebas (12, 13). Antimicrobials tested on RP-5 were rated as very effective (protecting the tissue culture cells from destruction by the amebas), effective, poorly effective, marginal, or ineffective (no protective value). There was little difference in response to the three drug concentrations tested (1, 5, and 10 μg/ml), with perhaps slightly less inhibition (more tissue cell destruction) at 1 μg/ml than at higher drug concentrations. With minor differences attributable to variations between testing experiments, the pattern of antimicrobial sensitivity of RP-5 and SAm was similar to that of other Balamuthia isolates from humans and animals (F. L. Schuster, unpublished observations). The comparative results of testing are summarized in Table 1, based upon a subjective estimate of ability of antimicrobials to spare the MKC monolayer from feeding and destruction by amebas. No attempt was made in these experiments to determine if the antimicrobials tested were merely amebastatic or were amebicidal.
TABLE 1.
Efficacy of selected antimicrobials tested against three isolates of B. mandrillarisa
| Drug | Result forb:
|
||
|---|---|---|---|
| Environmental isolate (RP-5) | Host isolate (SAm) | B. mandrillaris (CDC:V194)c | |
| Amphotericin B | +++ | ++ | ++ |
| Azithromycin | + | ++ | +++ |
| Fluconazole | + | + | ± |
| 5-Fluorocytosine | − | − | + |
| Pentamidine isethionate | ++++ | ++++ | +++ |
| Sulfadiazine | − | − | ± |
Estimate based on percentage of tissue culture cell sheet destroyed.
++++, very effective inhibitor of Balamuthia growth; +++ or ++, effective inhibitor; +, poorly effective inhibitor; ±, marginal inhibitor; −, ineffective inhibitor.
Human isolate (ATCC 50606) from Nevada from 1990 (15).
DNA sequencing.
Sequence analysis of the clinical and environmental isolates by using primer mt900 provided ∼230 bp of the 5′ end of the putative B. mandrillaris PCR amplimer. This region is Balamuthia specific and allows robust identification of the genus. This region is also useful for determination of the identity of Balamuthia samples, since some of the 16S mitochondrial rDNA variation between Balamuthia isolates is found in this region. In the present study, this region demonstrated that the 16S rRNA sequence from the environmental isolate was identical to that of the ameba isolated from the brain of the child. Furthermore, these two isolates were identical in this region to two other Balamuthia isolates: one from a patient who died in Nevada in 1990 and a second from a 6-year-old female patient in New York (15). This agreement among these four isolates provides strong molecular evidence that both RP-5 and SAm are Balamuthia amebas and suggests that they are the same strain.
DISCUSSION
This is the first report of isolation of a Balamuthia-like ameba from the environment. Our assumption that this isolate is Balamuthia is based upon several lines of evidence: (i) immunofluorescence staining with rabbit anti-Balamuthia serum; (ii) reactivity with human serum containing Balamuthia antibodies (in this case serum from the deceased child); (iii) a similar profile of sensitivity to six different antimicrobial agents tested, as seen with other isolates of Balamuthia obtained from human and animal sources; (iv) similar ultrastructural appearance of the trophic and cystic stages as seen by transmission electron microscopy; and (v) a 16S rDNA sequence identical to that of the ameba isolated from the brain of the child and identical in the region examined to the 16S mitochondrial rDNA of other known Balamuthia isolates. Evidence that the isolate is not an Acanthamoeba is based upon immunofluorescence staining and ultrastructural morphology, especially that of the cyst stage in the life cycle. Cloning of both the trophic and cystic stages of the isolate was performed to ensure that the cultures were indeed pure cultures and were not contaminated with other soil amebas. Amebas from such clonal populations appeared uniform when examined by both light and electron microscopy, whether derived from cysts or from trophozoites (Fig. 2), although they varied with respect to their growth rates in axenic medium.
Booton et al. (3, 4) used the PCR for analysis of seven clinical isolates of Balamuthia, using both nuclear (18S) and mitochondrial (16S) rDNA. No variation in nuclear rDNA and only slight variation (0 to 1.8%) in mitochondrial rDNA sequences were found, despite widely different geographical origins (Australia, New York, and California) and hosts (human, mandrill baboon, and horse) of isolates. They concluded that all Balamuthia isolates tested represented a single species and reaffirmed an earlier report that Balamuthia and Acanthamoeba share a close phylogenetic relationship (1). As applied to the environmental isolate, the 16S mitochondrial rDNA sequence fits with those of other isolates and is identical to those of two other Balamuthia clinical isolates.
Previous attempts at isolation of this ameba from soil samples collected from the vicinity of other human and animal cases of infection yielded no candidate amebas or possible balamuthias that could be separated from contaminating protists and fungi (10). Noteworthy in this isolation was the appearance of the balamuthias in plated soil samples several weeks after the initial plating and long after the first wave of protozoal growth. During this period, many plates became overgrown with fungi, complicating visualization and isolation of the amebas. A problem associated with isolation and growth of balamuthias is their extended doubling time, which can range from ∼20 to ∼50 h in axenic cultures (12). This, and their slow rate of movement (∼0.15 μm/s, as determined by time-lapse video microscopy), made separation of the amebas from contaminating fungal growth difficult.
In culture, Balamuthia amebas do not feed on bacteria (15). However, since there is no protocol to follow for isolation of Balamuthia, we used the conventional method of nonnutrient agar with E. coli as a food source, employed in isolation of Naegleria and Acanthamoeba from environmental or clinical samples (11, 14). The presumptive Balamuthia amebas appeared to be feeding upon other amebas on the isolation plate and not on the bacteria. In vitro, Balamuthia feeds on other soil amebas if provided, and Acanthamoeba can be used in lieu of tissue culture cells as a food source for Balamuthia (16). In this study, N. gruberi (and N. fowleri) also served as suitable food sources. In nature, it is likely that balamuthias feed on other soil amebas and, perhaps, other types of protozoa found in soil.
The particular soil sample from which the ameba was isolated is of interest regarding a possible source and/or route of infection of the child. The soil sample came from a potted plant on the floor in the living room of the child's home, adjacent to the television set where the child spent much time. According to her guardians, the child apparently handled or played with soil from the pot during periods of watching television. There is, therefore, a likelihood that the soil was the source of the ameba that infected the child, possibly via a nasopharyngeal route, and ultimately caused her death (2).
The isolation of Balamuthia amebas from soil affirms its status as a free-living ameba that, like Naegleria and Acanthamoeba, is an opportunistic pathogen. This conclusion is supported by direct or indirect contacts with soil in several previous infections (soil contamination of wounds or breaks in skin, suspected infection by inhalation of wind-blown soil, and infections of zoo animals). Still, the relative rarity of Balamuthia encephalitis cases, the difficulty in making and confirming diagnoses, and the problems associated with isolating and growing Balamuthia amebas make this ameba a challenge to identify and recover from suspected soil samples. Questions regarding the risk of Balamuthia infection from contact with any soil type will likely remain difficult to answer until more data become available on the presence of these amebas in environmental samples.
REFERENCES
- 1.Amaral Zettler, L. A., T. A. Nerad, C. J. O'Kelly, M. T. Peglar, P. M. Gillevet, J. D. Silberman, and M. L. Sogin. 2000. A molecular reassessment of the leptomyxid amoebae. Protist 151:275-282. [DOI] [PubMed] [Google Scholar]
- 2.Bakardjiev, A., C. Glaser, F. Schuster, and G. S. Visvesvara. 2002. Three-year-old girl with fever and coma. Pediatr. Infect. Dis. J. 21:75. [DOI] [PubMed] [Google Scholar]
- 3.Booton, G. C., J. R. Carmichael, G. S. Visvesvara, T. J. Byers, and P. A. Fuerst. 2003. Genotyping of Balamuthia mandrillaris based on nuclear 18S and mitochondrial 16S rRNA genes. Am. J. Trop. Med. Hyg. 68:65-69. [PubMed] [Google Scholar]
- 4.Booton, G. C., J. R. Carmichael, G. S. Visvesvara, T. J. Byers, and P. A. Fuerst. 2003. Identification of Balamuthia mandrillaris by PCR assay using the mitochondrial 16S rRNA gene as a target. J. Clin. Microbiol. 41:453-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabot, E. L., and A. T. Beckenbach. 1989. Simultaneous editing of multiple nucleic and protein sequences with ESEE. Comput. Appl. Biosci. 5:233-234. [DOI] [PubMed] [Google Scholar]
- 6.Casas, I., L. Powell, P. E. Klapper, and G. M. Cleator. 1995. New method for the extraction of viral RNA and DNA from cerebrospinal fluid for use in the polymerase chain reaction assay. J. Virol. Methods 53:25-36. [DOI] [PubMed] [Google Scholar]
- 7.Ledee, D. R., G. C. Booton, M. H. Awwad, S. Sharma, R. K. Aggarwal, I. A. Niszl, M. B. Markus, P. A. Fuerst, and T. J. Byers. 2002. Advantages of using mitochondrial 16S rDNA sequences to classify clinical isolates of Acanthamoeba. Investig. Ophthalmol. Vis. Sci. 44:1142-1149. [DOI] [PubMed] [Google Scholar]
- 8.Martinez, A. J., and G. S. Visvesvara. 1997. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 7:583-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page, F. C. 1988. A new key to freshwater and soil gymnamoebae. Freshwater Biological Association, Ambleside, Cumbria, United Kingdom.
- 10.Rideout, B. A., C. H. Gardiner, I. H. Stalis, J. R. Zuba, T. Hadfield, and G. S. Visvesvara. 1997. Fatal infections with Balamuthia mandrillaris (a free-living amoeba) in gorillas and other Old World primates. Vet. Pathol. 34:15-22. [DOI] [PubMed] [Google Scholar]
- 11.Schuster, F. L. 2002. Cultivation of pathogenic and opportunistic free-living amebas. Clin. Microbiol. Rev. 15:342-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuster, F. L., and G. S. Visvesvara. 1996. Axenic growth and drug sensitivity studies of Balamuthia mandrillaris, an agent of amebic meningoencephalitis in humans and other animals. J. Clin. Microbiol. 34:385-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuster, F. L., and G. S. Visvesvara. 1998. Efficacy of novel antimicrobials against clinical isolates of opportunistic amebas. J. Eukaryot. Microbiol. 45:612-618. [DOI] [PubMed] [Google Scholar]
- 14.Visvesvara, G. S. 1999. Pathogenic and opportunistic free-living amebae, p. 1383-1390. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 15.Visvesvara, G. S., A. J. Martinez, F. L. Schuster, G. J. Leitch, S. V. Wallace, T. K. Sawyer, and M. Anderson. 1990. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J. Clin. Microbiol. 28:2750-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visvesvara, G. S., F. L. Schuster, and A. J. Martinez. 1993. Balamuthia mandrillaris n. g. n. sp., agent of amebic meningoencephalitis in humans and other animals. J. Eukaryot. Microbiol. 40:504-514. [DOI] [PubMed] [Google Scholar]