Abstract
Neonatal toxic shock syndrome-like exanthematous disease (NTED) is a new neonatal disease caused by toxic shock syndrome toxin 1 (TSST-1). We conducted a prospective surveillance study and characterized the methicillin-resistant Staphylococcus aureus (MRSA) strains isolated from patients with NTED and compared them with the strains from patients with other MRSA infections and asymptomatic carriers. The study was performed in the neonatal intensive care unit and a general neonatal and maternal ward in the Tokyo Women's Medical University Hospital (TWMUH) from September to December 1998. Among 103 patients eligible for the study, MRSA was detected in 62 (60.2%) newborns; of these 62 newborns, 8 (12.9%) developed NTED, 1 (1.6%) had another MRSA infection, and 53 (85.5%) were asymptomatic MRSA carriers. Sixty-nine MRSA strains were obtained from the 62 newborns. DNA fingerprinting by pulsed-field gel electrophoresis showed two clusters: clone A with 8 subtypes and clone B. Sixty-seven of the 69 MRSA strains (97.1%) belonged to clone A, and type A1 was the most predominant (42 of 69 strains; 60.9%) in every neonatal and perinatal ward. All but one of the clone A strains had the TSST-1 and staphylococcal enterotoxin C genes. We also analyzed eight MRSA strains from eight NTED patients in five hospitals in Japan other than TWMUH. All the MRSA strains from NTED patients also belonged to clone A. These results suggest that a single clone that predominated in the neonatal wards of six hospitals might have caused NTED. However, the occurrence of NTED might not be dependent on the presence of an NTED-specific strain.
Toxic shock syndrome (TSS) is an acute life-threatening illness (39) caused by TSS toxin 1 (TSST-1), produced by Staphylococcus aureus (10, 40-42). Large outbreaks of TSS were seen in the United States from 1980 to 1985 (10). Cytokines produced by T cells activated by TSST-1, now classified as superantigenic toxins (10, 27, 40, 41), have been implicated in the pathogenesis of this illness (10, 40-42). The massive expansion in the number of TSST-1-reactive vβ2-positive T cells observed in patients with TSS supports this view (6, 10, 40). Since 1992, a number of neonates in Japan have been reported to have developed systemic exanthema, thrombocytopenia, and fever in the first week of life (36; N. Takahashi and H. Nishida, Arch. Dis. Child. 77:F79, 1997). Subsequently, microbiological examinations showed that most of the neonates with this illness were colonized by methicillin-resistant S. aureus (MRSA) strains that produced TSST-1 (36), suggesting that the pathogenesis of this illness is the same as that for TSS. Actually, the neonates exhibited a marked polyclonal expansion of vβ2-positive T cells in the acute phase of this illness (35, 36). As this neonatal disease did not match the clinical criteria for TSS, the disease was named neonatal toxic shock syndrome-like exanthematous disease (NTED) (36).
MRSA remains a major problem in nosocomial infections. The incidence of MRSA infection has been increasing among inpatients in many hospitals worldwide, especially neonatal, perinatal, and pediatric wards, as well as among outpatients in Japan (1, 20, 37). On the other hand, only 10 to 20% of MRSA carriers develop symptoms of NTED (35). Several recent reports have suggested that the development of NTED is dependent on host factors such as serum anti-TSST-1 levels (35). However, few reports have compared the epidemiological data for MRSA strains isolated from NTED patients with those for isolates from non-NTED patients. The question as to whether a specific clone(s) of MRSA is involved as the etiologic agent of NTED has been raised. Molecular typing techniques have introduced new possibilities for evaluation of the epidemiology of MRSA strains (1, 8, 11, 28, 32, 34).
In the present study, we characterized the MRSA strains isolated from NTED patients and compared them with MRSA strains isolated from patients with other MRSA infections and neonatal asymptomatic MRSA carriers.
MATERIALS AND METHODS
Prospective surveillance.
A prospective study was performed in two areas of a neonatal intensive care unit (NICU; 25 beds with 300 admissions per year) and a general maternal and perinatal ward (general ward; 37 beds with 700 admissions per year) in the Tokyo Women's Medical University Hospital (TWMUH; 1,500 beds) between 13 September and 28 December 1998. The NICU is divided into two areas: an intensive care area (with nine incubators) and an intermediate care area (with 15 beds). Each incubator in the intensive care area occupies 7.0 m2. A total of 103 patients were analyzed. Informed consent was obtained from all the parents. NTED was diagnosed on the basis of the clinical criteria established by Takahashi et al. (36), which consisted of erythema plus at least one of the following three conditions: thrombocytopenia with platelet counts below 150 × 109/liter, a C-reactive protein concentration above 10 mg/liter, and a rectal body temperature above 38°C. Nasal, oral mucosal, umbilical, and rectal swab specimens or fecal specimens were obtained from the neonates in the NICU on postnatal day 3 and once a week later. The same samples were collected from the neonates in the general maternal and perinatal ward on postnatal day 3. Other specimens requested by the patients' doctors were also used in the analyses.
Bacterial strains.
Each sample was plated onto a Trypticase soy agar plate with 5% defibrinated sheep blood (blood agar; Nissui Pharmaceutical Co, Ltd., Tokyo, Japan), and the plates were incubated at 35°C aerobically for 24 h. OPA Staphylococcus agar (Nippon Beckton-Dickinson Co., Ltd., Tokyo, Japan), which contained oxacillin, polymyxin B, aztreonam, amphotericin B, and egg yolk in brain heart infusion agar base, was also used for the selective isolation of MRSA strains if staphylococcal colonies were not found on the blood agar plates. S. aureus was identified by standard microbiological methods, including Gram staining, the catalase test, the latex slide agglutination test for clumping factor and protein A (PS test; Eiken Chemistry Co. Ltd., Tokyo, Japan), and the tube coagulase test (22). If similar S. aureus strains were obtained from different sites, the order of priority was blood, other infection sites, umbilicus, sputum, nostril, and rectal swab or feces, because the umbilicus is the primary site of neonatal colonization and infection (18). If different colonies of S. aureus were recovered from one patient, further antimicrobial susceptibility testing and molecular analysis were performed. Then, if phenotypic or genotypic differences were confirmed, both strains were counted and were included in the analysis as multiple isolates from the same patient. We also analyzed eight MRSA strains from eight patients with NTED in the NICUs of five other hospitals. Seibo Hospital (SH) is a TWMUHH-affiliated community hospital (192 beds) located 6.5 km from TWMUH. The other four hospitals are not affiliated with TWMUH. The status, number of beds, and distance from TWMUH are as follows: International Medical Center of Japan (IMCJ), a teaching hospital with 925 beds 1 km from TWMUH; Kawaguchi Municipal Medical Center (KMMC), a teaching hospital with 540 beds 12.5 km from TWMUH; Tokyo Metropolitan Kiyose Pediatric Hospital (TMKPH), a teaching hospital with 303 beds 20 km from TWMUH; and Nagoya Second Red Cross Hospital (NSRCH), a teaching hospital with 835 beds 380 km from TWMUH. Coagulase typing (44) was carried out with the MRSA strains isolated from NTED patients by using a coagulase typing kit (Denka Seiken, Niigata, Japan). The strains were stocked in 10% skim milk at −85°C until use.
Antimicrobial susceptibility testing.
Antimicrobial susceptibility testing was performed with the Pos Combo Panel Type 2J, and the results were read on a WalkAway System (Dade Behring Inc., West Sacramento, Calif.). The antibiotics tested were gentamicin (GEN), erythromycin (ERY), clindamycin (CLI), ofloxacin (OFX), vancomycin (VAN), and trimethoprim-sulfamethoxazole (SXT). The isolates were interpreted as sensitive or resistant according to the MICs, as described previously (12). Resistance to several drugs was determined by plating on Trypticase soy agar containing spectinomycin (SPT; 500 μg/ml) and tetracycline (TET; 40 μg/ml). After a 24-h incubation, growth of more than two colonies was determined as resistance (17).
PFGE analysis.
Preparation of chromosomal DNAs and pulsed-field gel electrophoresis (PFGE) analysis were carried out as described previously (8), with minor modifications, by using InCert agarose and SeaKem Gold agarose (FMC BioProducts, Rockland, Maine) instead of SeaPlaque and SeaKem LE agarose (FMC BioProducts), respectively. The PFGE run time was 20 h. The PFGE profiles were analyzed by visual inspection of the patterns according to the criteria of Tenover et al. (38). Isolates showing fragment differences of six or less were considered to be the same clone with different subtypes (38).
DNA extraction for amplification.
A single colony was suspended to a McFarland 1.0 standard in 100 μl of TE buffer (20 mM Tris chloride, 2 mM EDTA [pH 7.5]) with 10 U of achromopeptidase (Wako Chemical, Co. Ltd., Osaka, Japan), and the suspension was incubated at 55°C for 10 min. After centrifugation at 18,500 × g for 5 min, the supernatants were used as crude DNA extracts for PCR.
Detection of enterotoxins, exfoliative toxins, and TSST-1 by multiplex PCR.
The genes for staphylococcal enterotoxin A (SEA [sea]), SEB (seb), SEC (sec), SED (sed), SEE (see), exfoliative toxin A (ETA [eta]), ETB (etb), and TSST-1 (tst) were detected by using the multiplex PCR system with the oligonucleotide primers described by Becker et al. (3), with minor modifications. The PCR conditions were 30 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s.
Determination of SCCmec type.
The staphylococcal cassette chromosome mec (SCCmec) types of the MRSA strains isolated from NTED patients were determined by PCR of the mec and ccr gene complexes, as described previously (31).
Statistical analysis.
Differences between groups were analyzed by the Mann-Whitney U test (StatView 5.0; Abacus Concepts Inc., Berkeley, Calif.). P values less than 0.05 were considered significant.
RESULTS
Prospective surveillance.
The results of the prospective surveillance for S. aureus in neonates are summarized in Table 1. Among 103 patients eligible for the study, MRSA was detected in 62 (60.2%) newborns. No MRSA strain was isolated from the other 41 newborns. Among the 62 MRSA-positive newborns, 8 (12.9%) developed NTED, 1 (1.6%) had another MRSA infection, and 53 (85.5%) were asymptomatic MRSA carriers without any signs of infection. Six patients with NTED were in the intensive care area and two were in the intermediate care area. NTED did not occur in any of the newborns in the general ward. MRSA was isolated from all the NTED cases. The other case of MRSA infection was bacteremia and umbilicitis, which occurred in a patient in the intensive care area. The incidence of MRSA isolation in the intensive care area (22 of 25; 88.0%) was significantly higher than those in the intermediate care area (17 of 36; 47.2%; P < 0.002) and the general ward (23 of 42; 54.8%; P < 0.02). Methicillin-sensitive S. aureus (MSSA) was isolated from only six patients (one with umbilicitis in the intermediate care area and three and two asymptomatic carriers in the intermediate care area and the general ward, respectively), and only three patients were infected or colonized with MSSA strains without infection or colonization with MRSA strains (data not shown). Ten patients were simultaneously infected or colonized with multiple isolates of S. aureus. Two different MRSA strains were isolated from seven patients, and both MSSA and MRSA strains were isolated from three patients. Therefore, we obtained 69 MRSA strains from 62 patients and analyzed those strains in this study.
TABLE 1.
Results of prospective surveillance for MRSA in neonates
| Results of screening cultures | No. of patients
|
|||
|---|---|---|---|---|
| Ward
|
Total | |||
| General | NICU
|
|||
| Intermediate care | Intensive care | |||
| MRSA isolated from: | ||||
| NTED patients | 0 | 2 | 6 | 8 |
| Patients with other infections | 0 | 0 | 1 | 1 |
| Carrier | 23 | 15 | 15 | 53 |
| Total | 23 | 17 | 22 | 62 |
| MRSA not detecteda | 19 | 19 | 3 | 41 |
| Total | 42 | 36 | 25 | 103 |
No patients with NTED were in this category.
Antimicrobial susceptibility profiles.
The 69 MRSA strains from TWMUH and 8 MRSA strains from five other hospitals showed five different antibiogram types with a panel of eight antibiotics (GEN, ERY, CLI, OFX, VAN, SXT, SPT, and TET). Antibiogram type I was resistance to all drugs except VAN and SXT, and isolates with this antibiogram type were found in both areas in the NICU and the general ward in TWMUH (data not shown). Types I and II (type II was similar to type I, except for sensitivity to GEN) were also predominant (in all hospitals, 44 of 77 [57.1%] and 27 of 77 [35.1%] isolates, respectively; in TWMUH, 40 of 69 [57.9%] and 25 of 69 [36.2%] isolates, respectively; and in the other hospitals, 4 of 8 [50%] and 2 of 8 [25%] isolates, respectively). Only minor populations were of antibiogram type III (resistance to ERY, CLI, OFX, and SPT), type IV (resistance to ERY, OFX, and SPT), and type V (resistance to ERY and SPT): three, one, and two strains, respectively. Of all the antimicrobial agents tested, the ones to which resistance was most frequently observed were ERY (100%), SPT (100%), OFX (75 of 77; 97.4%), CLI (74 of 77; 96.1%), and TET (71 of 77; 92.2%). The percentage of isolates resistant to GEN was lower (44 of 77; 57.1%). None of the isolates were resistant to SXT or VAN. There was one unique MSSA strain (TWCC4372), which was resistant to GEN, ERY, CLI, OFX, SPT, and TET but sensitive to oxacillin (data not shown).
Molecular analysis of S. aureus strains.
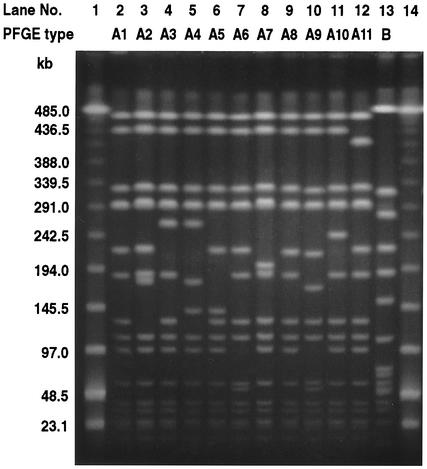
Table 2 shows the genotypic and phenotypic characteristics of 69 MRSA strains evaluated in the prospective study conducted at TWMUH. The PFGE patterns of MRSA strains revealed a single major clone, clone A (67 of 69 isolates; 97.1%), with eight subtypes (subtypes A1 to A6, A8, and A9), and a minor clone, clone B (2 of 89 isolates; 2.9%), according to the criteria of Tenover et al. (38) (Fig. 1). By using the antibiogram, clone A (types I, II, and III) could easily be differentiated from clone B (type V). The most common subtype found was A1 (42 of 69 isolates; 60.9%), followed by A3 (15 of 69 isolates; 21.7%). The remaining PFGE subtypes were found at low frequencies: A2, 4 of 69 isolates (6.0%); A4, 2 of 69 isolates (2.9%); and A5, A6, A8, and A9, 1 isolate each. Clone A strains were detected in all 62 patients colonized or infected with MRSA. Among all clone A strains, all except one A9 strain had tst and sec. None of the MRSA strains possessed sea, seb, sed, see, eta, or etb. No enterotoxin, tst, or exfoliative toxin gene was detected in the type B MRSA strains. Twelve MRSA strains were obtained from eight NTED patients. These strains belonged to subtype A1 (n = 5), A2 (n = 1), or A3 (n = 6). No specific MRSA clone(s) was associated exclusively with NTED. We found no correlation between the PFGE subtype and an MRSA carrier state, other MRSA infection, or NTED. Five different PFGE types (types A7, C, D, E, and F) were obtained for the MSSA strains (except for the data for subtype A7 in Fig. 1, data not shown). Except for the PFGE type A7 strain, the MSSA strains of the five PFGE types did not have any enterotoxin, exfoliative toxin, or TSST-1 genes; type A7 had sec and tst. The PFGE pattern of the multidrug-resistant MSSA strain (strain TWCC4372, which was resistant to GEN, ERY, CLI, OFX, SPT, and TET) was designated A7 and was quite similar to those of the other A subtypes. Strains of subtypes A1, A2, and A3 (n = 59) were gentamicin resistant and susceptible (subtype A1, 23 resistant and 17 susceptible strains; subtype A2, 3 resistant strains and 1 susceptible strain; subtype A3, 13 resistant and 2 susceptible strains). Two major subtypes (subtypes A1 and A3) were widely distributed in the general ward (15 and 4 of 23 strains, respectively), in the intermediate care area (13 and 4 of 19 strains, respectively), and in the intensive care area 12 and 8 of 25 strains, respectively).
TABLE 2.
Clonal distribution of MRSA strains from patients of different statuses
| PFGE type and subtype | Toxin type | Antibiogram type | No. of strains from:
|
|||
|---|---|---|---|---|---|---|
| NTED patients | Patients with other infections | Carriers | Total | |||
| A | ||||||
| A1 | tst, sec | I | 3 | 0 | 20 | 23 |
| A1 | tst, sec | II | 2 | 0 | 15 | 17 |
| A1 | tst, sec | III | 0 | 0 | 2 | 2 |
| A2 | tst, sec | I | 1 | 0 | 2 | 3 |
| A2 | tst, sec | II | 0 | 0 | 1 | 1 |
| A3 | tst, sec | I | 5 | 1 | 7 | 13 |
| A3 | tst, sec | II | 1 | 0 | 1 | 2 |
| A4 | tst, sec | II | 1 | 0 | 1 | 2 |
| A5 | tst, sec | I | 0 | 0 | 1 | 1 |
| A6 | tst, sec | II | 0 | 0 | 1 | 1 |
| A8 | tst, sec | II | 0 | 0 | 1 | 1 |
| A9 | Negative | II | 0 | 0 | 1 | 1 |
| Total | 12 | 1 | 54 | 67 | ||
| B | Negative | V | 0 | 0 | 2 | 2 |
| Total | 12 | 1 | 56 | 69 | ||
FIG. 1.
PFGE patterns of MRSA and isogenic MSSA strains isolated from newborns. Lanes 1 and 14, low-molecular-weight bacteriophage λ DNA ladder markers; lanes 2 to 13, results obtained with the different strains. Each lane shows a different PFGE type, with the patient's clinical status indicated in parentheses below. Lane 2, TWCC4382 (NTED); lane 3, TWCC3794 (asymptomatic carrier); lane 4, TWCC3908-1 (NTED); lane 5, TWCC3957 (asymptomatic carrier); lane 6, TWCC4142 (asymptomatic carrier); lane 7, TWCC4173 (asymptomatic carrier); lane 8, TWCC4372 (MSSA infection other than NTED); lane 9, TWCC3904 (asymptomatic carrier); lane 10, TWCC4077-1 (asymptomatic carrier); lane 11, TWCC4082 (NTED, from SH); lane 12, TWCC4410-1 (NTED, from IMCJ); and lane 13, TWCC3861 (asymptomatic carrier).
The genotypic and phenotypic characteristics of the 20 MRSA strains isolated from NTED patients at TWMUH and the five other hospitals are listed in Table 3. Four patients in TWMUH harbored multiple MRSA strains with different genotypes or phenotypes. All the strains were identified to be the same clone (clone A), to belong to coagulase type II and SCCmec type II, and to have tst and sec. Two new PFGE subtypes were detected (subtype A10 from SH and subtype A11 from IMCJ and TMKPH) (Fig. 1). Most of the strains showed multidrug resistance (antibiogram type I or II, 18 of 20 isolates [90%]). Fourteen of 20 strains (70%) were isolated from the umbilicus. Subtype A1 (9 of 20 isolates [45%]) was the most predominant PFGE subtype, followed by A3 (6 of 20 isolates [30%]). The remaining PFGE types were found at low frequencies: A10 (2 of 20 isolates [10%]), A11 (2 of 20 isolates [10%]), and A2 (1 isolate). The same PFGE subtype (subtype A1) was found in TWMUH, SH, NSCRH, and KMMC. NSCRH, KMMC, and TWMUH do not share medical staff. Strain TWCC4410-1 from IMCJ and strain TWCC4442 from TMKPH could not be differentiated by PFGE type, toxin type, or antibiogram.
TABLE 3.
Typing of MRSA strains isolated from NTED patients
| Patient no. | Hospital | TWCC strain | Site | PFGE type
|
Toxin type | Coagulase type | SCCmec type | Antibiogram | |
|---|---|---|---|---|---|---|---|---|---|
| Clone | Subtype | ||||||||
| 1 | TWMUH | 3812 | Umbilicus | A | A2 | tst, sec | II | II | I |
| 2 | TWMUH | 3908-1 | Nose | A | A3 | tst, sec | II | II | I |
| 2 | TWMUH | 3908-2 | Nose | A | A3 | tst, sec | II | II | II |
| 3 | TWMUH | 3800 | Umbilicus | A | A3 | tst, sec | II | II | I |
| 4 | TWMUH | 4087-1 | Umbilicus | A | A3 | tst, sec | II | II | I |
| 5 | TWMUH | 4350 | Nose | A | A1 | tst, sec | II | II | I |
| 5 | TWMUH | 4382 | Umbilicus | A | A1 | tst, sec | II | II | II |
| 6 | TWMUH | 4373 | Oropharynx | A | A1 | tst, sec | II | II | II |
| 7 | TWMUH | 4032-2 | Umbilicus | A | A1 | tst, sec | II | II | I |
| 7 | TWMUH | 4032-1 | Umbilicus | A | A3 | tst, sec | II | II | I |
| 8 | TWMUH | 4055-2 | Umbilicus | A | A1 | tst, sec | II | II | I |
| 8 | TWMUH | 4055-1 | Umbilicus | A | A3 | tst, sec | II | II | I |
| 9 | SH | 3631 | Umbilicus | A | A1 | tst, sec | II | II | I |
| 10 | SH | 3633 | Nose | A | A1 | tst, sec | II | II | I |
| 11 | SH | 4082 | Umbilicus | A | A10 | tst, sec | II | II | I |
| 12 | SH | 4139 | Umbilicus | A | A10 | tst, sec | II | II | I |
| 13 | NSRCH | 4148 | Umbilicus | A | A1 | tst, sec | II | II | IV |
| 14 | IMCJ | 4410-1 | Blood | A | A11 | tst, sec | II | II | II |
| 15 | TMKPH | 4442 | Umbilicus | A | A11 | tst, sec | II | II | II |
| 16 | KMMC | 4451-1 | Umbilicus | A | A1 | tst, sec | II | II | III |
DISCUSSION
NTED was first described in 1995 as a novel exanthematous disease caused by the staphylococcal superantigen TSST-1 (36). TSST-1-producing MRSA strains were isolated from most cases of NTED (36). In our study all the MRSA strains isolated from eight NTED patients at TWMUH had the TSST-1 and SEC genes. Moreover, the TSST-1 and SEC genes were also detected in the eight strains isolated from eight NTED patients at the five other hospitals. These results confirm those presented in a previous report (36) and support the evidence implicating TSST-1 as an etiologic factor for NTED.
Since 1992, the number of hospitals in Japan in which this disease has been encountered has increased, from 25.7% (19 of 74) in 1995 to 70.8% (63 of 89) in 1998 (36). The rate of methicillin resistance among the S. aureus strains in many hospitals in Japan was greater than 70% (1, 20, 37). Most of the strains produced TSST-1 and SEC and belonged to coagulase type II and mecA-Tn554 polymorph I-A (1, 20, 34, 37). In the 1990s, this new major clone replaced the former clone that was dominant and widespread during the 1980s, with less resistance to β-lactams and without TSST-1 production (1, 15, 37). These data suggest that the time of emergence and the increase in the incidence of NTED coincide with the emergence and spread of a new MRSA strain. In our hospital, methicillin resistance rates among S. aureus strains were 60 to 65% from 1996 to 1998, on the basis of one strain per one inpatient (20). In this study, MRSA was more prominent in the neonatal wards than in other wards, despite the implementation of several control measures. The reason is not fully known. However, overcrowding, understaffing, and increased workloads are well-known risk factors for the nosocomial transmission of MRSA (9, 19, 43). In our neonatal intensive care area, each incubator occupies 7.0 m2, which is slightly below the American Academy of Pediatrics standard of ≧7.4 m2/incubator in intensive care units (14).
The vast majority of MRSA strains belonged to a single clone that spread in three neonatal and perinatal wards. This clone was isolated from all patients with newly developed cases of NTED. However, in all three wards, many patients infected or colonized with this clone did not develop NTED. We could not identify any NTED-specific PFGE type that was not isolated from patients with other staphylococcal infections or asymptomatic carriers. The production of TSST-1 depends on environmental conditions such as the oxygen or carbon dioxide concentration (33, 46, 47) and is regulated by various regulatory access systems (23, 47). The possibility that some strains with the same PFGE subtype produce different amounts of TSST-1 remains to be examined. However, these results rather support the notion that the development of NTED is highly dependent on the patient's immunological background (35, 36). Neonates are generally protected against various infectious agents by specific immunoglobulin G (IgG) antibodies transferred transplacentally from their mothers (35). Exposure to TSST-1-producing S. aureus is commonplace in the general population, yet TSS is rare (5). The reason for this is that host susceptibility plays a key role in determining the occurrence of TSS (5). Anti-TSST-1 antibodies play a protective role against the development of TSS in adults (10, 45). The development of NTED was also dependent on the serum anti-TSST-1 IgG antibody titer (35). Whether MRSA carriers developed NTED was partly dependent on the presence of IgG-type anti-TSST-1 antibodies from their mothers (35).
Interestingly, the PFGE patterns of the MRSA strains from NTED patients in the other hospitals closely resembled those of the strains found in TWMUH (Table 3). This means that a single MRSA clone might be widespread in the neonatal wards of many hospitals, in association with an increase in the incidence of NTED. Saito et al. (34) also reported that a single PFGE type of MRSA producing TSST-1 and SEC was widely distributed in the NICUs of three different hospitals in Japan. Musser et al. (29) also reported on the isolation of a single clone from the majority of patients affected by TSS with a urogenital focus.
Clone A has also frequently been isolated in the United States (1, 32). However, there were only two reports of probable NTED suggested by laboratory data and clinical findings from countries other than Japan (7, 13). Both reports were postpartum cases among mother-infant pairs. Both the mothers and infants in each pair had thrombocytopenia, fever, and macular rash without desquamation, and the infections were self-limited. These characteristics are compatible with NTED. The S. aureus strain isolated from one patient did not produce TSST-1 but produced SEC, another superantigen of S. aureus (7). NTED cases might be underrecognized in other countries. Some investigators have reported that the prevalence of serum anti-TSST-1 antibodies was very high in adults (45). Therefore, newborns who have anti-TSST-1 antibodies transferred from the mothers may be protected from NTED. Moreover, tst and sec of MRSA N315 and Mu50, which were coagulase type II and SCCmec type II (31), were found to be located in the same pathogenic island (24). RN4282 had another different pathogenic island that encoded tst without sec (24, 30). The potential for TSST-1 production in Japanese clone A MRSA strains might be different from that for U.S. clone A strains. Further investigations of toxin production or the genetic background of tst-containing pathogenic islands should be conducted with clone A MRSA strains from different countries or continents.
There were some discrepancies between the antibiograms and the PFGE patterns, especially in terms of resistance to GEN. Resistance to GEN in S. aureus is mainly due to a bifunctional aminoglycoside-modifying enzyme, aminoglycoside-6′-N-acetyltransferase-2"-O-phosphoryltransferase [AAC(6′)-APH(2")] (2, 4); and the gene for this enzyme, aac(6′)-aph(2"), is present in 84.5% of MRSA strains in Japan (16). Lelièvre et al. (26) reported that some GEN-sensitive and GEN-resistant MRSA strains had closely related PFGE profiles and that the GEN-sensitive strains could have emerged from resistant ones by excision or deletion of the aac(6′)-aph(2") gene. Although we did not check our strains for the presence of aminoglycoside-modifying enzymes, the different GEN susceptibility patterns among strains with the same PFGE pattern may be attributed to the spontaneous loss or acquisition of this plasmid. Moreover, one MSSA strain (strain TWCC4372) was closely related to clone A (subtype A7) with tst and sec and resistance to GEN, ERY, OFX, SPT, and TET. It is well known that MRSA strains spontaneously lose methicillin resistance in vitro and in vivo (11, 17, 21, 25).
NTED regresses spontaneously without any active antistaphylococcal treatment, and the prognosis is good (36; Takahashi and Nishida, Arch. Dis. Child. 77:F79, 1997). We also did not experience any fatal cases in the period covered by this study. However, the high incidence of NTED and the asymptomatic carrier state reflect the wide dissemination of a single MRSA clone in our hospital. Strict infection control measures and new strategies (9, 43) are required to control and eliminate NTED.
Acknowledgments
We thank Izumi Sakuma, Department of Pediatrics, SH; Sadao Yamanami, Neonatal Center, KMMC; Tetsuo Yokoyama, TMKPH; Hirofumi Miyazawa, Neonatal Care Center, IMCJ; and Hisanori Sobajima, Neonatal Care Center, NSRCH, for providing the representative strains from NTED patients.
REFERENCES
- 1.Aires de Sousa, M., H. de Lencastre, I. Santos Sanches, K. Kikuchi, K. Totsuka, and A. Tomasz. 2000. Similarity of antibiotic resistance patterns and molecular typing properties of methicillin-resistant Staphylococcus aureus isolates widely spread in hospitals in New York City and in a hospital in Tokyo, Japan. Microb. Drug Resist. 6:253-258. [DOI] [PubMed] [Google Scholar]
- 2.Archer, G. L., D. R. Dietrick, and L. Johnston. 1985. Molecular epidemiology of transmissible gentamicin resistance among coagulase-negative staphylococci in a cardiac surgery unit. J. Infect. Dis. 151:243-251. [DOI] [PubMed] [Google Scholar]
- 3.Becker, K., R. Roth, and G. Peters. 1998. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for application and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes and toxic shock syndrome toxin 1 gene. J. Clin. Microbiol. 36:2548-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne, M. E., M. T. Gillespie, and R. A. Skurray. 1990. Molecular analysis of a gentamicin resistance transposonlike element on plasmids isolated from North American Staphylococcus aureus strains. Antimicrob. Agents Chemother. 34:2106-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chance, T. D. 1996. Toxic shock syndrome: role of the environment, the host and the microorganism. Br. J. Biomed. Sci. 53:284-289. [PubMed] [Google Scholar]
- 6.Choi, Y., J. A. Lafferty, J. R. Clements, J. K. Todd, E. W. Gelfand, J. Kappler, P. Marrack, and B. L. Kotzin. 1990. Selective expansion of T cells expressing Vβ2 in toxic shock syndrome. J. Exp. Med. 172:981-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow, A. W., B. K. Wittmann, K. H. Bartlett, and D. W. Sceidele. 1984. Variant postpartum toxic shock syndrome with probable intrapartum transmission to the neonate. Am. J. Obstet. Gynecol. 148:1074-1079. [DOI] [PubMed] [Google Scholar]
- 8.Chung, M., H. de Lencastre, P. Matthews, A. Tomasz, I. Adamsson, M. Aires de Sousa, T. Camou, C. Cocuzza, A. Corso, I. Couto, A. Dominguez, M. Gniadkowski, R. Goering, A. Gomes, K. Kikuchi, A. Marchese, R. Mato, O. Melter, D. Oliveira, R. Palacio, R. Sá-Leáo, I. Santos-Sanches, J.-H. Song, P. T. Tassios, and P. Villari. 2000. Molecular typing of methicillin-resistant Staphylococcus aureus by pulsed-field gel electrophoresis: comparison of results obtained in a multilaboratory effort using identical protocols and MRSA strains. Microb. Drug Resist. 6:189-198. [DOI] [PubMed] [Google Scholar]
- 9.Cosseron-Zerbib, M., A. M. R. Afonso, T. Naas, P. Durand, L. Meyer, Y. Costa, N. El-Helali, G. Huault, and P. Nordmann. 1998. A control programme for MRSA (methicillin-resistant Staphylococcus aureus) contaminant in a paediatric intensive care unit: evaluation and impact on infections caused by other microorganisms. J. Hosp. Infect. 40:225-235. [DOI] [PubMed] [Google Scholar]
- 10.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Extoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominguez, M. A., H. de Lencastre, J. Linares, and A. Tomasz. 1994. Spread and maintenance of a dominant methicillin-resistant Staphylococcus aureus (MRSA) clone during an outbreak of MRSA disease in a Spanish hospital. J. Clin. Microbiol. 32:2081-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferraro, M. J., W. A. Craig, M. N. Dudley, G. Eliopoulos, D. W. Hecht, J. F. Hindler, L. B. Reller, A. T. Sheldon, Jr., J. M. Swenson, F. C. Tenover, R. T. Testa, M. P. Weinstein, and M. A. Wikler. 2001. Performance standards for antimicrobial susceptibility testing; 11th informational supplement. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Green, S. L., and K. S. LaPeter. 1982. Evidence for postpartum toxic shock syndrome in a mother-infant pair. Am. J. Med. 72:169-172. [DOI] [PubMed] [Google Scholar]
- 14.Haley, R. W., N. B. Cushion, F. C. Tenover, T. L. Bannerman, D. Dryer, J. Ross, P. J. Sanchez, and J. D. Siegel. 1995. Eradication of endemic methicillin-resistant Staphylococcus aureus infections from a neonatal intensive care unit. J. Infect. Dis. 171:614-624. [DOI] [PubMed] [Google Scholar]
- 15.Hiramatsu, K. 1995. Molecular evolution of MRSA. Microbiol. Immunol. 39:531-543. [DOI] [PubMed] [Google Scholar]
- 16.Ida, T., R. Okamoto, C. Shimauchi, T. Okubo, A. Kuga, and M. Inoue. 2001. Identification of aminoglycoside-modifying enzymes by susceptibility testing: epidemiology of methicillin-resistant Staphylococcus aureus in Japan. J. Clin. Microbiol. 39:3115-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inglis, B., P. R. Matthews, and P. R. Stewart. 1991. Induced deletions within a cluster of resistance genes in the mec region of the chromosome of Staphylococcus aureus. J. Gen. Microbiol. 136:2231-2239. [DOI] [PubMed] [Google Scholar]
- 18.Jellard, J. 1957. Umbilical cord as a reservoir of infection in a maternity hospital. Br. Med. J. 94:925-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kibbler, C. C., A. Quick, and A.-M. O'Neill. 1998. The effect of increased bed numbers on MRSA transmission in acute medical wards. J. Hosp. Infect. 39:213-219. [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi, K. 2002. Overview and strategy for methicillin-resistant Staphylococcus aureus and other antimicrobial resistant bacteria. Jpn. J. Med. Assoc. (Nihon Ishikaizasshi) 127:347-352. (In Japanese.).
- 21.Kikuchi, K., H. de Lencastre, J. Eagan, N. Boone, N. Bernard, D. Armstrong, and A. Tomasz. 1996. Double inhibition zone phenomenon in methicillin-resistant Staphylococcus aureus (MRSA). Clin. Infect. Dis. 23:894. [Google Scholar]
- 22.Kloos, W. E., and T. L. Bannerman. 1999. Staphylococcus, p. 264-282. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 23.Kornbulum, J., B. N. Kreiswirth, S. J. Projan, H. Ross, and R. P. Novick. 1990. Agr: a polycistronic, locus regulating exoprotein synthesis in Staphylococcus aureus, p. 373-402. In R. P. Novick (ed.), Molecular biology of the staphylococci. VCH Publishers, New York, N.Y.
- 24.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Gosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Futuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence, C., M. Cosseron, P. Durand, Y. Costa, and R. Leclercq. 1996. Consecutive isolation of homologous strains of methicillin-resistant and methicillin-susceptible Staphylococcus aureus from a hospitalized child. J. Hosp. Infect. 33:49-53. [DOI] [PubMed] [Google Scholar]
- 26.Lelièvre, H., G. Lina, M. E. Jones, C. Olive, F. Forey, M. Roussel-Delvallez, M.-H. Nicolas-Chanoine, C. M. Bébéar, V. Jarlier, A. Andremont, F. Vandenesch, and J. Etienne. 1999. Emergence and spread in French hospitals of methicillin-resistant Staphylococcus aureus with increasing susceptibility to gentamicin and other antibiotics. J. Clin. Microbiol. 37:3452-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, H., A. Llera, E. L. Malchiodi, and R. A. Mariuzza. 1999. The structural basis of T cell activation by superantigens. Annu. Rev. Immunol. 17:435-466. [DOI] [PubMed] [Google Scholar]
- 28.Mitsuda, T., K. Arai, S. Fujita, and S. Yokota. 1995. Epidemiological analysis of strains of methicillin-resistant Staphylococcus aureus (MRSA) infection in the nursery: prognosis of MRSA carrier infants. J. Hosp. Infect. 31:123-134. [DOI] [PubMed] [Google Scholar]
- 29.Musser, J. M., P. M. Schlievert, A. W. Chow, P. Ewan, B. N. Kreiswirth, V. T. Rosdahl, A. S. Naidu, W. Witte, and R. K. Selander. 1990. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc. Natl. Acad. Sci. USA 87:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novick, R. P., P. Schlievert, and A. Ruzin. 2001. Pathogenicity and resistant islands of staphylococci. Microb. Infect. 3:585-594. [DOI] [PubMed] [Google Scholar]
- 31.Okuma, K., K. Iwakawa, J. D. Turnidge, W. B. Grubb, J. M. Bell, F. G. O'Brien, G. W. Coombs, J. W. Pearman, F. C. Tenover, M. Kapi, C. Tiensasitorn, T. Ito, and K. Hiramatsu. 2002. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J. Clin. Microbiol. 40:4289-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts, R. B., A. de Lencastre, W. Eisner, E. P. Severina, B. Shopsin, B. N. Kreiswirth, A. Tomasz, and the MRSA Collaborative Study Group. 1998. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in 12 New York hospitals. J. Infect. Dis. 178:164-171. [DOI] [PubMed] [Google Scholar]
- 33.Ross, R. A., and A. B. Onderdonk. 2000. Production of toxic shock syndrome toxin 1 by Staphylococcus aureus requires both oxygen and carbon dioxide. Infect. Immun. 68:5205-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito, Y., K. Seki, T. Ohara, C. Shimatsu, Y. Honma, M. Hayashi, S. Masuda, and M. Nakano. 1998. Epidemiologic typing of methicillin-resistant Staphylococcus aureus in neonate intensive care units using pulsed-field gel electrophoresis. Microbiol. Immunol. 42:723-729. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi, N., H. Kato, K. Imanishi, K. Miwa, S. Yamanami, H. Nishida, and T. Uchiyama. 2000. Immunopathophysiological aspects of an emerging neonatal infectious disease induced by a bacterial superantigen. J. Clin. Investig. 106:1409-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi, N., H. Nishida, H. Kato, K. Imanishi, Y. Sakata, and T. Uchiyama. 1998. Exanthematous disease induced by toxic shock syndrome toxin 1 in the early neonatal period. Lancet 351:1614-1619. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka, T., K. Okuzumi, A. Iwamoto, and K. Hiramatsu. 1995. A retrospective study of methicillin-resistant Staphylococcus aureus clinical strains in Tokyo University Hospital. J. Infect. Chemother. 1:40-49. [Google Scholar]
- 38.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, O. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Todd, J., M. Fishaut, F. Kapral, and T. Welch. 1978. Toxic shock syndrome associated with phage-group-I staphylococci. Lancet ii:1116-1118. [DOI] [PubMed] [Google Scholar]
- 40.Uchiyama, T., and H. Kato. 1999. The pathogenesis of Kawasaki disease and superantigens. Jpn. J. Infect. Dis. 52:141-145. [PubMed] [Google Scholar]
- 41.Uchiyama, T., T. Tadakuma, K. Imanishi, M. Araake, S. Saito, X.-J. Yan, H. Fujikawa, H. Igarashi, and N. Yamaura. 1989. Activation of murine T cells by toxic shock syndrome toxin-1: the toxin-binding structures expressed on murine accessory cells are MHC class II molecules. J. Immunol. 143:3175-3182. [PubMed] [Google Scholar]
- 42.Uchiyama, T., Y. Kamagata, X.-J. Yan, M. Kohno, M. Yoshioka, H. Fujikawa, H. Igarashi, M. Okubo, F. Kawano, T. Saito-Taki, and M. Nakano. 1987. Study of the biological activities of toxic shock syndrome toxin-1. II. Induction of the proliferative response and the interleukin 2 production by T cells from human peripheral blood mononuclear cells stimulated with the toxin. Clin. Exp. Immunol. 68:638-647. [PMC free article] [PubMed] [Google Scholar]
- 43.Uehara, Y., K. Kikuchi, T. Nakamura, H. Nakama, K. Agematsu, Y. Kawakami, N. Maruchi, and K. Totsuka. 2001. Inhibition of methicillin-resistant Staphylococcus aureus colonization of oral cavities in newborns by viridans group streptococci. Clin. Infect. Dis. 32:1399-1407. [DOI] [PubMed] [Google Scholar]
- 44.Ushioda, H., T. Terayama, S. Sakai, H. Zen-Yoji, M. Nishiwaki, and A. Hidano. 1981. Coagulase typing of Staphylococcus aureus and its application in routine work, p. 77-83. In J. Jeljaszewicz (ed.), Staphylococci and staphylococcal infections. Gustav Fisher Verlag, Stuttgart, Germany.
- 45.Vergeront, J. M., S. J. Stolz, B. A. Crass, D. B. Nelson, J. P. Davis, and M. S. Bergdoll. 1983. Prevalence of serum antibody to staphylococcal enterotoxin F among Wisconsin residents: implications for toxic-shock syndrome. J. Infect. Dis. 148:692-698. [DOI] [PubMed] [Google Scholar]
- 46.Yarwood, J. M., and P. M. Schlievert. 2000. Oxygen and carbon dioxide regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J. Clin. Microbiol. 38:1797-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yarwood, J. M., J. K. McCormick, and P. M. Schlievert. 2001. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J. Bacteriol. 183:1113-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]