Abstract
Whether highly active antiretroviral therapy (HAART) should be modified in patients with persistent increases in CD4+ T cells despite detectable viral loads is an unresolved question. Forty-three heavily pretreated human immunodeficiency virus (HIV)-infected patients with virologic failure during HAART were studied before a change of therapy guided by genotypic analysis and during follow-up. Patients with an increase in CD4+ cell count (>100 cells/ml) over pre-HAART values were considered to be discordant patients (20 individuals), whereas patients with a lower increase or no increase in CD4+ cell count were considered failing patients (23 individuals). Based on univariate analysis, a high CD4+ cell count before antiretroviral treatment, homosexual behavior as a risk factor for HIV infection, reduced drug exposure to nonnucleoside reverse transcriptase inhibitors, low replicative capacity of HIV isolates, and more frequent detection of HIV isolates with a non-B subtype, an R5 biological phenotype, and M184V and T215Y/F mutations were factors associated with a discordant response to HAART. Based on multivariate analysis, only the M184V mutation remained significantly associated with a viroimmunologic discordant response (odds ratio, 25.48; 95% confidence interval, 1.43 to 453.93). No difference in lamivudine exposure was found between discordant (95%) and failing (91%) patients. Twelve months after the genotypic analysis-guided change of therapy, 3 discordant (15%) and 6 failing patients (26%) achieved undetectable viral loads (<50 copies/ml), whereas in patients with HIV RNA loads of >500 copies/ml, discordant responses were observed in 5 out of 15 discordant patients and in 4 out of 16 failing patients. A relationship between the M184V mutation and a viroimmunologic discordant response to HAART was found. After the genotypic analysis-driven change of therapy, similar rates of virologic suppression were detected in the two groups.
The goal of highly active antiretroviral therapy (HAART) in patients with human immunodeficiency virus (HIV) infection is the complete suppression of viral replication. After initiation of HAART, the plasma viral load decreases to below the level of detection in many HIV-infected patients (3, 9, 13). On the other hand, in clinical practice, 40 to 70% of patients show virologic failure, generally defined as persistently detectable HIV RNA levels in plasma (5, 12).
To date, the clinical significance of virologic failure remains unclear, but during partially suppressive therapy, the presence of circulating infectious-competent HIV type 1 (HIV-1) implies ongoing viral replication with the likely selection of drug-resistant virus (6). A special subset of patients includes those exhibiting a sustained increase in CD4+-T-cell count over 1 year of HAART, despite persistently high viral loads (10, 18, 20). This subset of viroimmunologically discordant patients accounts for approximately 30% of individuals receiving HAART; among them, during an 18-month follow-up period, the incidence of death or AIDS-defining event was 14%, sevenfold higher than that observed in patients showing a full response yet lower than that in subjects with no immunologic or virologic response (19). Moreover, a recent report showed that in discordant patients, the median time to immunologic failure after the onset of virologic failure was 36 months (4).
From a clinical viewpoint, whether the ongoing HAART in patients with virologic failure and sustained CD4+ responses should be modified is an unresolved question. Published therapy guidelines do not provide any clues to the answer. In the present study, we addressed this issue by analyzing virologic features of a group of 20 patients with viroimmunologically discordant responses to HAART, who were monitored longitudinally after a change of regimen guided by genotypic analysis. The results were compared with those obtained for a parallel group of 23 individuals without immunologic or virologic responses to HAART.
(This work was presented in part previously [S. Vella, G. d’Etto, L. Palmisano, E. Nincastri, S. Giuseppe Parisi, M. Andreotti, L. Sarmati, C. Galluzzo, C. Mastroianni, V. Vullo, E. Concia, and M. Andreoni, Abstr. 9th Conf. Retroviruses Opportunistic Infect., abstr. 490-M, 2002].)
MATERIALS AND METHODS
Study population.
Forty-three HIV-infected patients failing antiretroviral treatment were consecutively enrolled in the study. The inclusion criteria were (i) an uninterrupted HAART regimen for at least 12 months, (ii) a viral load of >3,000 HIV RNA copies/ml in the two last consecutive samples, and (iii) an assay result indicating genotypic resistance before HAART was changed. During a mean treatment period of 58.7 months (range, 18 to 126 months), patients received a mean of 5.7 antiretroviral drugs (range, 3 to 10 drugs), and 84 and 58% of patients were treated with protease inhibitors (PI) and nonnucleoside reverse transcriptase inhibitors (NNRTI), respectively. Treatment for all patients was changed according to both genotypic assay results and expert panel suggestions.
The patients were divided into two groups according to their immunologic responses to HAART and the genotypic analysis-guided change of therapy. Patients with increases in CD4+ cell count (>100 cells/ml) with respect to pre-HAART values were considered to be discordant patients (20 individuals), whereas patients with lower or no increases in CD4+ cell count were considered to be failing patients (23 individuals). The adherence to HAART was evaluated on the basis of a patient-reported questionnaire.
All patients, who were enrolled at the Departments of Infectious Diseases of “La Sapienza” University of Rome and of the University of Verona, gave informed consent to blood sampling and processing.
Laboratory monitoring.
Patients were studied at the time of enrollment for antiretroviral drug resistance mutations in plasma, CD4 cell count, HIV viral load in plasma, and adherence to HAART by using a patient-reported questionnaire. After the genotypic analysis-guided change of therapy, the patients were evaluated at 1, 2, 4, 6, 8, 10, and 12 months of follow-up for CD4 cell count and HIV viral load in plasma.
HIV-RNA was quantified with the AMPLICOR Monitor assay (Roche Molecular Systems, Branchburg, N.J.).
HIV was isolated from peripheral blood mononuclear cells (PBMCs) as previously described (21). Briefly, the patients' PBMCs were cocultured with PBMCs obtained from HIV-1-seronegative donors. Cultures, placed in a humidified chamber at 37°C with 5% CO2, were maintained for 60 days and monitored twice a week for p24 antigen production by use of a commercially available enzyme immunoassay (Abbott Laboratories, North Chicago, Ill.). A culture was considered positive if the concentration of p24 exceeded 1,000 pg/ml in two consecutive determinations. Positive supernatants were harvested by centrifugation and stored in liquid nitrogen.
To determine the syncytium-inducing (X4) or non-syncytium-inducing (R5) biological phenotype of HIV isolates, an aliquot of viral stock supernatant containing 100 50% tissue culture infective doses was cultured in T25 flasks with 106 MT-2 cells. Cultures were maintained for up to 4 weeks and examined for syncytia twice a week. Syncytium formation was defined by the presence of at least 10 multinucleated giant cells in five high-power fields.
Replicative capacity of HIV isolates.
The replicative capacity of HIV isolates was determined as previously described (14). Briefly, 5 × 106 patient PBMCs were cocultured with 5 × 106 PBMCs combined from two HIV-seronegative donors. The time in days to reach a level of 100,000 pg of p24 antigen/ml in culture supernatants (replication constant K) was calculated by using nonlinear regression modelling. The relative replicative capacity of the primary isolates was determined from ratios of resistant-strain K (Krs) to wild-type-strain K (Kwt) multiplied by 100 and was expressed as a percentage of the K of the wild-type virus. For the evaluation of Kwt, two primary isolates obtained from PBMCs of patients who had never received antiretroviral treatment and without drug resistance mutations were used. The Kwt used in the determination of the relative replicative capacity of HIV isolates was 5 days, i.e., the mean of the K values of the two control viruses (4.8 and 5.2 days, respectively).
All assays were performed in duplicate, and the mean value was considered.
Sequencing of the HIV-1 pol gene.
A commercial kit was used to identify mutations in the pol gene of HIV-1 (ViroSeq HIV-1 Genotyping System version 2; PE Biosystems, Foster City, Calif.). Five hundred microliters of plasma was centrifuged at 4°C for 1 h at 21,000 to 25,000 × g. The resulting viral pellet was processed by a guanidinium-ethanol-isopropanol-based method and resuspended in 50 or 100 μl of RNA diluent, depending on whether the viral load was less or greater than 15,000 copies/ml. The reverse transcriptase (RT) PCR was carried out with a single primer and the enzyme murine leukemia virus reverse transcriptase. In a second PCR, the cDNA products from the RT reaction were amplified, resulting in an amplicon that encompassed the entire HIV protease (PR) gene and the first 324 codons of the RT gene. Big Dye Terminator chemistry and seven custom primers were used to sequence both strands of the amplicon. The sequencing product was analyzed on an ABI Prism 310 genetic analyzer (PE Biosystems). DNA base calling was performed by using the DNA Sequencing Analysis software (PE Biosystems). ViroSeq HIV-1 Genotyping System software was used to analyze the overlapping segments to give a consensus sequence, to compare it to the sequence of the HIV-1 pNL4-3 reference strain (GenBank accession number M19921), and to interpret the genotyping results. The HIV subtype was determined by comparison of the PR and RT gene sequences to the Stanford HIV Sequence Database (23).
Statistical analysis.
The association between potential determinant factors and viroimmunologic discordance was assessed by estimating crude and adjusted odds ratios (OR) and their 95% confidence intervals (CI) through univariate and multivariate models. In the multivariate analysis, those variables that were significantly associated with viroimmunologic discordance in the univariate analysis were considered. The associations with a P value of <0.05 were considered significant. Continuous variables were calculated by logistic regression analysis for each unit increase, except for CD4 cell counts, for which increases of 250 cells/mm3 were used. The virologic and immunologic differences between the two groups of patients after the genotypic analysis-guided change of therapy were evaluated by using analysis of variance. All statistical analysis is intended for intention-to-treat determinations. SPSS for Windows (version 10.1.3; SPSS Inc.) was used for the analysis.
RESULTS
The demographic and clinical parameters of 43 patients failing HAART, stratified according to immunologic responses to therapy, are presented in Table 1. Based on univariate analysis, no significant differences in terms of age, gender, or adherence to antiretroviral treatment were detected between the 20 viroimmunologically discordant patients and the 23 failing therapy patients. Homosexual behaviour as a risk factor for HIV infection was significantly associated with a viroimmunologically discordant response to HAART (OR, 5.65; 95% CI, 1.02 to 31.48), whereas heterosexual behavior was significantly more frequent in failing patients (OR, 0.23; 95% CI, 0.06 to 0.90). Discordant patients had significantly higher CD4 cell counts than failing patients before starting any antiretroviral treatment (OR, 5.94; 95% CI, 1.43 to 24.65). Based on genotypic analysis, no significant difference in HIV viral load was detected between the two groups.
TABLE 1.
Determinant factors related to immunologic response to HAART despite virologic failure
| Characteristic | Value for patients
|
Crude OR (95% CI) | P | |
|---|---|---|---|---|
| Discordant (n = 20) | Failing (n = 23) | |||
| Age (yr)a | 37 (20-51) | 38 (20-70) | 0.98 (0.92-1.04) | 0.48 |
| Gender male (%) | 14 (70) | 17 (73) | 0.82 (0.21-3.12) | 0.78 |
| Risk factor | ||||
| Homosexual (%) | 7 (91) | 2 (35) | 5.65 (1.02-31.48) | 0.048 |
| Heterosexual (%) | 4 (20) | 12 (52) | 0.23 (0.06-0.90) | 0.035 |
| IVDUd (%) | 8 (40) | 8 (35) | 1.25 (0.36-4.32) | 0.72 |
| Other | 1 | 1 | ||
| Time on HAART (mo)a | 51 (18-120) | 48 (24-126) | 0.99 (0.98-1.02) | 0.72 |
| CD4 pre-HAART (cells/μl)a | 276 (25-648) | 142 (2-420) | 5.94 (1.43-24.65) | 0.014 |
| HIV RNA at time of genotype analysis (copies/ml)a | 4.49 (3.59-5.18) | 5.07 (3.47-6.62) | 0.39 (0.14-1.08) | 0.069 |
| Subtype B HIV-1 isolate (%) | 18 (90) | 14 (61) | 5.78 (1.07-31.16) | 0.041 |
| R5 biological phenotype (%) | 15 (75) | 9 (39) | 4.67 (1.25-17.36) | 0.022 |
| Replicative capacity of HIV isolatea,b | 32.8 (14-54) | 52.0 (21-98) | 1.04 (1.01-1.09) | 0.038 |
| NNRTI exposure (%) | 10 (50) | 15 (65) | 0.53 (0.16-1.82) | 0.31 |
| PI exposure (%) | 15 (75) | 21 (91) | 0.29 (0.05-1.68) | 0.16 |
| Adherence (%)c | 100 (75-100) | 100 (67-100) | 1.08 (0.99-1.18) | 0.09 |
| Drug resistance mutationsa | ||||
| RT mutations | 5.0 (0-11) | 4.3 (1-10) | 1.08 (0.88-1.34) | 0.47 |
| PR mutations | 3.0 (0-9) | 2.0 (0-7) | 1.15 (0.87-1.50) | 0.33 |
| M184V mutation (%) | 17 (85) | 6 (26) | 14.1 (3.00-66.7) | 0.001 |
| T215Y/F mutation (%) | 15 (75) | 9 (39) | 4.00 (1.06-15.14) | 0.041 |
| K103N mutation (%) | 7 (35) | 12 (52) | 0.40 (0.11-1.43) | 0.16 |
| V82A mutation (%) | 8 (40) | 6 (26) | 1.67 (0.45-6.13) | 0.44 |
| L90M mutation (%) | 7 (35) | 5 (17) | 1.72 (0.44-6.72) | 0.43 |
Median (range).
Percent in comparison with value for wild-type HIV-1 strain.
Median (range) of the adherence to HAART (percentage of pills taken during the last 3 days).
IVDU, intravenous drug user.
Significantly higher prevalences of the R5 biological phenotype and the subtype B of HIV strains were detected in discordant patients than in failing subjects (OR of 4.67 and 95% CI of 1.25 to 17.36 versus OR of 5.78 and 95% CI of 1.07 to 31.16, respectively). All but two patients with non-subtype-B HIV infections listed heterosexual behavior as a risk factor for HIV infection. Moreover, a significantly lower replicative capacity (OR, 1.04; 95% CI, 1.01 to 1.09) was detected in HIV-1 strains isolated from discordant individuals than in failing patients.
All patients had been heavily pretreated, with a median number of six antiretroviral drugs having been received for a median of 51 and 48 months of treatment in discordant and failing patients, respectively. No significant differences in history of antiretroviral treatment with regard to PI and NRTI were observed between two groups; conversely, failing patients were significantly more heavily treated with NNRTI than were discordant patients (OR, 0.29; 95% CI, 0.09 to 0.97).
In both groups, the genotypic drug resistance analysis showed a high number of mutations either in the RT or the PR gene of HIV. However, a higher but not significant number of drug-resistant mutations was detected in discordant patients than in failing patients.
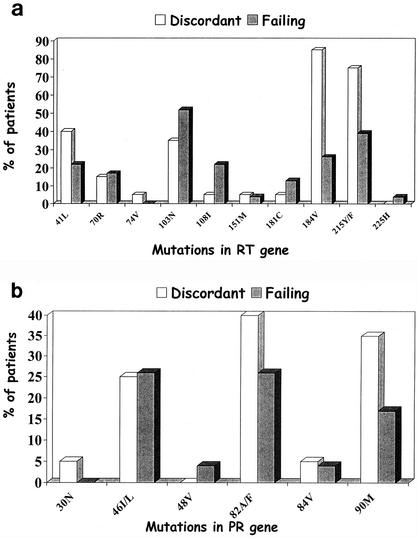
The prevalences of primary drug resistance mutations in RT and PR genes are reported in Fig. 1a and b, respectively. A significantly higher number of discordant patients than failing patients carried the M184V and the T215Y/F mutations (OR of 14.1 and 95% CI of 3.00 to 66.7 versus OR of 4.00 and 95% CI of 1.06 to 15.14, respectively). No significant difference in prevalences of other drug resistance mutations in both genes was detected in the two groups. Nevertheless, NNRTI-related primary mutations (K103N and Y181C) were more prevalent in failing patients than in discordant subjects.
FIG. 1.
Prevalence of primary drug resistance mutations in reverse transcriptase (a) and protease (b) genes of HIV-1 isolates from 20 patients with viroimmunologically discordant responses to therapy and in 23 patients failing therapy.
Using multivariate analysis, only the prevalence of the M184V mutation was confirmed to be significantly related to the viroimmunologically discordant response to HAART (OR, 25.48; 95% CI, 1.43 to 453.93).
No significant difference in lamivudine (3TC) exposure was found between discordant (19 out of 20) and failing (21 out of 23) patients. Moreover, 17 (85%) discordant and 17 (73%) failing patients were receiving HAART that included 3TC at the time of enrollment. The M184V mutation was detected in the HIV of 22 (64%) 3TC-treated patients. Among the 17 discordant patients carrying the M184V mutation, 14 of them were being treated with 3TC, whereas 2 subjects had been previously exposed to 3TC. The remaining patient had been heavily treated with dideoxyinosine (27 months) but had never been treated with 3TC. Conversely, only 6 out of 17 patients failing HAART that included 3TC harbored the M184V mutation.
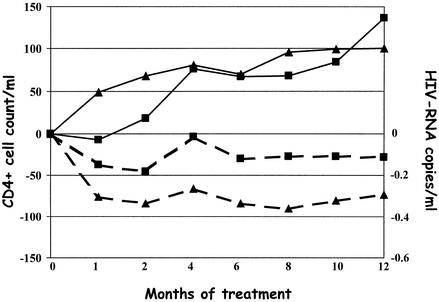
The viroimmunologic parameters of patients during the 12-month follow-up period after the genotypic analysis-guided change of therapy are reported in Fig. 2. No significant difference in decrease of HIV RNA viral load at 12 month between discordant and failing patients was detected (mean of −0.28 and −0.73 log HIV RNA copies/ml, respectively; P = 0.65). Furthermore, three out of five discordant patients and six out of seven failing patients with viral loads of less than 500 copies/ml reached viral loads that were undetectable (<50 copies/ml). A stronger but not significant increase in CD4 cell count compared to baseline value was reported for discordant patients than for failing subjects (mean increase of 136 and 100 cells/ml, respectively; P = 0.065).
FIG. 2.
CD4 cell count (solid lines) and HIV-RNA copy number (broken lines) after the plasma genotypic analysis-guided change of therapy in 20 patients with discordant viroimmunologic responses (▪) and in 23 patients failing therapy (▴).
In patients with detectable viral loads (>500 copies of HIV RNA/ml), viroimmunologically discordant responses to a genotypic analysis-guided change of therapy with increases of more than 100 CD4 cells/ml were observed in 5 out of 15 and 4 out of 16 discordant and failing patients, respectively. In both groups, approximately half of the patients showed a viroimmunologic failure.
No significant difference in number of newly prescribed drugs was observed in the two groups of patients (data not shown).
DISCUSSION
In this study, more than 50% of patients with virologic failure during HAART and two-thirds of patients receiving HAART that included 3TC carried the M184V mutation. Moreover, the M184V mutation was strictly associated based on multivariate analysis with the viroimmunologically discordant response to therapy despite similar frequencies of a 3TC-including regimen and of self-reported adherence to treatment in both the failing and discordant groups. The M184V mutation, related to 3TC resistance, could create a “less fit” virus that is slower to overcome suppression by antiretroviral therapy than the wild-type virus. Despite the emergence of a primary resistance mutation, this apparent beneficial effect could be related either to the reduced processivity and increased fidelity of the RT (25, 26) and to the reduced pyrophosphorolysis of the mutant variant (8). Nevertheless, the patients treated with 3TC monotherapy experienced a rapid virologic failure due to the emergence of the M184V mutation, although the viral load remained consistently suppressed below baseline values (7, 22).
In our study, viroimmunologically discordant patients carried HIV isolates with an impaired viral fitness compared to patients failing HAART. There is evidence that the viruses recovered from discordant individuals have decreased cytopathogenicity in thymic tissue that may permit the regeneration of T cells, despite a persistently elevated viral load (2, 15). The fitness of HIV in vivo depends on the interaction of a multitude of viral factors (e.g., replicative capacity, mutation rate, and host cell tropism) and host factors (e.g., genetic background, immune control, and target cell availability) (24). Among viral issues, the drug resistance-related mutations seem to play a major role in driving the viral fitness of isolates.
In this study, discordant patients carried HIV isolates with a higher number of drug resistance mutations, either in the RT or the PR gene, than did failing subjects, although this difference did not attain a statistical significance. A previous report showed that after 12 months of therapy, 66 and 82% of patients with discordant responses to treatment acquired both primary and secondary resistance mutations to PI, respectively (11).
In our study, the presence of drug resistance mutations does not interfere with immunologic restoration under HAART and the immune recovery occurs independently of the presence of resistant HIV variants and virologic failure. Furthermore, the CD4 cell count in a patient before the start of any antiretroviral therapy represents a variable that is predictive of immunologic recovery despite virologic failure during HAART.
Homosexual behavior as a risk factor for HIV infection was more frequently reported by discordant patients, whereas heterosexual behavior was prevalent among failing patients. The majority of patients with heterosexual behavior carried non-subtype-B HIV-1 isolates. An increasing prevalence of non-subtype-B HIV-1 isolates in these subjects has already been described (16), and previous reports showed that these clades might be less susceptible in vitro to antiretroviral drugs (1, 17).
Very limited data are available on the long-term outcome of patients with discordant responses to HAART. During 18 months of follow-up, 4 out of 28 discordant patients (14%) receiving HAART showed decreases in CD4 cell count and a progression to a new AIDS-defining event (19). Similarly, a recent report showed a median delay of 3 years between the onset of virologic failure and the return of the absolute CD4 cell count to pretherapy levels (4). In our study, by 12 months after the genotypic analysis-guided change of therapy, similar percentages of discordant (25%) and failing (30.4%) patients achieved undetectable levels of HIV-1 viral load (<500 copies/ml) whereas both groups showed a 50% rate of virologic failure.
Nevertheless, some of the limitations of our study should be discussed. First, the wide range of treatment duration (18 to 126 months) could result in a heterogeneous patient population in which mechanisms contributing to discordant responses could differ. However, the median treatment durations in failing and discordant patients are very similar, suggesting that patients with different pharmacological exposures are equally distributed in both groups. Second, this study was a longitudinal and not a randomized clinical trial designed to detect the determinant factors of discordant responses to HAART. A limited number of discordant patients was enrolled to evaluate the change in viroimmunologic markers of HIV infection after a genotypic analysis-guided change of therapy. In the near future, randomized clinical trials with large populations of discordant and failing patients should be performed to evaluate these preliminary conclusions. Finally, we cannot exclude the possibility that the higher prevalence of M184V mutations in discordant patients reflects their better adherence to the medication regimen than that by failing patients. However, the evaluation of the adherence based on the self-reported questionnaire did not show any difference between the two groups of patients.
Our observations cannot fully address the question of whether HAART should be modified in patients with persistent increases in CD4+ T cells despite detectable viral loads; however, they underline the role played by the drug resistance and particularly the presence of the M184V mutation in these subjects. Further studies are needed to clarify whether an antiretroviral therapy switch in discordant patients could represent a valid strategy in term of virologic and immunologic responses.
Acknowledgments
This work was supported by a grant from “Progetto Ricerche AIDS,” Istituto Superiore di Sanità, Ministry of Health (to M. Andreoni and S. Vella).
REFERENCES
- 1.Apetrei, C., D. Descamps, G. Collin, I. Loussert-Ajaka, F. Damond, M. Duca, F. Simon, and F. Brun-Vezinet. 1998. Human immunodeficiency virus type 1 subtype F reverse transcriptase sequence and drug susceptibility. J. Virol. 72:3534-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belec, L., C. Piketty, A. Si-Mohamed, C. Goujon, M. C. Hallouin, S. Cotigny, L. Weiss, and M. D. Kazatchkine. 2000. High levels of drug-resistant human immunodeficiency virus variants in patients exhibiting increasing CD4+ T cell counts despite virologic failure of protease inhibitor-containing antiretroviral combination therapy. J. Infect. Dis. 181:1808-1812. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter, C. C., D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, S. M. Hammer, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, P. G. Yeni, and P. A. Volberding. 2000. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA panel. JAMA 283:381-390. [DOI] [PubMed] [Google Scholar]
- 4.Deeks, S. G., J. D. Barbour, R. M. Grant, and J. N. Martin. 2002. Duration and predictors of CD4 T-cell gains in patients who continue combination therapy despite detectable plasma viremia. AIDS 16:201-207. [DOI] [PubMed] [Google Scholar]
- 5.Deeks, S. G., J. D. Barbour, J. N. Martin, M. S. Swanson, and R. M. Grant. 2000. Sustained CD4+ T cell response after virologic failure of protease inhibitor-based regimens in patients with human immunodeficiency virus infection. J. Infect. Dis. 181:946-953. [DOI] [PubMed] [Google Scholar]
- 6.Deeks, S. G. 2000. Determinants of virological response to antiretroviral therapy: implications for long-term strategies. Clin. Infect. Dis. 30:S177-S184. [DOI] [PubMed]
- 7.Eron, J. J., S. L. Benoit, J. Jemsek, R. D. MacArthur, J. Santana, J. B. Quinn, D. R. Kuritzkes, M. A. Fallon, M. Rubin, et al. 1995. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. N. Engl. J. Med. 333:1662-1669. [DOI] [PubMed] [Google Scholar]
- 8.Feng, J. Y., and K. S. Anderson. 1999. Mechanistic studies examining the efficiency and fidelity of DNA synthesis by the 3TC-resistant mutant (184V) of HIV-1 reverse transcriptase. Biochemistry 38:9440-9448. [DOI] [PubMed] [Google Scholar]
- 9.Hammer, S. M., K. E. Squires, M. D. Hughes, J. M. Grimes, L. M. Demeter, J. S. Currier, J. J. Eron, Jr., J. E. Feinberg, H. H. Balfour, Jr., L. R. Deyton, J. A. Chodakewitz, and M. A. Fischl. 1997. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infections and CD4 cell counts of 200 per cubic millimetre or less. N. Engl. J. Med. 337:725-733. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann, D., G. Pantaleo, P. Sudre, A. Telenti, et al. 1998. CD4-cell count in HIV-1-infected individuals remaining viraemic with highly active antiretroviral therapy (HAART). Lancet 351:723-724. [DOI] [PubMed] [Google Scholar]
- 11.Lecossier, D., F. Bouchonnet, P. Schneider, F. Clavel, and A. J. Hance. 2001. Discordant increases in CD4+ T cells in human immunodeficiency virus-infected patients experiencing virologic treatment failure: role of changes in thymic output and T cell death. J. Infect. Dis. 183:1009-1016. [DOI] [PubMed] [Google Scholar]
- 12.Ledergerber, B., M. Egger, M. Opravil, A. Telenti, B. Hirschel, M. Battegay, P. Vernazza, P. Sudre, M. Flepp, H. Furrer, P. Francioli, R. Weber, et al. 1999. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Lancet 353:863-868. [DOI] [PubMed] [Google Scholar]
- 13.Li, T. S., R. Tubiana, C. Katlama, V. Calvez, H. A. Mohand, and B. Autran. 1998. Long lasting recovery of CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet 351:1682-1686. [DOI] [PubMed] [Google Scholar]
- 14.Nicastri, E., L. Sarmati, G. d'Ettorre, L. Palmisano, S. G. Parisi, I. Uccella, A. Rianda, E. Concia, V. Vullo, S. Vella, and M. Andreoni. 2003. Replication capacity, biological phenotype, and drug resistance of HIV strains isolated from patients failing antiretroviral therapy. J. Med. Virol. 69:1-6. [DOI] [PubMed] [Google Scholar]
- 15.Nijhius, M., S. Deeks, and C. Boucher. 2001. Implications of antiretroviral resistance on viral fitness. Curr. Opin. Infect. Dis. 14:23-28. [DOI] [PubMed] [Google Scholar]
- 16.Op De Coul, E. L., A. A. Coutinho, A. van der Schoot, G. J. van Doornum, V. V. Lukashov, J. Goudsmit, M. Cornelissen, and the Dutch HIV-1 Subtype Surveillance. 2001. The impact of immigration on env HIV-1 subtype distribution among heterosexuals in The Netherlands: influx of subtype B and non-B strains. AIDS 15:2277-2286. [DOI] [PubMed] [Google Scholar]
- 17.Palmer, S., A. Alaeus, J. Albert, and S. Cox. 1998. Drug susceptibility of subtypes A, B, C, D, and E human immunodeficiency virus type 1 primary isolates. AIDS Res. Hum. Retrovir. 14:157-162. [DOI] [PubMed] [Google Scholar]
- 18.Piketty, C., P. Castiel, L. Belec, D. Batisse, A. Si Mohamed, J. Gilquin, G. Gonzalez-Canali, D. Jayle, M. Karmochkine, L. Weiss, J. P. Aboulker, and M. D. Kazatchkine. 1998. Discrepant responses to triple combination antiretroviral therapy in advanced HIV disease. AIDS 12:745-750. [DOI] [PubMed] [Google Scholar]
- 19.Piketty, C., L. Weiss, F. Thomas, A. S. Mohamed, L. Belec, and M. D. Kazatchkine. 2001. Long-term clinical outcome of human immunodeficiency virus-infected patients with discordant immunologic and virologic responses to a protease inhibitor-containing regimen. J. Infect. Dis. 183:1328-1335. [DOI] [PubMed] [Google Scholar]
- 20.Renaud, M., C. Katlama, A. Mallet, V. Calvez, G. Carcelain, R. Tubiana, M. Jouan, E. Caumes, H. Agut, F. Bricaire, P. Debre, and B. Autran. 1999. Determinants of paradoxical CD4 cell reconstitution after protease inhibitor-containing antiretroviral regimen. AIDS 13:669-676. [DOI] [PubMed] [Google Scholar]
- 21.Sarmati, L., E. Nicastri, G. el-Sawaf, L. Ercoli, S. Vella, and M. Andreoni. 1997. Increase in neutralizing antibody titer against sequential autologous HIV isolates after 16 weeks saquinavir (Invirase) treatment. J. Med. Virol. 53:313-318. [PubMed] [Google Scholar]
- 22.Schuurman, R., M. Nijhuis, R. van Leeuwen, P. Schipper, D. de Jong, P. Collis, S. A. Danner, J. Mulder, C. Loveday, C. Christopherson, S. Kwok, J. Sninsky, and C. A. B. Boucher. 1995. Rapid changes in human immunodeficiency virus type 1 RNA load and appearance of drug-resistant virus populations in persons treated with lamivudine (3TC). J. Infect. Dis. 171:1411-1419. [DOI] [PubMed] [Google Scholar]
- 23.Shafer, R. W., D. Stevenson, and B. Chan. 1999. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 27:348-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoddart, C. A.,T. J. Liegler, F. Mammano, V. D. Linquist-Stepps, M. S. Hayden, S. G. Deeks, R. M. Grant, F. Clavel, and J. M. McCune. 2001. Impaired replication of protease inhibitor-resistant HIV-1 in human thymus. Nat. Med. 7:712-718. [DOI] [PubMed] [Google Scholar]
- 25.Wainberg, M. A.,W. C. Drosopoulos, H. Salomon, M. Hsu, G. Borkow, M. Parniak, Z. Gu, Q. Song, J. Manne, S. Islam, G. Castriota, and V. R. Prasad. 1996. Enhanced fidelity of 3TC-selected mutant HIV-1 reverse transcriptase. Science 271:1282-1285. [DOI] [PubMed] [Google Scholar]
- 26.Wainberg, M. A., M. Hsu, Z. Gu, G. Borkow, and M. A. Parniak. 1996. Effectiveness of 3TC in HIV clinical trials may be due in part to the M184V substitution in 3TC-resistant HIV-1 reverse transcriptase. AIDS 10:S3-S10. [DOI] [PubMed]