Abstract
Recurrence of Clostridium difficile-associated diarrhea (CDAD) occurs in 15 to 20% of patients after discontinuation of treatment. Arbitrarily primed PCR was used to investigate the epidemiology of recurrent CDAD in 18 patients. Reinfection with a new strain occurred in 6 of 18 patients (33.3%), while 12 patients relapsed with the original strain shortly after discontinuation of treatment. These data suggest that reinfection with exogenous C. difficile is a common problem and that not all recurrences are due to relapse.
Clostridium difficile-associated diarrhea (CDAD) is the most common hospital-acquired infectious diarrhea. Treatment of CDAD is primarily with metronidazole or vancomycin; however, between 15 and 20% of patients have recurrences following discontinuation of therapy (10). It is believed that these are relapses due to persistence of spores in the gut after treatment (2). The possibility of recurrent disease due to environmental contamination and reinfection was considered early in 1980 (11). Only a limited number of studies have been performed that investigate the rate of reinfection in C. difficile infections. In a study reported by Johnson et al., 45% (5 of 11) of patients were reinfected with a new strain (7). O'Neill et al. found a 75% reinfection rate among eight inpatients and two outpatients analyzed by restriction endonuclease analysis (8). The goal of this study was to further investigate the epidemiology of recurrences of C. difficile infection by determining the rates of true relapses versus reinfections.
A total of 18 patients with a history of CDAD recurrences were analyzed in this study. All patients were diagnosed as having CDAD by the cytotoxicity assay. Multiple isolates (at least two) were obtained from each patient. Twelve of these patients were inpatients at the University of California, Davis Medical Center. Three were outpatients at University of California, Davis Medical Center, and three were outpatients at the University of Michigan Medical Center, Ann Arbor.
Stock cultures from patients were inoculated on cycloserine-cefoxitin-fructose agar plates (6) and were grown anaerobically at 37°C for 48 h. DNA was extracted from a single colony from each plate as described elsewhere (9). Arbitrarily primed PCR that relies on the T7 oligonucleotide was used to identify different strains (9). This method has been used before and has been validated to other typing methods (3, 4, 9). Amplification products were run by electrophoresis on 1.5% agarose gels (Gibco BRL, Grand Island, N.Y.). Gels were stained with ethidium bromide (0.5 μg/ml), destained, and analyzed visually by using the Quantity One software package with the Molecular Analyst Fingerprinting software (Bio-Rad, Hercules, Calif.).
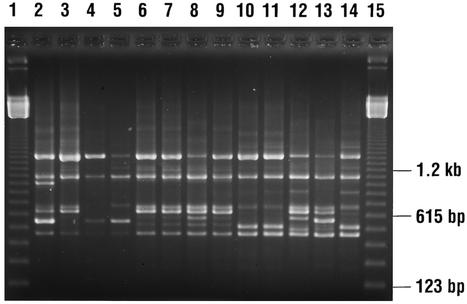
Six of the eighteen recurrences (33.3%) were due to reinfection with exogenous C. difficile strains, while 12 of 18 (66.7%) were true relapses. In the inpatient population, 4 of 12 (33.3%) CDAD cases were due to reinfection and 8 of 12 (66.6%) were relapses. In the outpatient population, 33.3% (two of six) of patients were found to have reinfection and 66% (four of six) relapsed approximately 1 to 7 months afterward (Table 1). Figure 1 shows the DNA-banding patterns of selected isolates representing reinfection or true relapses.
TABLE 1.
Epidemiology of recurrent C. difficile-associated diarrhea
| Patient population | No. of patients | Time range between recurrences |
|---|---|---|
| Inpatients | 12 | 2-5 wk |
| With relapse | 8 | |
| With reinfection | 4 | |
| Outpatients | 6 | 1-7 mo |
| With relapse | 4 | |
| With reinfection | 2 |
FIG. 1.
DNA-banding patterns of selected C. difficile isolates from patients with recurrent CDAD. Lanes 1 and 15, 123-bp DNA ladder; lanes 2 to 5 and lanes 12 to 14, DNA-banding patterns of isolates from three patients (lanes 2 and 3, lanes 4 and 5, and lanes 12 to 14) with recurrent CDAD due to reinfection; and lanes 6 to 11, DNA fingerprints of two patients (lanes 6 to 9 and lanes 10 and 11) with relapsing CDAD.
Reinfection with C. difficile occurred both in inpatient and outpatient settings. True relapses were common in inpatients whose recurrent CDAD was diagnosed shortly after discontinuation of treatment. This observation suggests the persistence of spores in the gastrointestinal tract of these patients. One outpatient from whom four isolates were obtained was reinfected with multiple strains during a 7-month period. It is possible that these outpatients are colonized by multiple isolates even when they are asymptomatic or that they acquire new strains from environmental sources. Our results are in agreement with those of others who have investigated the nature of relapses. The rate of recurrences varies in reports from 5 to 66%, with a rate of 20% considered average (5, 7). Johnson et al. and O'Neill et al. found that 45 to 60% of recurrences were due to reinfection with new strains (7, 8). Most of these studies have been done on inpatients. A study of CDAD recurrences in human immunodeficiency virus-infected patients found that 64% of the recurrences were due to relapses, while 32% were reinfections and 4% were a combination of relapse and reinfection (1). In a number of studies, an association has been established between the persistence of the organism and relapses. The finding that 66.6% of inpatients had a true relapse shortly after discontinuation of treatment may be explained as incomplete eradication of the organism and its persistence in spore form.
In summary, our results show that reinfection with exogenous C. difficile is a common problem and that not all recurrent CDAD is due to true relapses.
Acknowledgments
We thank F. Robert Fekety for providing some of the isolates used in this study (F. Robert Fekety, C. difficile Culture Collection, Ann Arbor, Michigan).
REFERENCES
- 1.Alonso, R., S. Gros, T. Pelaez, D. Garcia-de-Viedma, M. Rodriguez-Creixems, and E. Bouza. 2001. Molecular analysis of relapse vs. re-infection in HIV-positive patients suffering from recurrent Clostridium difficile associated diarrhoea. J. Hosp. Infect. 48:86-92. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, J. G., F. J. Tedesco, S. Shull, et al. 1980. Symptomatic relapse after oral vancomycin therapy of antibiotic-associated pseudomembranous colitis. Gastroenterology 78:431-434. [PubMed] [Google Scholar]
- 3.Brazier, J. S. 2001. Typing of Clostridium difficile. Clin. Microbiol. Infect. 7:428-431. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, S. H., Y. J. Tang, and J. Silva, Jr. 2001. Molecular typing methods for the epidemiological identification of Clostridium difficile strains. Expert Rev. Mol. Diagn. 1:61-70. [DOI] [PubMed] [Google Scholar]
- 5.Fekety, R., L. V. McFarland, C. M. Surawicz, R. N. Greenberg, G. W. Elmer, and M. E. Mulligan. 1997. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin. Infect. Dis. 24:324-333. [DOI] [PubMed] [Google Scholar]
- 6.George, W. L., V. L. Sutter, D. Citron, and S. M. Finegold. 1979. Selective and differential medium for isolation of Clostridium difficile. J. Clin. Microbiol. 9:214-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson, S., A. Adelmann, C. R. Clabots, L. R. Peterson, and D. N. Gerding. 1989. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J. Infect. Dis. 159:340-343. [DOI] [PubMed] [Google Scholar]
- 8.O'Neill, G. L., M. H. Beaman, and T. V. Riley. 1991. Relapse versus reinfection with Clostridium difficile. Epidemiol. Infect. 107:627-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang, Y. J., S. T. Houston, P. H. Gumerlock, M. E. Mulligan, D. N. Gerding, S. Johnson, F. R. Fekety, and J. Silva, Jr. 1995. Comparison of arbitrarily primed PCR with restriction endonuclease and immunoblot analyses for typing Clostridium difficile isolates. J. Clin. Microbiol. 33:3169-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilcox, M. H., and R. C. Spencer. 1992. Clostridium difficile infection: responses, relapses, and re-infections. J. Hosp. Infect. 22:85-92. [DOI] [PubMed] [Google Scholar]
- 11.Young, G. P., N. Bayley, P. Ward, D. J. St. John, and M. I. McDonald. 1986. Antibiotic-associated colitis caused by Clostridium difficile: relapse and risk factors. Med. J. Aust. 144:303-306. [DOI] [PubMed] [Google Scholar]