Abstract
In order to evaluate the LightCycler-based PCR (LC-PCR) as a diagnostic assay technique, a classical pp65 antigenemia assay and the commercially available COBAS Amplicor CMV Monitor (CACM) assay were compared to the LC-PCR assay for the detection and quantitation of cytomegalovirus (CMV) load in 404 parallel specimens of peripheral blood from 66 patients after solid organ transplantation. A good correlation existed among these three assays (r ≅ 0.6, P < 0.0001). The LC-PCR assay was the most sensitive (54% of specimens positive) compared to the CACM (48.6%) and the pp65 antigenemia (26%) assays. The LC-PCR assay detected all samples found positive by using both the CMV pp65 antigenemia assay and the CACM assay. The LC-PCR also had the widest dynamic range (from 250 to 107 DNA copies/ml of plasma). No cross-reactions were found among CMV and Epstein-Barr virus, varicella-zoster virus, or herpes simplex virus in the LC-PCR by using amplification with specifically designed primer pairs. Precision, expressed as the coefficient of variation, was <3% with standard DNA from cell cultures and between 6.55 and 14.1% with clinical specimens in repeat LC-PCR runs. One run of the LC-PCR took half of the time required for the semiautomated CACM procedure. Because of its sensitivity, specificity, cost-effectiveness, and simplicity, the LC-PCR assay could replace the pp65 antigenemia and the CACM assays as the preferred technique for the surveillance, diagnosis, and monitoring of response of CMV diseases in high-risk populations.
Despite significant advances resulting from the use of preemptive and prophylactic antiviral therapy for the prevention of cytomegalovirus (CMV) disease after solid organ transplantation, CMV continues to cause morbidity and mortality in this setting (15, 29, 30, 31). Appropriate management of these patients is dependent on the early detection and quantitation of CMV viremia and/or CMV DNAemia to identify patients at greatest risk for CMV disease, diagnose infection, and determine response and duration of antiviral therapy (1, 8, 13, 23, 24, 25). The CMV pp65 antigenemia assay, a specific quantitative method developed in the early 1990s, has been widely used for the diagnosis of CMV disease and monitoring of responses to antiviral therapy (3, 6, 11). However, this assay sometimes shows false-negative results due to a low-level expression of the pp65 antigen in white blood cells in a small number of patients with definite CMV disease (20, 22, 28). The labor-intensive nature of the procedure, the requirement for immediate sample processing, and the subjective interpretation of slides place limitations on this assay as a routine diagnostic procedure (4, 16, 22). In addition, the clinical utility of these assays in assessing response to antiviral therapy is uncertain (7, 22). PCR-based qualitative detection of CMV DNA in blood samples provides 100% sensitivity for the diagnosis of CMV disease, but the specificity is generally 50% or less (17, 29, 33). The NucliSens assay (Organon Teknika Diagnostics, Boxtel, The Netherlands), an isothermal nucleic acid amplification reaction assay, detects the presence of CMV late-mRNA pp67. The presence of mRNA pp67 indicates active viral replication, and its detection is a marker for active CMV infection (18, 19). Nevertheless, this assay has less sensitivity than DNA amplification and antigenemia assays for detection of CMV infection. The lower sensitivity of the assay may result in failure to detect or predict CMV disease in all patients (22). In one study, the assay did not detect the mRNA transcripts in 4 of 11 patients who developed CMV disease (19). A commercially available COBAS Amplicor CMV Monitor (CACM) assay has been developed by Roche Diagnostics (Branchburg, N.J.) for the determination of CMV DNA load (5, 6, 30). This semiautomated PCR amplification system provides clinical laboratories with a standardized assay for detection and quantitation of CMV in plasma, featuring high sensitivity and specificity. However, some disadvantages, such as the cost, the narrow dynamic range of the assay and the time-consuming nature of the procedure, limit the use of CACM as a surveillance tool in high-risk populations (22, 26, 35).
Recently, a fluorescence-tagged, real-time quantitative PCR assay in a closed tube system has been introduced into research and diagnostic laboratories. Two systems are available, the LightCycler-based PCR (LC-PCR; Roche Diagnostics) and the ABI Prism 7700 sequence detection system (PE Applied Biosystems). A few studies have demonstrated that real-time quantitative PCR is accurate, rapid, and cost-effective with high sensitivity and specificity for the determination of CMV DNA load (10, 11, 14, 17, 22, 26, 32, 35). Since “in-house” PCR assays may result in different outcomes due to variations in targeting sequences, primer pairs, DNA extraction methods, and amplification conditions, the real-time PCR method, for detecting and monitoring CMV viral DNA load, needs to be further developed, evaluated, and validated before its widespread introduction into diagnostic laboratories. We designed this clinical study to evaluate the potential of using the LC-PCR assay as an alternative to the CACM test for quantitation of CMV load in plasma collected from patients after solid organ transplantation. In the present study, we report our comparisons of critical parameters, such as sensitivity, specificity, dynamic range, required-time and cost of the LC-PCR, CACM, and pp65 antigenemia assays, running in parallel with the same clinical specimens. The precision of the “in-house” LC-PCR assay was also determined.
MATERIALS AND METHODS
Patients and clinical specimens.
All solid organ transplant recipients at the University of Alberta Hospitals undergo laboratory-based buffy coat surveillance for CMV infection. The frequency of surveillance specimens submitted is determined by protocol and is dependent on the pretransplant donor-recipient serostatus, use of antibody therapy for induction and rejection, and use of antiviral therapy for prophylaxis and treatment. Additional samples are submitted in the setting of suspected clinical disease. The CMV pp65 antigenemia assay (3) and viremia quantified by using a shell vial technique (9) have been used as routine surveillance and diagnostic tools.
A total of 66 patients, including 16 heart transplant, 18 liver transplant, 23 renal transplant, 5 renal or pancreas transplant, and 4 lung transplant recipients, were enrolled in the present study from November 1999 to February 2001. A total of 404 plasma samples from these patients were assayed. At each sampling point, two 4-ml tubes of EDTA-treated blood were collected. Plasma was collected after centrifugation for 10 min at 500 × g.
LC-PCR.
The preparation of DNA from plasma and the LC-PCR were carried out with minor modifications from the methods previously described by Schaade et al. (26). Briefly, double-stranded DNA was extracted from 200 μl of plasma by using a Qiagen DNA Minikit according to the manufacturer's protocol (Qiagen, Inc., Mississauga, Ontario, Canada) and eluted from the column with 50 μl of distilled H2O. A forward primer gpB1 (5′-TACCCCTATCGCGTGTGTTC-3′; Tm = 57.1°C) and reverse primer gpB2 (5′-ATAGGAGGCGCCACGTATTCT-3′, Tm = 57.6°C) were expected to yield a 254-bp product from the CMV glycoprotein B gene by PCR amplification (2). The hybridization donor probe (Tm = 62°C) with a fluorescein 3′-end label and the acceptor probe (Tm = 62.5°C) with a LC-Red 640 5′-end label were used for real-time detection during the LC-PCR (26). All primers and probes were synthesized in TIB Molbiol LLC. The sequence and location of the PCR primers and probes are shown in Fig. 1B. A 20-μl portion of the PCR mixture containing 5 μl of DNA solution, 4 mM MgCl2, 0.5 μM concentrations of each primer, 0.2 μM concentrations of each probe, and 2 μl of the reagent from a LC-FastStart DNA Master hybridization probe kit (Roche Diagnostics) was added to the capillaries (Roche Diagnostics). The capillaries were centrifuged, mounted onto the carousel, and loaded into the LightCycler. The thermal cycles were as follows: an initial 10 min at 95°C, followed by 45 cycles of 15 s of denaturing at 95°C, 10 s of annealing at 55°C, and a 10-s extension at 72°C. During the annealing period the data were collected in the single mode, with channel setting F2/F1. For data analysis, the baseline adjustment was done in the arithmetic mode, and the fluorescence curve analysis was done in the fit points mode with two points of the LC software (version 3). Specimens with fluorescence signal higher than the background were considered positive. The specificity of the fluorescence signal was checked by a melting curve analysis after each run. The Tm of the specific probes was 67.5°C.
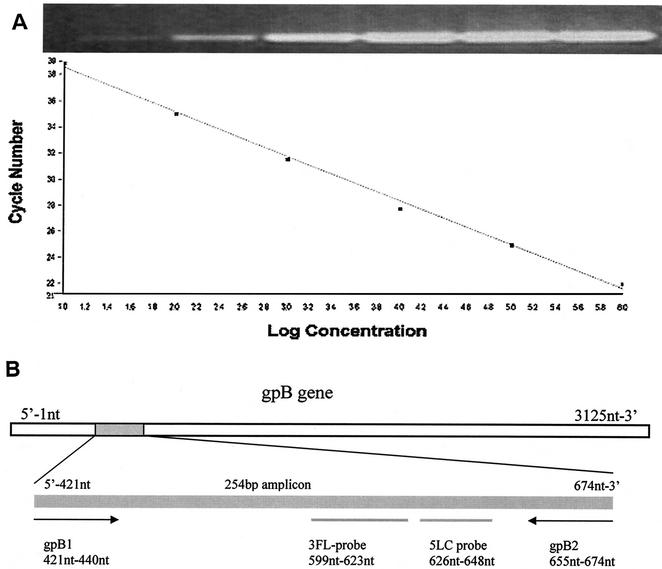
FIG. 1.
(A) Standard curve for CMV LC-PCR. Standard DNA from the CMV glycoprotein B gene, in serial dilutions ranging from 106 to 101 copies, was amplified in the LC-PCR assay. The fluorescence intensity was plotted against cycle numbers. Slope, −3.305; intercept, 41.38; error, 0.132; r, −1. The insert shows the PCR products after agarose gel electrophoresis and ethidium bromide staining. (B) The sequence and location of PCR primers and probes in the CMV glycoprotein B (gpB) gene (accession no. A13758).
To prepare a stock of the positive control, CMV DNA was extracted from known CMV-containing cultured cells. A PCR product (320 bp) was obtained by using a forward primer gpB1 and a reverse primer gpB3 (5′-CCTCCTATAACGCGGCTGTA-3′) from the same region of the CMV glycoprotein B gene (2). The product was analyzed by electrophoresis on a 2% agarose gel and further purified by using a Qiagen PCR purification kit. The purified product was quantified by spectrophotometry, dispensed in aliquots containing 106 genome copies per tube, and stored at −70°C. These aliquots were used to prepare a fresh series of log dilutions (106 to 101 genome copies) in order to establish a positive standard curve for the LC-PCR.
The precision and sensitivity of the LC-PCR were evaluated in five LC-PCR runs carried out on different days with both positive CMV DNA fragments and five known CMV-positive clinical specimens. DNA isolated from cultured cells infected with Epstein-Barr virus (EBV), varicella-zoster virus (VZV), and herpes simplex virus (HSV) was analyzed, with the same primer pairs, in the LC-PCR to define the specificity of the assay. To detect the presence of any inhibitors, 100 copies of CMV DNA were added to negative specimens, as determined by the first run of the LC-PCR, and the LC-PCR was rerun. Negative results obtained in the second run of the LC-PCR would indicate that the specimens or the reactions contained inhibitors.
CACM test.
The CACM test, a quantitative PCR assay, amplifies a 365-bp fragment of the CMV polymerase gene. The PCR was carried out according to the manufacturer's instructions. The amplicors and quantitative standards were detected separately with specific probes by using a semiautomated COBAS system (30). Results were expressed as CMV DNA copies/ml of plasma. The quantitative assay has a lower limit of detection at 400 CMV DNA copies/ml.
Antigenemia assay.
The CMV pp65 antigenemia test was carried out with commercial antibody according to the standard protocol (Clonab CMV; Biotest AG, Dreieich, Germany). Briefly, a suspension of peripheral blood leukocytes was prepared and adjusted to a concentration of 2 × 105 cells per ml. The suspension (0.1 ml) was used to prepare a cell spot on a slide. The slide was fixed in acetone and the CMV antigen was detected by immunofluoresence with a monoclonal antibody against the CMV pp65 antigen. The positive cells were enumerated and reported as the number of positive cells per 2 × 105 leukocytes.
Costs of three assays.
The costs of three assays were measured in terms of procedure requirements for time, reagents and/or kits, and labor.
Statistical methods.
Differences between CMV viral load detected with the three assays were analyzed by using the Pearson chi-square test. Quantitative correlation among the antigenemia assay, the LC-PCR and the CACM assays was calculated by using the Pearson product format correlation. The precision of the LC-PCR for detecting CMV was expressed by the coefficient of variation. The confidence interval (CI) was at 95%, and the significance level was set at P < 0.05.
RESULTS
Evaluation of the LC-PCR assay.
One copy of standardized DNA from cultured cells could be detected in one out of five repeat LC-PCR runs (data not shown), whereas 101, 102, 103, 104, 105, and 106 DNA copies were detected in all five LC-PCR runs (100%). Since the DNA from 20 μl of plasma was used for one PCR, the lower threshold for CMV DNA detection is 250 copies per ml of plasma. The correlation between the noise band crossing points and log DNA copy numbers revealed a good negative linear relationship (r = −1, P < 0.0001) (Fig. 1A). The other viruses tested-EBV, HSV, and VZV-did not cross-react with CMV in the LC-PCR. All negative specimens were tested for PCR inhibition, and none was observed.
To check precision of the assay, standard DNA was diluted to 101, 102, 103, 104, 105, and 106 copies per PCR; the coefficients of variation of the noise band cross-points were 1.07% (CI 0.87 to 1.27%), 1.35% (CI 1.05 to 1.65%), 1.29% (CI 0.98 to 1.60%), 1.9% (CI 1.37 to 2.43%), 2.87% (CI 1.99 to 3.75%), and 2.08% (CI 1.37 to 2.79%), respectively, in five replicate LC-PCR runs. However, in five replicate LC-PCR runs the reproducibility of five clinical samples with unknown copy numbers of CMV DNA showed coefficients of variation of cycle cross-points from 6.55% (CI 2.79 to 10.31%) to 14.1% (CI 11.14 to 17.06%). Therefore, a greater variation was observed with DNA from clinical specimens than with standard DNA fragments.
Detection of CMV by the pp65 antigenemia, the LC-PCR, and the CACM assays.
In a total of 404 plasma samples from 66 patients who underwent solid organ transplantation, 391 specimens were tested by all three of the assay methods—the pp65 antigenemia, LC-PCR, and CACM tests—and the other 13 specimens were assayed by two tests—the pp65 CMV antigenemia and the LC-PCR assays. Positive samples were observed in 21% of the patients tested by using the pp65 antigenemia assay, while the CMV LC-PCR and CACM assays detected positive samples in 38% of patients (25 of 66). Of 404 specimens, 26% (105 of 404) were positive for CMV in the pp65 antigenemia assay. All antigenemia-positive specimens were confirmed to be positive by both the LC-PCR and the CACM assays. Using the LC-PCR assay, 228 of 404 samples were determined to be positive. Of the 224 CMV DNA-positive specimens detected by LC-PCR (4 samples of the 228 were not tested), only 203 were found to be positive (90%) by the CACM assay. None of the specimens determined to be negative by LC-PCR were positive by the CACM assay. The results of these three assays for detection of CMV from clinical specimens are summarized in Table 1.
TABLE 1.
CMV detection by pp65 antigenemia assay and by the CACM and LC-PCR tests
| Assay and result | Antigenemia assay
|
|||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients
|
No. of samples
|
|||||||
| Positive | Negative | Total | Pa | Positive | Negative | Total | Pa | |
| CACM | ||||||||
| Positive | 14 | 11 | 25 | 0.0401 | 105 | 98 | 203 | <0.001 |
| Negative | 0 | 41 | 41 | 0 | 188 | 188 | ||
| Total | 14 | 52 | 66 | 105 | 286 | 391 | ||
| LC-PCR | ||||||||
| Positive | 14 | 11 | 25 | 0.0401 | 105 | 123 | 228 | <0.001 |
| Negative | 0 | 41 | 41 | 0 | 176 | 176 | ||
| Total | 14 | 52 | 66 | 105 | 299 | 404 | ||
As determined by the Pearson chi-square test.
Regression analysis of CMV quantitation by the pp65 antigenemia, the LC-PCR, and the CACM assays.
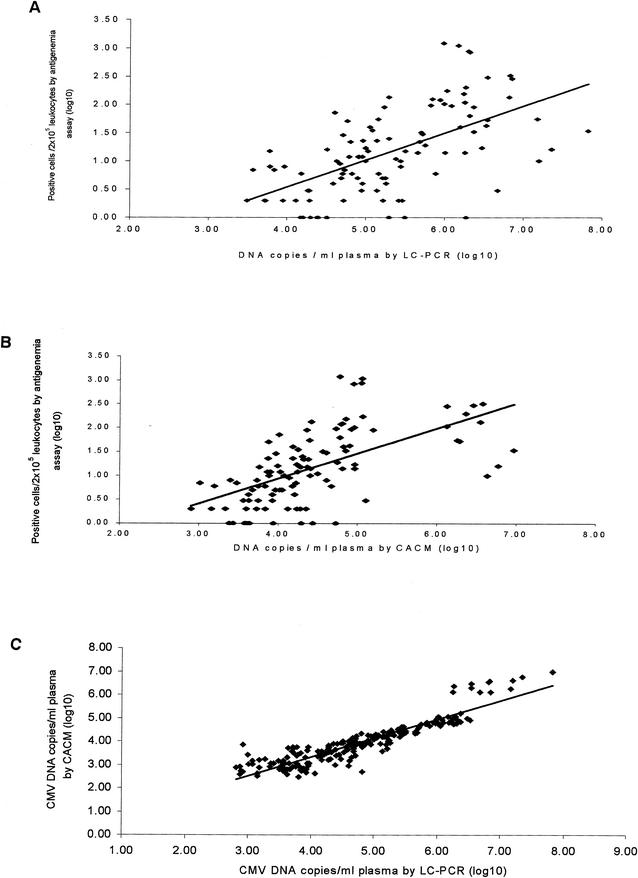
The correlation of CMV quantitation between the three assays was analyzed in 105 positive specimens, and the results are shown in Fig. 2. The number of positive cells per 2 × 105 leukocytes (log mean ± the standard deviation [SD]) in the CMV antigenemia assay and the CMV DNA copy numbers per milliliter of plasma (log mean ± the SD) in the LC-PCR and the CACM assays were 1.17 ± 0.76 (CI 1.03 to 1.31), 5.31 ± 0.93 (CI 5.13 to 5.49), and 4.44 ± 0.87 (CI 4.27 to 4.61), respectively. A significant statistical correlation was observed between the results of the CMV antigenemia and the LC-PCR assays (r = 0.59, P < 0.0001) and also between the results of the CMV antigenemia and the CACM assays (r = 0.60, P < 0.0001). The number of CMV-positive cells in the antigenemia assay and CMV DNA copy number showed similar patterns of variation with time after solid organ transplantation, regardless of the CMV serologic status of the donor and recipient. Results from three representative patients are shown in Fig. 3.
FIG. 2.
Scatter diagrams and regression lines show the correlation between the CMV pp65-positive cell numbers in leukocytes determined by antigenemia assays and the CMV DNA copy numbers in plasma determined by LC-PCR (r = 0.59, P < 0.0001) (A) and CACM (r = 0.60, P < 0.0001) (B) assays. (C) The correlation coefficient (r) of quantitative CMV DNA values between LC-PCR and CACM results was 0.91 (P < 0.001).
FIG. 3.
Fluctuations of CMV viral load after transplantation measured by pp65 antigenemia, LC-PCR and CACM assays in three patients with pretransplant donor (D)-recipient (R) serostatus. The viral load in sequential samples collected was plotted. The values below zero on the y axis indicate negative antigenemia assay results or CMV DNA copy numbers below the threshold of detection of the assays (CACM < 400 copies and LC-PCR < 250 copies/ml of plasma).
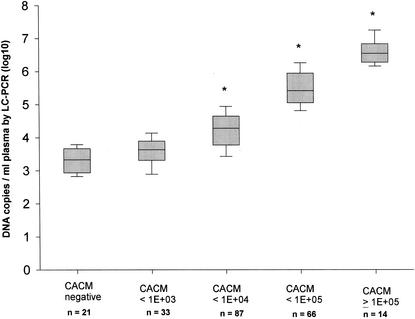
The data shown in this figure also illustrate that the numbers of CMV DNA copies detected by the LC-PCR were higher in most samples than those detected by the CACM assay. CMV DNA was detected in 203 specimens by both the CACM and the LC-PCR assays. The DNA copy number per ml of plasma (log mean ± the SD) was 3.91 ± 0.92 (CI 3.79 to 4.03) in the CACM assay versus 4.73 ± 1.05 (CI 4.59 to 4.87) in the LC-PCR. The results of CMV DNA load generated by LC-PCR were ca. 10-fold greater than those generated by the commercially available CACM test. A very good linear correlation existed between the CACM and the LC-PCR tests (Fig. 2C). To further elucidate the relationship between the CACM and LC-PCR for quantitative detection of CMV DNA, 203 specimens assayed with the CACM were subgrouped according to their CACM results (negative, <103, <104, <105, and ≥105). DNA copy numbers obtained by the LC-PCR for the specimens in each of these subgroups were compared to the results from the CACM. The r value revealed a statistical significance at 0.64, 0.92, and 0.71 for the subgroups with DNA copy numbers, as measured by the CACM assay, of <104, <105 and ≥105 (P < 0.001), respectively, illustrating good correlation at high copy numbers. There was no correlation in the subgroups with DNA copy numbers of <103 (r = 0.18, P > 0.3) (Fig. 4), illustrating much poorer correlation at low copy numbers. The LC-PCR was more sensitive than the CACM assay for detection of CMV; 21 specimens found to be negative by the CACM assay were found to be positive for CMV DNA by using the LC-PCR method.
FIG. 4.
The vertical box shows the correlation of CMV DNA copy numbers between the CACM and LC-PCR assays when the CACM results were subgrouped as follows (negative, <103, <104, <105, and ≥105 DNA copies/ml of plasma). Horizontal lines within the boxes represent the log median. ∗, P < 0.001.
The LC-PCR assay is a one-step procedure and easy to use. The entire CMV viral load procedure by using LC-PCR takes a maximum of 120 min, while the antigenemia and CACM procedures require 180 and 240 min, respectively, for a single test. The costs per sample with the antigenemia, LC-PCR, and CACM tests are $35.8, $39.8, and $104.7 (Canadian dollars), respectively.
DISCUSSION
CMV viral load assessment in the peripheral blood of a solid organ transplant is a potentially powerful tool for use as a surveillance tool, along with preemptive antiviral strategies for the prevention of CMV disease, as a diagnostic tool for the detection of CMV disease and as a monitoring tool for the effectiveness of antiviral treatment. In the present study, we compared three assays—the LC-PCR, CACM, and pp65 antigenemia tests—for detection and quantitation of CMV viral load with respect to sensitivity, specificity, dynamic range, technical required time, and cost in a diagnostic setting. The results generated demonstrated a good correlation between these three methods. The sensitivities of the LC-PCR and CACM assays were superior to that of the antigenemia test (P < 0.001). The LC-PCR method was more sensitive than the CACM method for detection of viral DNA load, especially in patients with lower viral loads. This may be an attractive feature when high-risk patients such as those in the negative-positive, recipient-donor subgroup are undergoing routine posttransplant surveillance. The antigenemia test was least sensitive. This lack of sensitivity may result in false-negative results and delay early diagnosis and initiation of therapy for CMV disease (22, 28). In contrast, the LC-PCR and the CACM assays detected a wide range of viral DNA loads: from 250 to 107 DNA copies per ml of plasma by the LC-PCR and 400 to 105 by the CACM. Even minor fluctuations of viral DNA load within these ranges could be detected. Two characteristics of the LC-PCR and the CACM assays—their sensitivity and wide dynamic range—may provide a considerable advantage in identifying and predicting patients at high risk for the development of CMV disease after transplantation.
The LC-PCR and CACM tests are based on the detection of viral DNA load in plasma by PCR amplification. The target sequences for amplification, however, were designed differently. In comparing the results of CMV DNA load measurements obtained by the LC-PCR and the CACM methods, we found a very good correlation between the results with the LC-PCR protocol developed in our laboratory and the commercially available CACM test widely used in clinical diagnostic laboratories (r = 0.91, P < 0.001). The CMV DNA load was ∼10-fold greater when assayed by using the LC-PCR compared to results obtained by using the CACM in the present study. Schaade et al. reported similar results when the CMV viral load was determined by the CACM assay to be >104 copies per ml of plasma (26). The differences are thought to stem both from the different efficiencies in amplification of DNA fragments located in the glycoprotein B gene for the LC-PCR versus the polymerase gene for the CACM assay and from the different detection strategies. In the LC-PCR test, amplified sequences were detected on a cycle by cycle basis during thermal cycles (real-time PCR), whereas the CACM test determines only the final DNA copy numbers at the end of the analysis (endpoint assay). Therefore, a high DNA load can be detected by the LC-PCR with fewer cycles, since a negative linear correlation exists between viral DNA load and the numbers of amplification cycles required. Additionally, the CACM method cannot properly estimate the viral DNA load when the DNA concentration in original specimens is greater than 105 copies per ml, as demonstrated in the test with calibrated CMV DNA standards in the present study. With the narrower dynamic range of the CACM, dilutions are needed for specimens with high viral DNA load in order to accurately determine the real peak load. Diluting specimens and repeating runs may introduce errors and are time-consuming and expensive. Accurate determination of peak viral load may be useful in the monitoring of response to antiviral therapy. Considering the dynamics of CMV replication in vivo when the viral load may rise quickly, a “frequent follow-up strategy” for CMV load determination may improve the clinical management of patients at high risk for CMV disease. Thus, from a clinical point of view there is a great need for an assay that is time- and cost-effective, that is able to detect a wide range of clinically relevant CMV DNA load, and that is a one-step procedure. The present study demonstrated that the LC-PCR was a one-step assay and that the entire testing procedure with the LC-PCR was faster and cheaper than the CACM assay. Schaade et al. reported that the cost of the supplies required for the commercially available CACM test exceeds that of the supplies required for the LC-PCR by a factor of 20 (26).
Prior to the introduction and validation of PCR techniques for detecting DNA load, the antigenemia test was considered to be the “gold standard” for detecting CMV disease (3, 6, 9). In the present study all specimens testing positive by the pp65 antigenemia assay were confirmed to be positive by the LC-PCR. However, the positive rate for 404 specimens in the LC-PCR test (56%) was double that obtained in the pp65 antigenemia assay (26%). This difference is not unexpected given that the monoclonal antibody only directly detects immediate-early CMV protein in white blood cells infected by CMV, whereas the LC-PCR assay detects and amplifies specific CMV DNA in plasma. We also observed that the fluctuations in CMV load in peripheral blood detected by the antigenemia, the LC-PCR and the CACM assays demonstrated similar patterns with sequential samples in all patients with solid organ transplantation. When CMV was below detectable levels in the antigenemia assay, the CMV DNA load determined by the LC-PCR and the CACM assays were low, although still detectable. This supports our conclusion that the high sensitivity of the LC-PCR assay was not due to false positives or contamination.
We observed a melting point of 67.5°C for the hybridization probes with the PCR amplicons of all of the positive specimens and CMV DNA-positive controls in the present study. This melting point was higher than that previously published by Schaade et al. (27). In that study, two different melting temperatures, 59.2 and 53.1°C, were observed and could be explained by sequence variation of the amplicon where the probe binds. Other factors that could affect melting temperature, such as the primer and probe purification, DNA extraction, and PCR conditions, were similar in Schaade's and our study except for a difference in the annealing temperature (58 and 55°C, respectively). Genetic heterogeneities of CMV glycoprotein B gene from patients with different clinical backgrounds were previously described (12, 34). While all of the patients in our study had solid organ transplantation, Schaade et al. did not specify the clinical histories of their patients. The homogeneous patient population in our study would explain the absence of variation of melting temperature in our data.
Precision of LC-PCR showed a greater coefficient of variation in clinical specimens compared to samples of purified CMV DNA. The reason for this is not clear. In general, it is accepted that DNA isolated from clinical specimens contains some impurities that may inhibit the PCR and signal detection (21).
The specificity of the LC-PCR protocol for detecting CMV load was confirmed in the present study by demonstrating negative results with EBV-, HSV-, and VZV-infected cultured cells. An analysis of the correlation between clinical features of CMV disease and CMV DNA copy numbers in plasma is currently ongoing in order to establish a clinically relevant “cutoff” threshold of the LC-PCR that can be used to predict CMV disease. The present study focused on a comparison of three available techniques for determining human CMV load. Based on the data from the present study, we conclude that the real-time LC-PCR assay developed in our laboratory allows for sensitive detection and an accurate quantitation of CMV DNA load over a large dynamic range (10 to 106 copies). With good sensitivity, specificity, relatively low cost, and a simple procedure, we believe that the real-time LC-PCR assay with its advantages could replace the commercial CACM test for quantitation and monitoring of CMV DNA in the diagnostic virology laboratory.
Acknowledgments
We thank D. L. Tyrrell for the use of the LC-PCR facilities, Roche Diagnostics, Inc. (Canada), for providing us with the CACM assay kits used in this comparative study, and B. Lee for reviewing the manuscript.
REFERENCES
- 1.Aitken, C., W. Barrett-Muir, C. Millar, K. Templeton, J. Thomas, F. Sheridan, D. Jeffries, M. Yaqoob, and J. Breuer. 1999. Use of molecular assays in diagnosis and monitoring of cytomegalovirus disease following renal transplantation. J. Clin. Microbiol. 37:2804-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai, X., G. Hosler, B. B. Rogers, D. B. Dawson, and R. H. Scheuermann. 1997. Quantitation polymerase chain reaction for human herpesvirus diagnosis and measurement of Epstein-Barr virus burden in posttransplant lymphoproliferative disorder. Clin. Chem. 43:1843-1849. [PubMed] [Google Scholar]
- 3.Boeckh, M., R. A. Bowden, J. M. Goodrich, M. Pettinger, and J. D. Meyers. 1992. Cytomegalovirus antigen detection in peripheral blood leukocytes after allogeneic marrow transplantation. Blood 80:1358-1364. [PubMed] [Google Scholar]
- 4.Boeckh, M., and G. Boivin. 1998. Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin. Microbiol. Rev. 11:533-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caliendo, A. M., R. Schuurman, B. Yen-Lieberman, S. A. Spector, J. Andersen, R. Manjiry, C. Crumpacker, N. S. Lurain, and A. Erice. 2001. Comparison of quantitative and qualitative PCR assays for cytomegalovirus DNA in plasma. J. Clin. Microbiol. 39:1334-1338. [DOI] [PMC free article] [PubMed]
- 6.Caliendo, A. M., K. St. George, S. Y. Kao, J. Allega, B. H. Tan, R. LaFontaine, L. Bui, and C. R. Rinaldo. 2000. Comparison of quantitative cytomegalovirus (CMV) PCR in plasma and CMV antigenemia assay: clinical utility of the prototype Amplicor CMV Monitor test in transplant recipients. J. Clin. Microbiol. 38:2122-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crossi, P., S. Kusne, K. St. George, M. Magnone, J. Rakela, J. Fung, and T. E. Starzl. 1996. Guidance of ganciclovir therapy with pp65 antigenemia in cytomegalovirus-free recipients of livers from seropositive donors. Transplantation 61:1659-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emery, V. C., C. A. Sabin, A. V. Cope, D. Gor, A. F. Hassan-Walker, and P. D. Griffiths. 2000. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 355:2032-2036. [DOI] [PubMed] [Google Scholar]
- 9.Gerna, G., D. Zipeto, M. Parea, M. G. Revello, E. Silini, E. Percivalle, M. Zavattoni, P. Grossi, and G. Milanesi. 1991. Monitoring of human cytomegalovirus infections and ganciclovir treatment in heart transplant recipients by determination of viremia, antigenemia, and DNAemia. J. Infect. Dis. 164:488-498. [DOI] [PubMed] [Google Scholar]
- 10.Greenlee, D. J., H. Fan, K. Lawless, C. R. Harrison, and M. L. Gulley. 2002. Quantitation of CMV by real-time PCR in transfusable RBC units. Transfusion 42:403-408. [DOI] [PubMed] [Google Scholar]
- 11.Guiver, M., A. J. Fox, K. Mutton, N. Mogulkoc, and J. Egan. 2001. Evaluation of CMV viral load using TaqMan CMV quantitative PCR and comparison with CMV antigenemia in heart and lung transplant recipients. Transplantation 71:1609-1615. [DOI] [PubMed] [Google Scholar]
- 12.Haberland, M., U. Meyer-König, and F. T. Hufert. 1999. Variation within the glycoprotein B gene of human cytomegalovirus is due to homologous recombination. J. Gen. Virol. 80:1495-1500. [DOI] [PubMed] [Google Scholar]
- 13.Humar, A., D. Kumar, G. Boivin, and A. M. Caliendo. 2002. Cytomegalovirus (CMV) virus load kinetics to predict recurrent disease in solid-organ transplant patients with CMV disease. J. Infect. Dis. 186:829-833. [DOI] [PubMed] [Google Scholar]
- 14.Kearns, A. M., A. J. Turner, and R. Freeman. 2002. Rapid detection and quantification of CMV DNA in urine using LightCycler-based real-time PCR. J. Clin. Virol. 24:131-134. [DOI] [PubMed] [Google Scholar]
- 15.Kizilisik, T. A., J. K. Preiksaitis, and N. M. Kneteman. 1993. Cytomegalovirus disease in liver transplant recipients: impact of acyclovir prophylaxis. Transplant Proc. 25:2282-2283. [PubMed] [Google Scholar]
- 16.Landry, M. L., D. Ferguson, S. Cohen, K. Huber, and P. Wetherill. 1995. Effect of delayed specimen processing on cytomegalovirus antigenemia test results. J. Clin. Microbiol. 33:257-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nitsche, A., N. Steuer, C. A. Schmidt, O. Landt, and W. Siegert. 1999. Different real-time PCR formats compared for the quantitative detection of human cytomegalovirus DNA. Clin. Chem. 45:1932-1937. [PubMed] [Google Scholar]
- 18.Patel, R., T. F. Smith, M. Espy, D. Portela, R. H. Wiesner, R. A. Krom, and C. V. Paya. 1995. A prospective comparison of molecular diagnostic techniques for the early detection of cytomegalovirus in liver transplant recipients. J. Infect. Dis. 171:1010-1014. [DOI] [PubMed] [Google Scholar]
- 19.Pellegrin, I., I. Garrigue, D. Ekouevi, L. Couzi, P. Merville, P. Merel, G. Chene, M. H. Schrive, P. Trimoulet, M. E. Lafon, and H. Fleury. 2000. New molecular assay to predict occurrence of cytomegalovirus disease in renal transplant recipients. Infect. Dis. 25:36-42. [DOI] [PubMed] [Google Scholar]
- 20.Piiparinen, H., K. Hockerstedt, M. Lappalainen, J. Suni, and I. Lautenschlager. 2002. Monitoring of viral load by quantitative plasma PCR during active cytomegalovirus infection of individual liver transplant patients. J. Clin. Microbiol. 40:2945-2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preiser, W., H. F. Rabenau, J. U. Vogel, V. Brixner, and H. W. Doerr. 2002. Performance characteristics of an automated PCR assay for the quantification of cytomegalovirus DNA in plasma. J. Virol. Methods 101:149-157. [DOI] [PubMed] [Google Scholar]
- 22.Razonable, R. R., C. V. Paya, and T. F. Smith. 2002. Role of the laboratory in diagnosis and management of cytomegalovirus infection in hematopietic stem cell and solid-organ transplant recipients. J. Clin. Microbiol. 40:746-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson, W. P., R. B. Colvin, S. H. Cheeseman, N. E. Tolkoff-Rubin, J. T. Herrin, A. B. Cosimi, A. B. Collins, M. S. Hirsch, R. T. McCluskey, P. S. Russell, and R. H. Rubin. 1981. Glomerulopathy associated with cytomegalovirus viremia in renal allografts. N. Engl. J. Med. 305:57-63. [DOI] [PubMed] [Google Scholar]
- 24.Roberts, T. C., D. C. Brennan, R. S. Buller, M. Gaudreault-Keener, M. A. Schnitzler, K. E. Sternhell, K. A. Garlock, G. G. Singer, and G. A. Storch. 1998. Quantitative polymerase chain reaction to predict occurrence of symptomatic cytomegalovirus infection and assess response to ganciclovir therapy in renal transplant recipients. J. Infect. Dis. 178:626-635. [DOI] [PubMed] [Google Scholar]
- 25.Rollag, H., S. Sagedal, K. I. Kristiansen, D. Kvale, E. Holter, M. Degre, and K. P. Nordal. 2002. Cytomegalovirus DNA concentration in plasma predicts development of cytomegalovirus disease in kidney transplant recipients. J. Clin. Microbiol. 8:431-434. [DOI] [PubMed] [Google Scholar]
- 26.Schaade, L., P. Kockelkorn, K. Ritter, and M. Kleines. 2000. Detection of cytomegalovirus DNA in human specimens by LightCycler PCR. J. Clin. Microbiol. 38:4006-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaade, L., P. Kockelkorn, K. Ritter, and M. Kleines. 2001. Detection of cytomegalovirus DNA in human specimens by LightCycler PCR: melting point analysis is mandatory to detect virus strains with point mutations in the target sequence of the hybridization probes. J. Clin. Microbiol. 39:3809. [DOI] [PMC free article] [PubMed]
- 28.Seropian, S., D. Ferguson, E. Salloum, D. Cooper, and M. L. Landry. 1998. Lack of reactivity to CMV pp65 antigenemia testing in a patient with CMV disease following allogeneic bone marrow transplant. Bone Marrow Transplant. 22:507-509. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro, A. M., V. G. Bain, J. K. Preiksaitis, M. M. Ma, S. Issa, and N. M. Kneteman. 2000. Ogilvie's syndrome associated with acute cytomegaloviral infection after liver transplantation. Transplant. Int. 13:41-45. [DOI] [PubMed] [Google Scholar]
- 30.Sia, I. G., J. A. Wilson, M. J. Espy, C. V. Paya, and T. F. Smith. 2000. Evaluation of the COBAS Amplicor CMV Monitor test for detection of viral DNA in specimens taken from patients after liver transplantation. J. Clin. Microbiol. 38:600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stratta, R. J., M. S. Shaeffer, R. S. Markin, R. P. Wood, A. N. Langnas, E. C. Reed, J. P. Donovan, G. L. Woods, K. A. Bradshaw, T. J. Pillen, et al. 1992. Cytomegalovirus infection and disease after liver transplantation: an overview. Dig. Dis. Sci. 37:673-688. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka, N., H. Kimura, K. Iida, Y. Saito, I. Tsuge, A. Yoshimi, T. Matsuyama, and T. Morishima. 2000. Quantitative analysis of cytomegalovirus load using a real-time PCR assay. J. Med. Virol. 60:455-462. [DOI] [PubMed] [Google Scholar]
- 33.Weber, B., U. Nestler, W. Ernst, H. Rabenau, J. Braner, A.Birkenbach, E. H. Scheuermann, W. Schoeppe, and H. W. Doerr. 1994. Low correlation of human cytomegalovirus DNA amplification by polymerase chain reaction with cytomegalovirus disease in organ transplant recipients. J. Med. Virol. 43:187-193. [DOI] [PubMed] [Google Scholar]
- 34.Wirgart, B. Z., M. Brytting, A. Linde, B. Wahren, and L. Grillner. 1998. Sequence variation within three important cytomegalovirus gene regions in isolates from four different patient populations. J. Clin. Microbiol. 36:3662-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yun, Z., I. Lewensohn-Fuchs, P. Ljungman, and A. Vahlne. 2000. Real-time monitoring of cytomegalovirus infections after stem cell transplantation using the TaqMan polymerase chain reaction assays. Transplantation 69:1733-1736. [DOI] [PubMed] [Google Scholar]