Abstract
Pulsed-field gel electrophoresis (PFGE) is considered the “gold standard” for molecular typing of methicillin-resistant Staphylococcus aureus (MRSA). However, the method is time-consuming and expensive, and its discriminatory power may not be necessary in outbreak situations. We used a rapid multiplex PCR-based method with published primers and compared the results with those obtained by PFGE. A total of 75 clinical isolates were typed: 59 strains originated from our prospectively collected clinical strains and were epidemiologically unrelated; 16 strains came from an outbreak that was epidemiologically well defined in time and space. A primer mix of the spa gene, the coa gene, and the hypervariable region adjacent to mecA gene was used for multiplex PCR. Both PFGE and PCR clustered the 75 strains into 41 different genotypes. Concordance of the results was 100% for strains originating from the outbreak. Overall, both methods produced concordant results in 72% of cases. A total of 16% were clustered together by PFGE, but not by PCR and 12% were clustered together by PCR but not by PFGE, respectively. The turnaround time was only 8 h for PCR but 5 days for PFGE. This PCR-based method is excellent for rapid and inexpensive typing of MRSA in an outbreak setting, but the discriminatory power and reproducibility are still insufficient to replace PFGE in longitudinal studies in the endemic setting.
Nosocomial infections caused by methicillin-resistant strains of Staphylococcus aureus (MRSA) belong to the most important multiresistant pathogens worldwide (5). Monitoring and limiting the intra- and interhospital spread of MRSA strains requires the use of rapid and accurate epidemiologic typing systems. The emergence of recently documented vancomycin-resistant S. aureus (VRSA) amplifies the need for control of MRSA and VRSA (2). Some MRSA strains, epidemic MRSA, have been observed to disseminate quickly (7). Their prompt identification is crucial to control and eradicate an outbreak, while saving resources for unnecessary isolations of patients unrelated to an outbreak. A large number of molecular methods have been developed for typing MRSA strains. DNA fingerprinting by pulsed-field gel electrophoresis (PFGE) is considered to be one of the most reliable, discriminatory, and reproducible typing procedures to allow the detection of high degree DNA polymorphism (8, 10, 14, 21-23), but it is technically demanding, time-consuming, and expensive. In an outbreak setting a rapid typing tool should allow one to cluster epidemiologically linked cases and separate them from sporadic cases.
The aim of the present study was to adapt a rapid PCR method to investigate MRSA strains in an outbreak setting. We compared PCR results from epidemiologically well-defined unrelated strains and outbreak strains with the results of PFGE.
MATERIALS AND METHODS
Bacterial isolates.
A total of 75 MRSA isolates were investigated. Of these, 59 isolates originated from our prospectively collected epidemiologically unrelated clinical isolates; no association was found in terms of time and location. They were collected between 2000 and 2002. In February 2002, 16 isolates came from an outbreak on a single ward. Classification of the cases was done by our prospective MRSA surveillance system (5). All strains were stored at −70°C.
Identification of MRSA.
Colonies suspected to be S. aureus were screened with the latex agglutination test Pastorex Staph Plus (Bio-Rad) and confirmed as such with Gram stain, catalase, and aurease (Rapidec Staph; bioMérieux). Resistance to oxacillin was determined according to the NCCLS guidelines by using an oxacillin screen agar (MRSA screen agar; bioMérieux) and confirmed by detection of penicillin-binding protein 2a (MRSA-Screen; Denka Seiken). Any discrepant results were resolved by PCR testing with primers targeting femA and mecA gene.
PFGE.
The procedure was performed as described by Pfaller et al. (13). Briefly, a washed bacterial suspension was mixed with 2% SeaPlaque GTG agarose (FMC BioProducts) at 58°C and allowed to solidify into plug molds at room temperature. Chromosomal DNA was prepared in several incubating and washing steps by using EC buffer (6 mM Tris-HCl, 1 M NaCl, 0.1 M EDTA, 0.5% Brij-58, 0.2% deoxycholate, 0.5% Sarkosyl), lysostaphin, and proteinase K. After digestion of the genomic DNA by SmaI (New England Biolabs), the restriction fragments were separated by PFGE by using a temperature-controlled CHEF DR III system (Bio-Rad) with the following conditions: 6.0 V/cm for 24 h at switch times ramped from 5 to 60 s. After staining with ethidium bromide, the fragments were visualized by using a UV transilluminator and then documented by using a video gel documentation system (MWG-Biotech). For PFGE pattern analysis, the software GelCompar (Applied Maths) was applied. The dendrograms were calculated by the unweighted pair group method by using arithmetic averages. Isolates were considered identical and closely related when there were 0 and ≤2 banding differences, respectively. PFGE typing has routinely been performed in our laboratory since 1992.
PCR.
DNA was isolated as previously described (24). One loopful of S. aureus cells was harvested from agar plates. Cells were resuspended in 50 μl of lysostaphin (Sigma Chemical) at 100 μg/ml and incubated for 10 min at 37°C. Then, 50 μg of proteinase K solution/ml and 150 μl of 0.1 M Tris buffer (pH 7.5) were added, followed by incubation for 10 min at 37°C and then 5 min at 95°C. DNA was stored at −20°C until reuse.
A PCR was applied to simultaneously amplify a part of the hypervariable region (HVR) adjacent to the mecA gene, a part of the spa gene coding for protein A, and a part of the coa gene coding for coagulase, based on established primers (25). The PCR was performed with 2 μl of the previously prepared cell lysates, with HotStarTaq (Qiagen) in a final volume of 25 μl. The primer mix contained 170 pmol of HVR1 and HVR2, 3.6 pmol of SPA1 and SPA2, and 6.0 pmol of COA1 and COA2. Cycling was performed in the GeneAmp PCR system 9600 (Perkin-Elmer). Samples were denaturated at 95°C for 15 min, followed by 35 cycles with the following parameters: denaturation at 94°C for 1 min, annealing at 56°C for 1 min, and extension at 72°C for 3 min, with the final extension at 72°C for 5 min. With each run strain number 84 was amplified as an internal control. Next, 8 μl of each amplicon was electrophoresed in a 2% agarose gel (NuSieve3:1; BioWhittaker) with a subsequent ethidium bromide staining and visualized under UV light. A 100- to 1,500-bp ladder (Roche Applied Sciences) was used as a molecular weight marker. Images of gel lanes were normalized, and dendrograms prepared by using GelCompar software were printed. The interpretation criterion for identifying different strains was a single band difference (25). To evaluate the reproducibility, PCR was repeated with all of the strains.
Comparison and cost.
Correlation of banding between PFGE and PCR was done independently by two investigators blinded to the results of the epidemiological investigations. The material costs for PCR and PFGE were estimated based on data from accounting, the real cost of the disposable, and the cost of labor. Costs for equipment and resources usually available in microbiology laboratories were not included.
RESULTS
Comparison of PCR with PFGE.
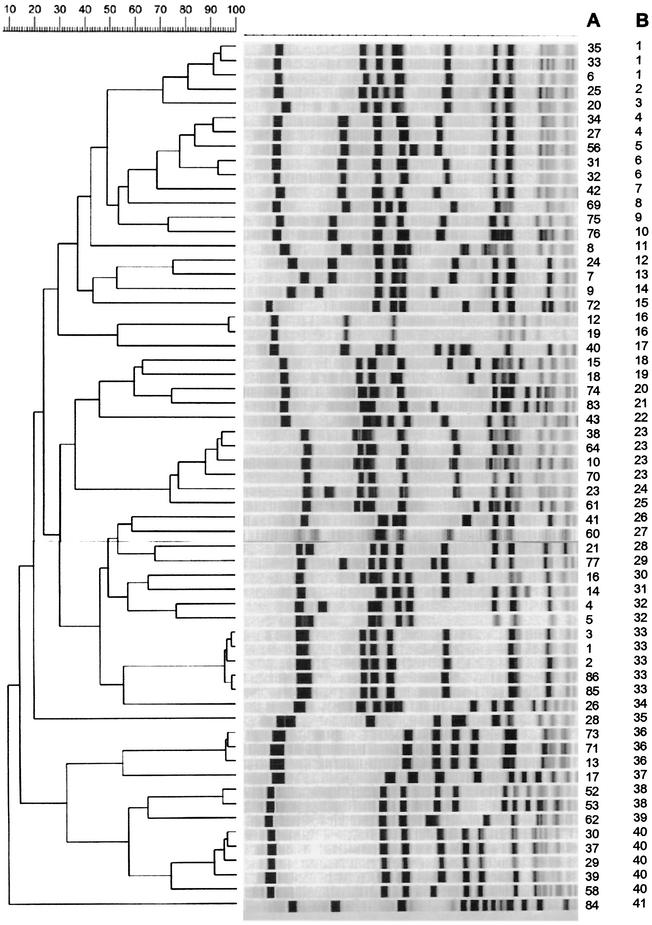
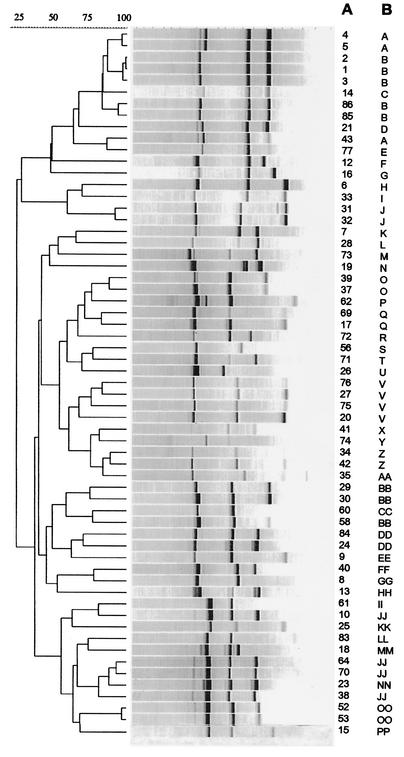
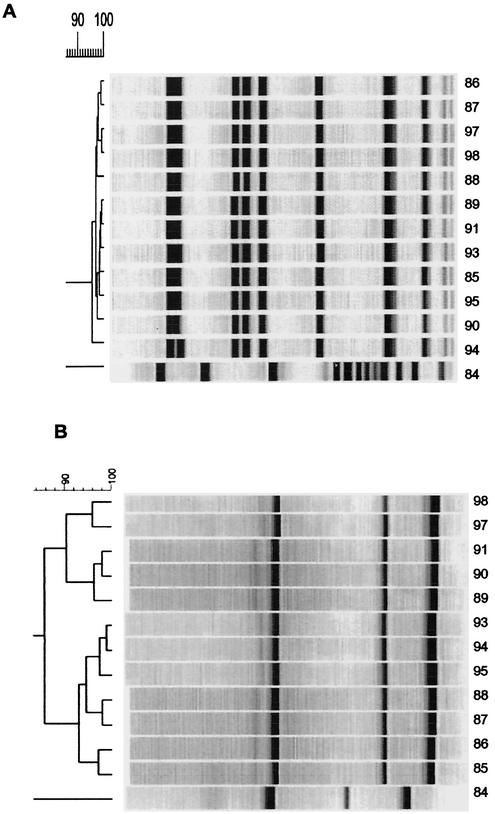
PFGE grouped the 75 isolates into 41 types (Fig. 1), the first PCR run grouped the 75 isolates into 41 types (Fig. 2), and the second PCR run grouped the 75 isolates into 35 types. The concordance for the 16 isolates from the outbreak was 100% by PFGE versus PCR (first or second run) (Fig. 3).
FIG. 1.
Dendrogram of SmaI-PFGE of epidemiologically unrelated strains. Strain numbers are indicated in column A; PFGE types are indicated in column B.
FIG. 2.
Dendrogram of PCR of epidemiologically unrelated strains. Strain numbers are indicated in column A; PCR types are indicated in column B.
FIG. 3.
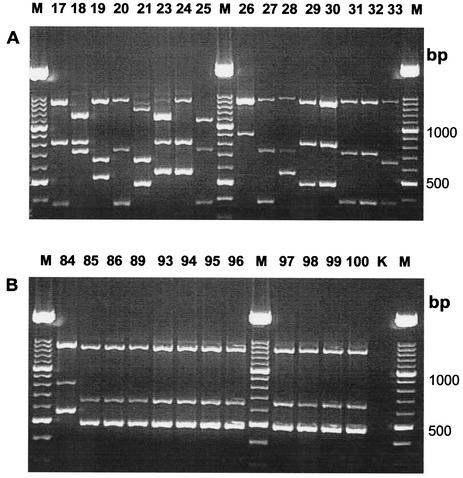
Dendrogram of SmaI-PFGE (A) and PCR results (B) from epidemic strains. Strain numbers are indicated on the right side. Strain 84 is the unrelated control.
PFGE and the first run produced concordant results in 72% of the overall strains, PFGE and the second run produced concordant results in 76.7% of the overall strains (Table 1). In the first run 16% were clustered together by PFGE but not by PCR, and in the second one 8% were clustered together by PFGE but not by PCR. A total of 12% of the isolates were clustered together by PCR but not by PFGE in the first run versus a total of 15% clustered by the second run. In the first PCR run all isolates were typeable; in the second PCR run two strains (2.6%) were classified as not typeable, although only the band corresponding to the spa gene could be detected.
TABLE 1.
Comparison of PCR versus PFGE typing resultsa
| PCR round and result | No of strains tested by PFGE
|
|
|---|---|---|
| Positive | Negative | |
| First | ||
| Positive | 32 | 9 |
| Negative | 12 | 22 |
| Second | ||
| Positive | 35 | 11 |
| Negative | 6 | 21 |
Strains typed by both PFGE and PCR (+/+ strains) were clustered together by PFGE and PCR. Strains typed by neither method (−/− strains) were unrelated by PFGE and PCR. The +/− or −/+ strains were clustered together by PFGE but not by PCR or by PCR but not by PFGE as indicated. In the PCR second run, two strains were not typeable.
Reproducibility of the described PCR method.
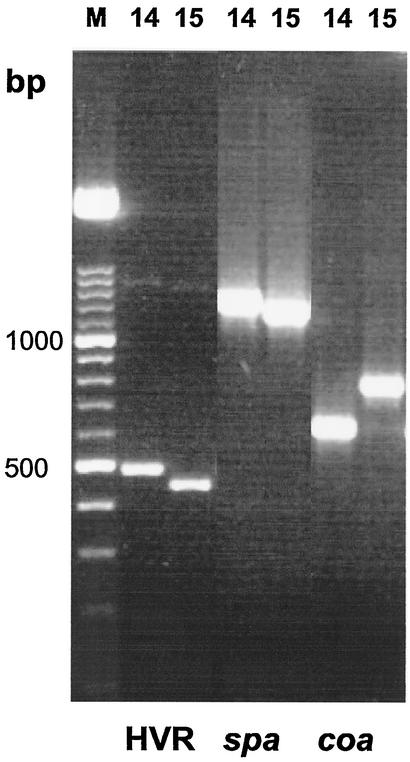
The concordance for the 16 isolates from the outbreak was 100% in both PCR runs. The two PCR runs produced concordant results in 72%, including both nonepidemic and epidemic strains. A total of 12% were clustered together by the first run but not by the second run, and 13.3% were clustered together by the second run but not by the first run. Figure 4 shows the PCR products for each primer. The first band, located between 1,200 and 1,500 bp, corresponded to the spa gene and could be detected in all strains. The second band, located between 600 and 900 bp, corresponded to the coa gene and could be detected in all strains in the first run but was absent in two strains in the second run. The third band, located between 300 and 600 bp, corresponded to the HVR and was absent in 8 of 75 strains in the first run and in 18 strains in the second run. The intensity of HVR expression may vary between different runs. With each run, strain number 84 was amplified as an internal control and showed constant amplification fragment sizes in all reactions. Figure 5 shows the PCR products for primer mix for clinical unrelated and epidemic strains.
FIG. 4.
Agarose gel electrophoresis of PCR-amplified HVR adjacent to the mecA, spa, and coa genes. Strain numbers are indicated on the top of the lanes. Lane M, size marker. Molecular sizes are indicated at the left in base pairs.
FIG. 5.
Agarose gel electrophoresis of PCR products of clinical unrelated isolates (A) and epidemic strains (B), with strain 84 as a positive amplification control. K, negative control. Strain numbers are indicated at the top of the lanes. Lane M, molecular size marker.
Cost and labor.
By PCR DNA extraction, amplification and agarose gel electrophoresis were performed within 8 h, with a hands-on time of 2.5 h for a 17-sample batch. The material cost was estimated to be U.S. $2.5 per sample. PFGE was performed in 1 week, with a hands-on time of 9.5 h for a 17-sample batch. The material cost was estimated to be U.S. $6.0 per sample. In contrast to PCR, the hands-on time for PFGE is similar for 2 or 17 strains.
DISCUSSION
The PCR method with published primers described here proved to be reliable in an outbreak setting. It allows rapid separation of sporadic and epidemic cases. This method complements PFGE, which can be delayed until a dozen strains have been collected. Early detection of outbreaks is crucial to allocate resources in infection control (1). In the United States, MRSA has become endemic, and institutions frequently have several MRSA patients on their wards. Expensive interventions are warranted if the isolated strains are identical, thus providing strong evidence for an outbreak. In contrast, resources, such as those used in the screening of health care workers, are wasted if there is only a coincidence of multiple MRSA patients.
PFGE is considered the gold standard technique for MRSA typing due to its high discriminatory power, its excellent reproducibility, and its good correlation with epidemiologically linked data (11, 13, 14, 17, 21-23). However, PFGE is a slow and time-consuming procedure that requires specifically trained personnel and sophisticated equipment. It also fails to provide results in a timely fashion. Faster molecular typing approaches have been published over the last decade; most of these are based on DNA amplification by PCR (3, 4, 6, 7, 10, 12, 18, 19). The PCR method used in the present study is quick, inexpensive, and reliable in an outbreak setting and uses established primers (25). In such situations, rapid and reliable results are more important than an excellent discriminatory power that allows longitudinal studies on relatedness of strains over long time periods. Both methods gave 41 different patterns. However, PCR grouped the same strains in 35 patterns in the second run, indicating limited reproducibility of the PCR method. We used the primer mix of the spa gene, the coa gene, and the HVR adjacent to mecA gene for multiplex PCR. All three gene targets are known to be heterogeneous. The spa gene encoding protein A and the coa gene encoding coagulase contain repeat units and are highly polymorphic with regard to the number and sequence of repeats (6, 16, 20). The DNA sequence between mecA and IS431 consists of direct repeat units and is hypervariable due to its length polymorphism (12, 15). The variability in the spa gene has been considered as good marker for short-term epidemiological studies during nosocomial outbreaks, being able to distinguish between the epidemic and sporadic strains (7). The slower rate of change associated with coa gene is more appropriate for answering questions of global epidemiology (19). The reproducibility of the spa gene was 100%, that of the coa gene was 97%, and that of the mecA HVR varied between 76 and 89% in the present study. In the nonepidemic setting, the differences in concordance of the two typing systems are not surprising: the mutation rate (including point mutations), genetic rearrangements, and horizontal transfers of mobile DNA elements strongly influence the stability of typing patterns in longitudinal studies. Therefore, PCR is more prone to variable results over long time periods. PFGE is more appropriate in this setting. Deplano et al. (3) reported inter-IS256 PCR typing that achieved intracenter reproducibility and discrimination similar to those of PFGE. However, we could not confirm these good results for this PCR typing method (data not shown). Moreover, Deplano et al. also noted limited discriminatory power for strains originating from the United States. At this time, the method appears not yet suitable for a clinical microbiology laboratory.
A few limitations of this typing technique deserve consideration. (i) The reproducibility of 72% is less than optimal, although it is in a range concordant with previously published data (3, 4). (ii) Multiplex PCR is more prone to variable PCR products and may explain the unstable band corresponding to HVR. In fact, all but one banding pattern difference in the second run was due to changes in HVR. (iii) The study here was performed in a single laboratory, which facilitates reproducibility of the results.
This PCR method turned out to be significantly less expensive than PFGE, as also reported in a study by Montesinos et al. (9).
In conclusion, the PCR-based method described here is excellent for rapid and inexpensive typing of MRSA in an outbreak setting. In addition, it provides reasonable results for epidemiologically unrelated strains. PFGE still provides more information due to its high discriminatory power, established interpretation criteria, and intra- and interlaboratory reproducibility (23). However, the PCR-based method presented here can assist in an outbreak investigation and help to guide infection control interventions long before PFGE results become available. These results provide additional evidence that PCR-based methods can complement PFGE but cannot yet replace PFGE for MRSA typing in longitudinal studies.
Acknowledgments
We are grateful for the technical support of E. Schultheiss and for critical reading of the manuscript by J. Pettypool.
REFERENCES
- 1.Boyce, J. M. 1995. Strategies for controlling methicillin-resistant Staphylococcus aureus in hospitals. J. Chemother. 7(Suppl. 3):81-85. [PubMed] [Google Scholar]
- 2.Centers for Disease Control. 2002. Staphylococcus aureus resistant to vancomycin—United States. JAMA 288:824-825. [PubMed] [Google Scholar]
- 3.Deplano, A., A. Schuermans, J. Van Eldere, W. Witte, H. Meugnier, J. Etienne, H. Grundmann, D. Jonas, G. T. Noordhoek, J. Dijkstra, A. van Belkum, W. van Leeuwen, P. T. Tassios, N. J. Legakis, A. van der Zee, A. Bergmans, D. S. Blanc, F. C. Tenover, B. C. Cookson, G. O'Neil, M. J. Struelens, et al. 2000. Multicenter evaluation of epidemiological typing of methicillin-resistant Staphylococcus aureus strains by repetitive-element PCR analysis. J. Clin. Microbiol. 38:3527-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deplano, A., M. Vaneechoutte, G. Verschraegen, and M. J. Struelens. 1997. Typing of Staphylococcus aureus and Staphylococcus epidermidis strains by PCR analysis of inter-IS256 spacer length polymorphisms. J. Clin. Microbiol. 35:2580-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fluckiger, U., and A. F. Widmer. 1999. Epidemiology of methicillin-resistant Staphylococcus aureus. Chemotherapy 45:121-134. [DOI] [PubMed] [Google Scholar]
- 6.Goh, S. H., S. K. Byrne, J. L. Zhang, and A. W. Chow. 1992. Molecular typing of Staphylococcus aureus on the basis of coagulase gene polymorphisms. J. Clin. Microbiol. 30:1642-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoefnagels-Schuermans, A., W. E. Peetermans, M. J. Struelens, S. Van Lierde, and J. Van Eldere. 1997. Clonal analysis and identification of epidemic strains of methicillin-resistant Staphylococcus aureus by antibiotyping and determination of protein A gene and coagulase gene polymorphisms. J. Clin. Microbiol. 35:2514-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maslow, J. N., M. E. Mulligan, and R. D. Arbeit. 1993. Molecular epidemiology: application of contemporary techniques to the typing of microorganisms. Clin. Infect. Dis. 17:153-162. [DOI] [PubMed] [Google Scholar]
- 9.Montesinos, I., E. Salido, T. Delgado, M. Cuervo, and A. Sierra. 2002. Epidemiologic genotyping of methicillin-resistant Staphylococcus aureus by pulsed-field gel electrophoresis at a university hospital and comparison with antibiotyping and protein A and coagulase gene polymorphisms. J. Clin. Microbiol. 40:2119-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nada, T., S. Ichiyama, Y. Osada, M. Ohta, K. Shimokata, N. Kato, and N. Nakashima. 1996. Comparison of DNA fingerprinting by PFGE and PCR-RFLP of the coagulase gene to distinguish MRSA isolates. J. Hosp. Infect. 32:305-317. [DOI] [PubMed] [Google Scholar]
- 11.Na'was, T., A. Hawwari, E. Hendrix, J. Hebden, R. Edelman, M. Martin, W. Campbell, R. Naso, R. Schwalbe, and A. I. Fattom. 1998. Phenotypic and genotypic characterization of nosocomial Staphylococcus aureus isolates from trauma patients. J. Clin. Microbiol. 36:414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishi, J., H. Miyanohara, T. Nakajima, I. Kitajima, M. Yoshinaga, I. Maruyama, and K. Miyata. 1995. Molecular typing of the methicillin resistance determinant (mec) of clinical strains of Staphylococcus based on mec hypervariable region length polymorphisms. J. Lab. Clin. Med. 126:29-35. [PubMed] [Google Scholar]
- 13.Pfaller, M. A., R. J. Hollis, and H. S. Sader. 1992. Chromosomal restriction fragment analysis by pulsed-field gel electrophoresis, p. 10.5.c.1.-10.5.c.12. In H. D. Isenberg et al. (ed.), Clinical microbiology procedures handbook. American Society for Microbiology, Washington, D.C.
- 14.Prevost, G., B. Jaulhac, and Y. Piemont. 1992. DNA fingerprinting by pulsed-field gel electrophoresis is more effective than ribotyping in distinguishing among methicillin-resistant Staphylococcus aureus isolates. J. Clin. Microbiol. 30:967-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryffel, C., R. Bucher, F. H. Kayser, and B. Berger-Bachi. 1991. The Staphylococcus aureus mec determinant comprises an unusual cluster of direct repeats and codes for a gene product similar to the Escherichia coli sn-glycerophosphoryl diester phosphodiesterase. J. Bacteriol. 173:7416-7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakurada, J., Z. Li, K. Seki, M. Murai, A. Usui, M. Morihigashi, H. Jitsukawa, H. K. Seong, M. Mutou, and S. Masuda. 1994. Biochemical and genetic heterogeneity of staphylococcal protein A. FEMS Microbiol. Lett. 119:59-63. [DOI] [PubMed] [Google Scholar]
- 17.Schlichting, C., C. Branger, J. M. Fournier, W. Witte, A. Boutonnier, C. Wolz, P. Goullet, and G. Doring. 1993. Typing of Staphylococcus aureus by pulsed-field gel electrophoresis, zymotyping, capsular typing, and phage typing: resolution of clonal relationships. J. Clin. Microbiol. 31:227-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senna, J.-P. M., C. A. Pinto, L.-P. S. Carvalho, and D. S. Santos. 2002. Comparison of pulsed-field gel electrophoresis and PCR analysis of polymorphisms on the mec hypervariable region for typing methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 40:2254-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shopsin, B., M. Gomez, M. Waddington, M. Riehman, and B. N. Kreiswirth. 2000. Use of coagulase gene (coa) repeat region nucleotide sequences for typing of methicillin-resistant Staphylococcus aureus strains. J. Clin. Microbiol. 38:3453-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shuttleworth, H. L., C. J. Duggleby, S. A. Jones, T. Atkinson, and N. P. Minton. 1987. Nucleotide sequence analysis of the gene for protein A from Staphylococcus aureus Cowan 1 (NCTC8530) and its enhanced expression in Escherichia coli. Gene 58:283-295. [DOI] [PubMed] [Google Scholar]
- 21.Struelens, M. J., R. Bax, A. Deplano, W. G. Quint, and A. van Belkum. 1993. Concordant clonal delineation of methicillin-resistant Staphylococcus aureus by macrorestriction analysis and polymerase chain reaction genome fingerprinting. J. Clin. Microbiol. 31:1064-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Struelens, M. J., A. Deplano, C. Godard, N. Maes, and E. Serruys. 1992. Epidemiologic typing and delineation of genetic relatedness of methicillin-resistant Staphylococcus aureus by macrorestriction analysis of genomic DNA by using pulsed-field gel electrophoresis. J. Clin. Microbiol. 30:2599-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenover, F. C., R. Arbeit, G. Archer, J. Biddle, S. Byrne, R. Goering, G. Hancock, G. A. Hebert, B. Hill, R. Hollis, et al. 1994. Comparison of traditional and molecular methods of typing isolates of Staphylococcus aureus. J. Clin. Microbiol. 32:407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unal, S., J. Hoskins, J. E. Flokowitsch, C. Y. Wu, D. A. Preston, and P. L. Skatrud. 1992. Detection of methicillin-resistant staphylococci by using the polymerase chain reaction. J. Clin. Microbiol. 30:1685-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wichelhaus, T. A., K. P. Hunfeld, B. Boddinghaus, P. Kraiczy, V. Schafer, and V. Brade. 2001. Rapid molecular typing of methicillin-resistant Staphylococcus aureus by PCR-RFLP. Infect. Control Hosp. Epidemiol. 22:294-298. [DOI] [PubMed] [Google Scholar]