Abstract
The first case of Alternaria infectoria ocular infection is reported. Keratitis and endophthalmitis developed after eye-perforating trauma from a lemon tree branch. Two months after surgery and empirical steroid and antibiotic treatment, diagnosis by molecular methods was performed. PCR amplification was positive for a fungus after 4 h. Antifungal treatment with amphotericin B and fluconazole was initiated immediately. DNA sequence analysis showed Alternaria infectoria to be the causal agent. After topical and systemic administration of antifungal treatment, ocular inflammation disappeared and visual acuity improved. DNA typing was found to be a useful tool to achieve early identification of the causal agent.
Alternaria spp., together with other species of the genera Bipolaris, Curvularia, and Exserohilum, have sometimes been involved in human infections (1, 21, 22, 24, 25). These agents are cosmopolitan phaeoid fungi commonly isolated from plants, soil, food, and indoor air environments. At present, their importance as opportunistic pathogens is clearly increasing with the growing number of patients who are immunocompromised (1, 14, 18, 20, 22). The species identification of these fungi is somewhat difficult because of their special growth conditions, subtle morphological differences, and the need for correct interpretation of their morphological features. To obtain necessary conidiation, a week or more may be required for incubation of cultures and use of appropiate sporulation medium (12, 24). Molecular biology can be a useful tool for rapid detection and identification of fungal species involved in opportunistic mycosis (7, 15, 16, 19). Methods based on detection and analysis of the internal transcribed spacer (ITS)-5.8S ribosomal DNA(rDNA) region are currently proving to be a powerful tool for rapid and precise laboratory diagnosis (9, 10). This work reports the efficiency of PCR diagnosis and DNA typing to identify the causal agent in a case of Alternaria infectoria torpid keratitis and its subsequent success in treatment.
Case report.
A 66-year-old agricultural worker attended his local hospital after a perforating trauma in his right eye with a lemon tree branch. Corneal wound closure was performed. Intravitreal vancomycin (1 mg) was injected, and systemic and topical steroids (deflazacort and dexamethasone) and broad-spectrum antibiotics (topical ciprofloxacin, fortified tobramycin, and systemic ciprofloxacin) were given. A posttraumatic cataract together with intraocular inflammation developed, and 20 days later phacoemulsification and three-port vitrectomy were performed. The patient was treated again with systemic steroids (betamethasone) and broad-spectrum antibiotics, including systemic ciprofloxacin and topical tetracycline, with no improvement of his condition. After 20 days and since the eye showed no signs of improvement, the patient came to our center for a second opinion.
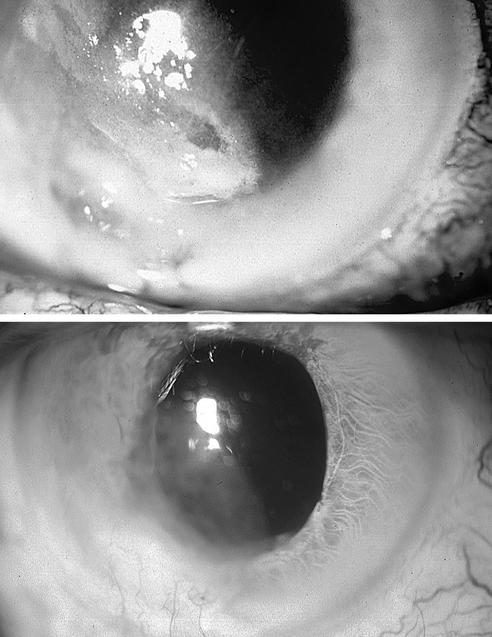
The patient's best corrected visual acuity detected hand movements. Slit lamp examination showed conjunctival hyperemia and corneal leukoma with ulceration and deep stromal infiltrates and an inferior descemetocele (Fig. 1, upper panel). Hypopyon and posterior synechiae were present. Fundus examination showed vitreous haze, and a B scan showed involvement of the posterior segment.
FIG. 1.
(Upper panel) Right eye showing opacification of the cornea due to abscess, with inferior descemetocele, deep stromal infiltrates, and hypopyon; (lower panel) right eye showing the leukomatous cornea with complete resolution of the abscess and descemetocele (6 months after treatment).
On suspicion of an insidious infection process, two corneal samples (scrapings) were taken. PCR was performed on one of them to amplify the ITS-5.8S region of fungal DNA (10). The universal primers used for fungal DNA amplification were ITS1 (5′ TCC GTA GGT GAA CCT GCG G 3′) and ITS4 (5′ TCC TCC GCT TAT TGA TAT GC 3′) (26) (Life Technologies, Barcelona, Spain). The other corneal sample was inoculated into thioglycolate medium and sent to the microbiology laboratory.
Molecular biology results.
The ITS-5.8S rDNA fragment amplification with specific fungal primers was positive, showing a band of about 600 bp. The result was obtained 4 h after the sample was taken. Amplified DNA from the PCR was sequenced and compared with sequences in the BLAST alignment program of the GenBank database (National Institutes of Health) and the EMBL fungal DNA database by using Fasta3 sequence homology searches, which allowed species identification 24 h after the sample was obtained. DNA database comparison of the sequence showed 99.8% identity with A. infectoria strains (STE-U4271, ATCC 12054) when the sequence was compared with any of the databases (EMBL or GenBank). Since, as some authors have inferred, about 14% of the sequences deposited in GenBank are misidentified (6), an A. infectoria type strain (CBS 21086) was also sequenced and compared with our isolate. The homology of the ITS-5.8S rDNA sequences of both strains was 99.5%. The access codes in the GenBank and EMBL database for our isolates are AY168773 and AJ549824, respectively.
Microbiology results.
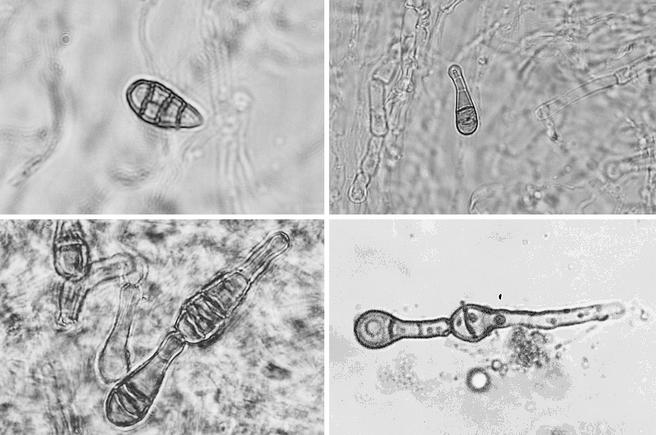
Based on molecular biology-based rapid fungal detection, thioglycolate broth was subcultured on Sabouraud dextrose-chloramphenicol agar and onto malt extract agar and incubated at 25°C. After 6 days of incubation, dark filamentous growth was detected. Subcultures on Sabouraud and malt extract agar were incubated at 30°C and room temperature. After 7 days at 30°C, a black-olivaceous to grey woolly colony developed. Microscopic observation with lactophenol blue stain showed straight or geniculate dark conidiophores. The conidia were mainly obclavate or pyriform, walled with several transverse septa, usually close to each other, and sometimes with longitudinal or oblique septa (Fig. 2, upper panels and lower left panel). Conidia were arranged in chains that tapered gradually to a beak that was always longer than one-third the length of the conidium (Fig. 2, lower panels). The overall conidium was 20 to 30 μm long and 10 to 12 μm thick in the broadest part; the apical beak of the conidium was 9 to 13 μm long. These morphological characteristics confirmed the identification of A. infectoria (8). Morphological identification was carried out by an external mycology laboratory (University Miguel Hernández).
FIG. 2.
Light photomicrographs showing typical conidia of A. infectoria. (Upper left panel) Free pigmented conidium showing transverse and longitudinal septa (magnification, ×400); (upper right panel) free pigmented conidium with a typical beak end (magnification, ×200); (lower panels) conidia in chains showing very close transverse septa and typical beaks (magnification, ×400).
Antifungal treatment was prescribed immediately after the first PCR detected a fungal genome. Topical amphotericin B (one 5-mg/ml drop, six times a day) and oral fluconazole (200 mg/day) were administered the same day the sample was taken. Three days later, an intravitreal injection of a 50-μg/ml concentration of amphotericin B in 0.1 ml was also administered. Intravenous injection of amphotericin B (1 mg/kg of body weight/day, starting with 0.25 mg/kg/day) was recommended to the patient, who accepted the treatment. Intravenous injection was administered for 21 days. Oral fluconazole and topical amphotericin B were maintained for 3 months. At that time, the corneal ulcer was healed, the anterior chamber and vitreous showed no inflammatory activity, and the patient's best corrected visual acuity had improved to 20/80 (Fig. 1, lower panel).
Due to the diversity of fungi that have been reported as opportunistic pathogens, it is imperative that their specific identification be made correctly by an experienced microbiologist or mycologist (21, 22, 24). In the majority of cases of keratitis and endophthalmitis caused by Alternaria species, the specific identification of the etiologic agents was not done (3, 4, 5, 17). Although Alternaria alternata has been described as a causal agent in such cases (2, 11, 23), A. infectoria has not been previously described as causing keratitis.
Application of PCR and molecular methods in this case provided a rapid diagnosis and resulted in the administration of specific and effective therapy, and our results are concordant with conventional phenotypic identification. To our knowledge, the present report describes the fourth known case of human infection caused by A. infectoria (13, 14, 18).
Our results affirm that DNA typing is a useful tool in the diagnosis and treatment of fungal infections, especially those caused by opportunistic filamentous fungi.
Acknowledgments
This work was supported by a grant from Fundación Navarro-Trípodi (Alicante, Spain) and a grant from Instituto Oftalmológico de Alicante (Alicante, Spain).
REFERENCES
- 1.Anaissie, E. J., G. P. Bodey, and M. G. Rinaldi. 1989. Emerging fungal pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 8:323-330. [DOI] [PubMed] [Google Scholar]
- 2.Ando, N., and K. Takatori. 1987. Keratomycosis due to Alternaria alternata corneal transplant infection. Mycopathologia 100:17-22. [DOI] [PubMed] [Google Scholar]
- 3.Arrese, J. E., C. Pierard-Franchimont, and G. E. Pierard. 1996. Onychomycosis and keratomycosis caused by Alternaria sp. A bipolar opportunistic infection in a wood-pulp worker on chronic steroid therapy. Am. J. Dermatopathol. 18:611-613. [DOI] [PubMed] [Google Scholar]
- 4.Azar, P., J. V. Aquavella, and R. S. Smith. 1975. Keratomycosis due to an Alternaria species. Am. J. Ophthalmol. 79:881-882. [DOI] [PubMed] [Google Scholar]
- 5.Chang, S. W., M. W. Tsai, and F. R. Hu. 1994. Deep Alternaria keratomycosis with intraocular extension. Am. J. Ophthalmol. 117:544-545. [DOI] [PubMed] [Google Scholar]
- 6.de Hoog, G. S., and R. Horre. 2002. Molecular taxonomy of the Alternaria and Ulocladium species from humans and their identification in the routine laboratory. Mycoses 45:259-276. [DOI] [PubMed] [Google Scholar]
- 7.Einsele, H., H. Hebart, G. Roller, J. Loffler, I. Rothenhofer, C. A. Muller, R. A. Bowden, J. van Burik, D. Engelhard, L. Kanz, and U. Schumacher. 1997. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 35:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis, M. B. 1971. Dematiaceous hyphomycetes, p. 468-469. CABI Publishing, New York, N.Y.
- 9.Esteve-Zarzoso, B., C. Belloch, F. Uruburu, and A. Querol. 1999. Identification of yeast by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol. 49:329-337. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer, C., F. Colom, S. Frases, M. E. Mulet, J. L. Abad, and J. L. Alió. 2001. Detection and identification of fungal pathogens by PCR and by ITS2 and 5.8S ribosomal DNA typing in ocular infections. J. Clin. Microbiol. 39:2873-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer, C., G. Munoz, J. L. Alio, J. L. Abad, and F. Colom. 2002. Polymerase chain reaction diagnosis in fungal keratitis caused by Alternaria alternata. Am. J. Ophthalmol. 133:398-399. [DOI] [PubMed] [Google Scholar]
- 12.Fothergill, A. W. 1996. Identification of dematiaceous fungi and their role in human disease. Clin. Infect. Dis. 22:S179-S184. [DOI] [PubMed] [Google Scholar]
- 13.Gerdsen, R., M. Uerlich, G. S. De Hoog, T. Bieber, and R. Horre. 2001. Sporotrichoid phaeohyphomycosis due to Alternaria infectoria. Br. J. Dermatol. 145:484-486. [DOI] [PubMed] [Google Scholar]
- 14.Halaby, T., H. Boots, A. Vermeulen, A. van der Ven, H. Beguin, H. van Hooff, and J. Jacobs. 2001. Phaeohyphomycosis caused by Alternaria infectoria in a renal transplant recipient. J. Clin. Microbiol. 39:1952-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes, K. A., T. J. Westerneng, J. W. Fell, and W. Moens. 1995. Rapid detection and identification of pathogenic fungi by polymerase chain reaction amplification of large subunit ribosomal DNA. J. Med. Vet. Mycol. 33:319-325. [DOI] [PubMed] [Google Scholar]
- 16.Jaeger, E. E., N. Carroll, S. Choudhury, A. A. Dunlop, H. M. Towlern, M. M. Matheson, P. Adamson, N. Okhravi, and S. Lightman. 2000. Rapid detection and identification of Candida, Aspergillus, and Fusarium species in ocular samples using nested PCR. J. Clin. Microbiol. 38:2902-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koc, A. N., K. Erkilic, N. Evrensel, and A. Coskun. 1997. A case of Alternaria keratitis treated with fluconazole. Eur. J. Clin. Microbiol. Infect. Dis. 16:322-323. [DOI] [PubMed] [Google Scholar]
- 18.Laumaillé, C., F. Le Gall, B. Degeilh, E. Gueho, and M. Huerre. 1998. Cutaneous Alternaria infectoria infection after liver transplantation. Ann. Pathol. 18:192-194. [PubMed] [Google Scholar]
- 19.Makimura, K., S. Y. Murayama, and H. Yamaguchi. 1994. Detection of a wide range of medically important fungi by the polymerase chain reaction. J. Med. Microbiol. 33:358-364. [DOI] [PubMed] [Google Scholar]
- 20.Morrison, V. A., R. J. Haake, and D. J. Weisdorf. 1993. The spectrum of non-Candida fungal infections following bone marrow transplantation. Medicine 72:78-89. [DOI] [PubMed] [Google Scholar]
- 21.Pritchard, R. C., and D. B. Muir. 1987. Black fungi: a survey of dematiaceous hyphomycetes from clinical specimens identified over a five year period in a reference laboratory. Pathology 19:281-284. [DOI] [PubMed] [Google Scholar]
- 22.Rees, J. R., R. W. Pinner, R. A. Hajjeh, M. E. Brandt, and A. L. Reingold. 1998. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992-1993: results of population-based laboratory active surveillance. Clin. Infect. Dis. 27:1138-1147. [PubMed] [Google Scholar]
- 23.Rummelt, V., K. W. Ruprecht, H. J. Boltze, and G. O. Naumann. 1991. Chronic Alternaria alternata endophthalmitis following intraocular lens implantation. Arch. Ophthalmol. 109:178. [DOI] [PubMed] [Google Scholar]
- 24.Schell, W. A. 1995. New aspects of emerging fungal pathogens. Clin. Lab. Med. 15:365-387. [PubMed] [Google Scholar]
- 25.Vartivarian, S. H., E. L. Anaissie, and G. P. Bodey. 1993. Emerging fungal pathogens in immunocompromised patients: classification, diagnosis, and management. Clin. Infect. Dis. 17:S487-S491. [DOI] [PubMed] [Google Scholar]
- 26.White, T. J., T. Bruns, S. Lee, and S. Tailor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innins, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, San Diego, Calif.