Abstract
To overcome host defenses, bacterial pathogens of the genus Yersinia inject specific effector proteins into colonized mammalian cells. One such virulence factor, YopJ, inhibits the host inflammatory response and induces apoptosis of immune cells by blocking multiple signaling pathways, including the MAPK and NF-κB pathways. In this study, we show that YopJ exerts its deleterious effects by catalyzing the acetylation of two serine residues in the activation loop of the MAP kinase kinase, MEK2. This covalent modification prevents the phosphorylation of these serine residues that is required for activation of MEK2 and downstream signal propagation. We also show that YopJ causes acetylation of a threonine residue in the activation loop of both the α and β subunits of the NF-κB pathway kinase, IKK. These results establish a hitherto uncharacterized mode of action for bacterial toxins and suggest the possibility that serine/threonine acetylation may occur even under nonpathogenic conditions and may be a widespread protein modification regulating protein function in eukaryotic cells.
Keywords: inflammation, MEK
Understanding the mode of action of bacterial toxins has provided insight into the working of mammalian cells especially with regard to signal transduction pathways that impinge upon the activation of the innate immune system (1, 2). Historically, plague has been one of the most devastating diseases to humans, second only to smallpox. The bacillus Yersinia pestis is the causative agent of plague, and two other Yersinia species, Yersinia pseudotuberculosis and Yersinia enterocolitica, cause septicaemic and gastrointestinal disorders (3). These pathogens inject a bouquet of six effector proteins into the mammalian cell cytosol using a type III secretion apparatus (4). These Yersinia outer proteins (Yops) help the pathogen multiply extracellularly in the host by preventing its phagocytosis and by slowing down the onset of the inflammatory response (5). YopE, YopT, and YopO target the Rho family of GTP-binding proteins that control actin cytoskeleton dynamics whereas YopH dephosphorylates focal adhesion proteins, thus inhibiting focal adhesion disassembly. Together, the action of these Yops contributes to the resistance of Yersinia to undergo phagocytosis, a process known to require remodeling of the actin cytoskeleton and of focal adhesions. Suppression of phagocytosis enables Yersinia to evade the macrophage defense network, thereby allowing them to proliferate in Peyer's patches as extracellular microcolonies. The leucine-rich protein, YopM, has been shown to bind to several host cell kinases, resulting in their activation (6). The remaining outer protein, YopJ (also called YopP in Y. enterocolitica) has emerged as an important agent that leads to the reduced host inflammatory response characteristic of Yersinia infections (5). Exposure of macrophages to lipopolysaccharide leads to the activation of NF-κB and of several members of the MAPK family that promote the production of proinflammatory cytokines such as TNF-α. YopJ induces apoptosis in macrophages (7), and it was shown that inhibition of both MAPK and NF-κB pathways was necessary for this effect (8), suggesting that Yersinia might neutralize and eliminate macrophages without inducing an inflammatory response. Suppression of the inflammatory response is mediated in part by the inhibition of the transcription factor NF-κB, the activation of which is central to the onset of inflammation. YopJ has been shown to bind directly to many members of the MAPK kinase superfamily and also to IKK (IκB kinase), preventing signaling through MAP kinases as well as through NF-κB (9).
Results
Modification of MEK by YopJ.
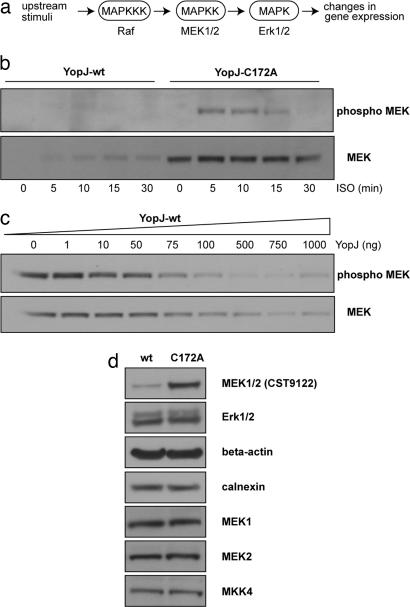
In an effort to understand the biochemical basis of the effects of YopJ on cellular signal transduction, we examined MAP kinase signaling in cultured mammalian cells. The Erk MAP kinase cascade is a three-component cascade in which the MAP kinases, Erk1/2, are activated upon phosphorylation by the MAP kinase kinases, MEK1/2, which are in turn, activated upon phosphorylation by the MAP kinase kinase kinase, Raf (Fig. 1a). HeLa cells are a well established system for the study of MAPK signaling and show basal nonphosphorylated Erk1/2 and MEK1/2 levels after serum starvation. Treatment of cells with EGF, phorbol ester (TPA), or the β2 adrenergic receptor agonist isoproterenol (ISO) leads to transient activation of the MAPK cascade. Treatment with 10 μM ISO at 37°C causes an activation of Erk and MEK within 5 min with a return to basal unphosphorylated levels by 30 min (see Fig. 4, which is published as supporting information on the PNAS web site). Treatment with EGF and TPA also gave rise to robust activation of Erk1/2 and MEK1/2; however, the return to baseline levels was not complete even 60 min after stimulation (see Fig. 4). It was thus decided to use ISO activation for experiments involving a time-course of MAPK activation.
Fig. 1.
MEK modification by YopJ. (a) The three-component MAPK cascade is an important signaling module that is shared by many signal transduction pathways; the MAPK pathway investigated in this study focuses on the MAP kinase kinases MEK1/2 that, in turn, activate the MAP kinases Erk1/2; this and similar pathways mediate the immediate host response to infection by Yersinia species. (b) Immunoblots from HeLa cells transfected with either YopJ-wt or the C172A mutant. Transfected cells were treated with 10 μM ISO at 37°C for the indicated times. (Upper) In YopJ-expressing cells, there is no signal corresponding to phospho-MEK, indicating that MEK activation is blocked by YopJ; in cells transfected with the C172A mutant of YopJ MEK, activation is clearly seen with a return to baseline levels by 30 min. (Lower) Loss of immunoreactivity with the CST9122 antibody against MEK1/2 in YopJ-wt expressing cells. (c) The block of MEK activation depends on the dosage of transfected YopJ. Indicated amounts (in ng) of plasmid pSFV-YopJ were transfected into a 35 mm dish of HeLa cells. Twenty-four hours after transfection, cells were serum-starved for 6 h and then treated with 10 μM ISO for 5 min at 37°C. Total lysates were examined by western analysis for phospho MEK1/2 and for total MEK1/2 (using the CST9122 antibody). (d) The loss of MEK signal is specific for the MEK1/2 (CST9122) antibody. Lysates from HeLa cells expressing either WT YopJ or the C172A mutant were immunoblotted by using the indicated antibodies. Whereas the MEK1/2 (CST9122) antibody discriminates between WT and C172A lanes, the other antibodies do not. This observation suggests that expression of active YopJ modifies MEK1/2 in a manner that masks the epitope recognized by the MEK1/2 (CST9122) antibody.
YopJ has previously been shown to interact with MEK2 and to inhibit its activation (9). We examined the effect of YopJ on MAPK signaling by expressing either WT YopJ or the inactive C172A mutant of YopJ in HeLa cells. MEK1/2 activation after ISO stimulation was inhibited in cells expressing WT YopJ (Fig. 1b), whereas cells expressing the inactive C172A mutant of YopJ showed the expected activation of MEK1/2 within 5 min of agonist stimulation, with a return to basal unphosphorylated levels by 30 min. Curiously, when the same cell lysates were examined (Fig. 1b Lower) for total MEK1/2 protein levels by using a MEK1/2 antibody (CST9122), no signal was detected in cells expressing WT YopJ, whereas cells expressing the C172A mutant of YopJ showed robust signals for total MEK1/2 protein. This effect of YopJ on the immunodetection of total protein levels was specific for MEK1/2 because total amounts of Erk1/2 and of other control proteins like beta-actin and calnexin were not observed to be different between YopJ WT and YopJ-C172A cells (Fig. 1d). The effect of YopJ on the activation of MEK1/2 was dose-dependent (Fig. 1c). Consistent with the results of Fig. 1b, the dose dependence of MEK1/2 inhibition correlated precisely with the loss of total MEK1/2 immunosignal as detected by the CST9122 antibody. Taken at face value these results would suggest that expression of YopJ in cells leads to degradation of MEK1/2 proteins. This interpretation was clearly inadmissible because previous studies had clearly shown that whereas YopJ expression had an effect on the amount of phosphoMEK it had no effect of on total level of MEK proteins (9, 10). We began to suspect that expression of YopJ might be resulting in the masking of the epitope on MEK1/2 recognized by the CST9122 anti-MEK1/2 antibody. We thus probed lysates of HeLa cells expressing WT or inactive YopJ using antibodies directed against other epitopes on MEK1 and MEK2. Because YopJ had been shown to interact with the MAPK kinases MKK3 and MKK4 (9), we also blotted with antibodies against MKK3 and MKK4 to examine whether any of them might show a reduction in total protein levels. By using these antibodies, it was determined that there was equal signal for total MEK protein levels from cells expressing either WT or mutant YopJ proteins (Fig. 1d). Under our experimental conditions, endogenous levels of MKK3 protein could not be detected by using the anti-MKK3 antibody.
The CST9122 MEK1/2 antibody used in Fig. 1 b and c was directed against a 20-aa peptide (conserved in MEK1 and -2) centered about amino acid 220 of MEK1 whereas the MEK1 and MEK2 antibodies used in Fig. 1d were directed against peptides taken from the amino and carboxyl termini of the respective proteins (Cell Signaling Technology). It was thus clear that expression of YopJ was not leading to degradation of MEK1/2 proteins but was causing a masking of the epitope of the anti-MEK1/2 CST9122 antibody that was directed against a peptide near the activation loops of the proteins. This observation was potentially very interesting because it suggested that YopJ expression might prevent activation of MEK1/2 by causing covalent modification of the activation loops of these kinases.
YopJ Leads to Acetylation of MEK2.
To elucidate the nature of modification, lysates from YopJ expressing cells were subjected to treatment with phosphatase, reducing agents, mild acid and mild alkali. However, none of these treatments restored the MEK1/2 signal as detected by the CST9122 antibody. Prolonged (5 h) incubation of the lysate in Laemmli Sample Buffer at 95°C did restore the signal, confirming that YopJ was indeed adding some covalent modification to MEK1/2 (data not shown). It must be noted here that given the nature of our readout wherein a loss of signal on a Western blot was diagnostic of the modification, YopJ must certainly be a very highly active enzyme in vivo and that most of the MEK molecules in the cell must be modified by YopJ. This effect of expressed YopJ on endogenous MEK1/2 was seen as early as 6 h after transfection of cells (data not shown).
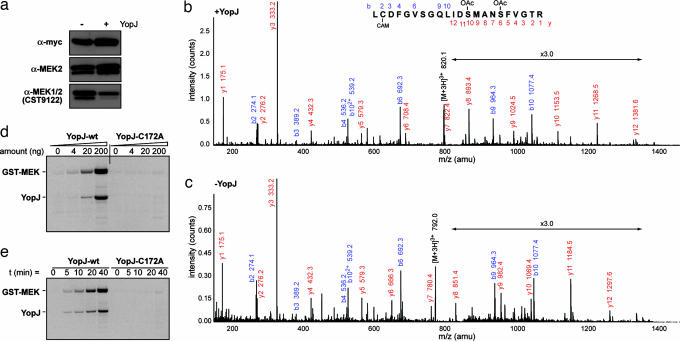
To identify the modification it was decided to isolate MEK from YopJ expressing cells for analysis by mass spectrometry. MEK2 bearing a myc epitope tag at its amino terminus and a hexahistidine tag at the carboxyl terminus was overexpressed in HeLa cells. It was verified from immunoblots by using the MEK1/2 CST9122 antibody that cotransfection of YopJ was sufficient to modify overexpressed myc-MEK2-(His)6 in a similar fashion to endogenous MEK (data not shown). Myc-MEK2-(His)6 was prepared by Ni-NTA affinity from lysates of HeLa cells coexpressing YopJ WT or a control plasmid. The prepared samples were analyzed for the presence of modification by Western blots by using anti-myc, anti-MEK2, and the discriminating anti-MEK1/2 CST9122 antibodies (Fig. 2a) and subjected to analysis by mass spectrometry.
Fig. 2.
YopJ modifies MEK2 by acetylation. (a) Before mass spectrometric analysis, myc-MEK2-(His)6 prepared from YopJ-coexpressing cells (+) and control cells (−) was blotted against different antibodies to ensure it was modified. (b and c) Mass spectrometric analysis of a peptide from MEK2 spanning amino acid residues 210–231. The fragmentation of the modified peptide is shown in b and for the unmodified peptide in c. It can be seen that, whereas Cys-211 is carbamidomethylated (CAM) in each case, Ser-222 and Ser-226 are O-acetylated (OAc) only in YopJ-coexpressing cells. (d) GST-MEK2 (0.5 μg) is acetylated in vitro by YopJ-wt in the presence of [14C]acetyl coenzymeA. Increasing amounts of YopJ lead to higher acetyl transfer. (e) Time course of acetylation of GST-MEK (0.5 μg) by YopJ (80 ng). Progressively greater acetyl transfer is seen over time. The inactive C172A mutant of YopJ does not show any activity in these assays.
Analysis of tryptic digests of the two samples revealed a peptide in MEK2 that was modified by YopJ. Triply charged ions with a mass/charge (m/z) ratio of 792.0, corresponding to the amino acid sequence of the activation loop L210C*DFGVSGQLIDSMANSFVGTR231 (where C* is carbamidomethylcysteine) was isolated for the control sample and fragmented to confirm the sequence. The corresponding triply charged ion from the YopJ-expressing cells had an m/z ratio of 820.1, with an additional mass of 84 atomic mass units (amu) (consistent with the addition of two acetyl groups). Peaks within the fragmentation series of the modified peptide (m/z 820.1) were compared with the unmodified one (m/z 792.0). The y6 and y10 ions of the modified peptide (Fig. 2b) have masses that are, respectively, 42 amu and 84 amu greater than the corresponding ions in the unmodified peptide (Fig. 2c). This result indicates the addition of acetyl groups on the serine residues at amino acid positions 222 and 226 of MEK2. Subsequent ions in the series y7 to y9 showed a mass increase of 42 amu, and in y11 to y12 an increase of 84 amu, supporting the assignment of these acetylation sites. This finding provides a mechanistic explanation for the ability of YopJ to inhibit activation of MEK as Ser-222 and Ser-226 lie in the activation loop of MEK2 and normally undergo phosphorylation by an upstream kinase to be activated. Prior acetylation of the hydroxyl groups of these serine residues leaves them incapable of accepting phosphates, thereby not permitting MEK2 activation.
Additionally, it was observed that Thr-13 of MEK2 is also a target for O-acetylation by YopJ (see Fig. 5, which is published as supporting information on the PNAS web site). The functional consequence of the acetylation of Thr-13 on MEK2 is currently uncertain. We expect that this acetylation event does not have a large impact on MEK activity because signaling by a constitutively active mutant of the closely related MAP kinase kinase MEK1 (made by replacing the activation loop serines with acidic residues) was not inhibited by expression of YopJ (9).
YopJ has Acetyltransferase Activity.
YopJ could either be an acetyl transferase by itself or act as an adaptor between MEK2 and some endogenous enzyme. We next examined whether acetyl transferase activity was an inherent property of YopJ. Recombinant MEK2 expressed as a GST-fusion protein was purified from Escherichia coli as were hexahistidine-tagged YopJ-wt and YopJ-C172A. Acetyl transferase activity of WT YopJ is shown in Fig. 2d where it can be seen that progressively higher amounts of YopJ result in greater acetylation of GST-MEK2 in the presence of 14C-labeled acetyl CoA (where the radiolabel is on the acetyl group). Control experiments were performed to verify that GST was not a substrate for acetyl transfer under our assay conditions. Fig. 2e shows a time course of the acetylation reaction over 40 min of incubation of YopJ with MEK2 at 30°C. It is to be noted that in addition to acetyl transfer to MEK2, attachment of the radiolabel to WT YopJ is also seen. The C172A mutant of YopJ does not serve as an acceptor of the acetyl group and thus does not transfer it to the substrate MEK2. These results demonstrate that acetyl transferase activity is an inherent enzymatic property of YopJ.
YopJ Acetylates IKKα and IKKβ.
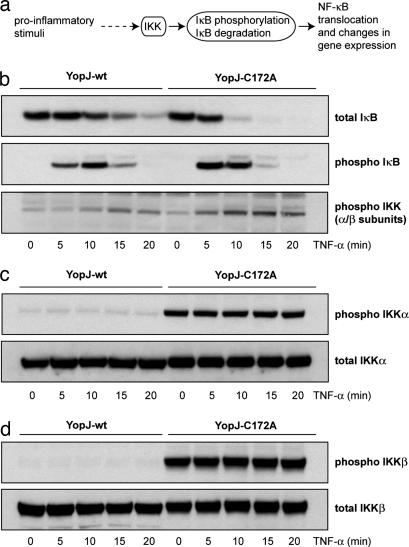
In addition to its effect on MAPK signaling, YopJ is also known to exert an inhibitory effect on NF-κB signaling (11). NF-κB is a collective designation for a family of dimeric transcription factors. In resting cells NF-κB is complexed with its inhibitor IκB and retained in the cytoplasm. IκB can be phosphorylated by IκB kinase, IKK (Fig. 3a). Phosphorylation of IκB leads to its ubiquitination and subsequent degradation. NF-κB is then freed to enter the nucleus and affect transcription. IKK is a multisubunit complex comprising two structurally similar catalytic subunits (IKKα and IKKβ) and a variable number of associated regulatory gamma subunits. The canonical pathway of NF-κB activation relies on activation of IKKβ which has been shown to phosphorylate IκB proteins. The kinase activity of IKKα, on the other hand, is required in the so called noncanonical pathway of NF-κB activation that operates mainly in B cells (12, 13). IKKα and IKKβ are highly homologous proteins (52% sequence identity, >70% sequence similarity) (14) and can be considered to be MAPKK-like molecules in that they are activated by MAPKKK-like molecules (15) and by the fact that the activation loops of MAPKK, IKKα, and IKKβ all contain two serine residues (or one serine and one threonine residue) within the sequence SxxxS/T that are phosphorylated by upstream kinases (14). Furthermore, a direct interaction of MAPKKs and IKKβ with YopJ has been reported (9).
Fig. 3.
YopJ modifies IKKα and IKKβ. (a) Various proinflammatory stimuli activate the NF-κB-signaling pathway by activating the IκB kinase (IKK) complex. IKK activation results in the phosphorylation and subsequent ubiquitin-mediated degradation of IκB, the inhibitor or NF-κB. The removal of IκB results in NF-κB translocation to the nucleus where it can regulate gene expression. (b) HEK293 cells transfected with YopJ-wt or YopJ-C172A were stimulated with TNF-α. IκB degradation is slowed down (Top) by the expression of YopJ-wt (but not the C172A inactive mutant of YopJ). This effect is due to reduced phosphorylation of IκB in these cells (Middle) caused by inhibition of activation of IKK (Bottom) caused by WT YopJ. (c and d) Overexpression of IKKα (c) or IKKβ (d) results in their activation in cells expressing inactive YopJ-C172A but this activation is blocked by WT YopJ (Upper). Total levels of IKKα and IKKβ are not affected by expression of YopJ (Lower).
We investigated the effect of YopJ expression on the NF-κB pathway by examining the response of YopJ-transfected mammalian cells to the proinflammatory cytokine TNF-α. TNF-α treatment induces a rapid activation of IKK which in turn phosphorylates IκB leading to the ubiquitination and proteasome-mediated degradation of IκB. Fig. 3b shows that expression of YopJ results in a reduction in this response when HEK293 cells are exposed to TNF-α. Phosphorylation and degradation of IκB are markedly retarded in cells expressing WT YopJ when compared with cells expressing the inactive C172A mutant of YopJ, which, in turn, correlates well with delayed activation (by phosphorylation) of IKK in these cells (Fig. 3b Bottom). These results demonstrate that YopJ affects the activation of IKK in mammalian cells.
Overexpression of either IKKα or IKKβ is known to result in autophosphorylation of their activation loops leading to activation of their kinase activities (16, 17). We coexpressed Flag-tagged IKKα or Flag-tagged IKKβ along with YopJ in mammalian cells. These cells were then examined for their response to stimulation with TNF-α (see Fig. 6, which is published as supporting information on the PNAS web site). It was observed that total levels of IκB protein were lower in IKKβ plus YopJ-wt cells compared with IKKβ plus YopJ-C172A cells, whereas no such diminution in IκB levels was seen in cells overexpressing IKKα (see Fig. 6 Upper). This observation is consistent with the fact that activation of IKKβ and not IKKα is required for IκB degradation (12). Furthermore, it suggests that WT YopJ “protects” IκB from degradation (presumably by affecting IKK activity), whereas inactive YopJ does not. Fig. 3 c and d Upper shows that IKKα and IKKβ are fully autophosphorylated and activated in cells coexpressing inactive YopJ. Activation of these cells with TNF-α does not lead to any detectable increase in the amount of phosphorylated IKKα or IKKβ. Interestingly it is observed (on these same panels) that recognition of expressed IKKα and IKKβ by the phospho-specific antibody is abrogated in cells expressing WT YopJ. As control, the total amounts of IKKα and IKKβ proteins were verified to be the same in cells expressing either form of YopJ (Fig. 3 c and d Lower). This observation immediately suggests that YopJ modifies IKKα and IKKβ within the activation loop, most probably by acetylating one or more serine/threonine residues within it.
To identify the site(s) of modification we immunoprecipitated Flag-tagged IKKα and Flag-tagged IKKβ from cells coexpressing YopJ. Mass spectrometric analysis (see Fig. 7, which is published as supporting information on the PNAS web site) revealed that both IKKα and IKKβ were indeed acetylated on the activation loop. In each case, the modification was on a threonine residue (indicated in bold) located between the two (underlined) phospho-acceptor serine residues (AKDVDQGSLC(OAc)T179SF in IKKα and AKELDQGSLC(OAc)T180SF in IKKβ). In addition to Thr-179, IKKα was also found to be acetylated on Ser-119 in the kinase domain and on Thr-722 located near the carboxyl terminus of IKKα. These two residues are, however, not conserved in IKKβ and the significance of their modification is not presently clear.
The cysteine residue adjacent to the acetyl-acceptor threonine residue in the activation loops of the catalytic IKKα and IKKβ subunits has previously been reported to be covalently modified by cyclopentenone prostaglandins (18) and other thiol-reactive agents (19, 20) resulting in the inhibition of IKK activation.
YopJ-mediated acetylation of the activation loop of the α and β subunits of IKK also prevents activation of the kinase activity of the IKK complex thus preventing phosphorylation of IκB in response to exposure of cells to TNF-α. These results are consistent with an earlier report (21) wherein it was demonstrated that phosphorylation of the activation loop of IKKβ was inhibited in YopJ-expressing mammalian cells. Our results thus provide a mechanistic basis for the observed inhibition of the canonical NF-κB proinflammatory pathway by YopJ.
Taken together, our results establish that YopJ is an acetyl transferase that catalyses O-acetylation of serine and threonine residues in two important kinases involved in the innate immune response of mammals, thereby inhibiting their activities.
Discussion
Interestingly, the YopJ-like molecule from Salmonella typhimurium, AvrA, has been reported to inhibit the NF-κB pathway (22) and also to inhibit the degradation of β-catenin (23). Phosphorylation of β-catenin occurs within a motif that is highly similar to the phosphorylation motif of IκB (14) and it has been suggested that IKKα may phosphorylate and regulate the degradation of β-catenin (24). It is thus possible that YopJ-related AvrA may also possess an acetyl transferase activity allowing it to inactivate IKKα thereby exerting its effect on β-catenin degradation.
Whereas acetylation of lysine residues in histones and transcription factors has been widely described there have also been reports of regulated acetylation of nonnuclear proteins (25). Furthermore, it has been suggested that acetylation events may temporally modulate IKK activity (26). Our elucidation of the mechanism of action of the bacterial toxin YopJ proposes the hypothesis that serine/threonine acetylation may be a widespread mode of biochemical regulation of endogenous processes in mammalian cells.
The analgesic and antiinflammatory effects of acetyl salicylic acid (Aspirin) administration are attributed to the transfer of the acetyl group to active site serine residues of cyclooxygenase enzymes resulting in the inhibition of their enzymatic activities. However, the cellular enzymes that (possibly) mediate the acetyl transfer have not been identified. Interestingly, there are by now numerous reports of cyclooxygenase-independent actions of acetyl salicylic acid that impinge primarily upon MAP kinase- and NF-κB-signaling pathways (27, 28). In the light of our results, we speculate that some of these effects might be due to the transfer of acetyl groups to the active sites of MAPKKs/IKKs catalyzed by yet uncharacterized serine/threonine acetyl transferases.
Materials and Methods
Reagents.
Isoproterenol and EGF were from Sigma (Dorset, U.K.), TPA was from Cell Signaling Technology (Danvers, MA), TNF-α was from R & D Systems (Minneapolis, MN). Primary antibodies against Erk, phosphoErk, MEK1/2, phosphoMEK1/2, MEK1, MEK2, MKK3, MKK4, IκB, phosphoIκB, IKKα, IKKβ, and phosphoIKKα/β were from Cell Signaling Technology; primary antibodies against calnexin were from Affinity BioReagents (Golden, CO); and primary antibodies against β-actin were from Abcam (Cambridge, U.K.).
Plasmid Constructs.
Plasmids encoding WT YopJ (pSFV-YopJ) and the inactive C172A mutant (pSFV-YopJ-CA) were the kind gifts of K. Orth (University of Texas Southwestern Medical Center, Dallas, TX); pCMV-MEK2 was provided by K.-L. Guan (University of Michigan, Ann Arbor, MI); and pCDNA3-Flag-IKKα and pCMV-Flag-IKKβ were kindly provided by D. Ballard (Vanderbilt University Medical Center, Nashville, TN).
pCMV-myc-MEK2-(His)6 coding for MEK2 with a hexahistidine tag at its carboxyl terminus was constructed by amplifying the MEK2 sequence with appropriate primers and ligating into pCMV-myc (a modified pCMV5 plasmid). GST-MEK2 was made by amplifying the coding region of MEK2 and ligating in-frame with GST in the bacterial expression plasmid pGEX6P. (His)6-YopJ-wt and (His)6-YopJ-C172A were made by ligating the coding region of YopJ in-frame with the amino-terminal hexahistidine tag of the bacterial expression vector pET28. All constructs used were verified by plasmid sequencing.
Cell Culture and Transfections.
HeLa and HEK293 cells were cultured in DMEM (Invitrogen) with 10% FBS. Transfections were typically done with GeneJuice (Novagen) in 35-mm dishes by using 1 μg of plasmid DNA per dish. Twenty-four hours after transfection, cells were serum-starved for 12–16 h and then stimulated with either ISO (10 μM) or TNF-α (20 ng/ml) for different times. Cells were harvested in Laemmli Sample Buffer, and lysates were resolved by NuPAGE 4–12% Bis-Tris Gels (Invitrogen). Western Blots were probed with primary antibodies at a typical dilution of 1:10,000 (except 1:500 for the phosphoIKKα/β antibody) at 4°C for 16 h and then incubated with horseradish peroxidise-linked secondary antibodies at room temperature for an hour followed by ECL (Amersham) detection on Kodak BioMax XAR film.
Mass Spectrometry.
Identification of modification on MEK2.
Ten micrograms of the pCMV-myc-MEK2-(His)6 construct was transfected either alone or along with 5 μg of pSFV-YopJ-wt into each of three 10-cm dishes by using GeneJuice (Novagen). Forty-eight hours after transfection, cells were harvested, and hexahistidine-tagged MEK2 was purified by using NiNTA affinity chromatography. The modified and unmodified MEK2 preparations were resolved by SDS/PAGE and the gel stained by Coomassie blue. Gel slices containing bands corresponding to MEK2 were excised and washed, alkylated, and in-gel digested with trypsin (29). A portion of the extracted tryptic peptides mixture was desalted and concentrated by using a GELoader tip filled with Poros R2 sorbent (Perseptive Biosystems, Framingham, MA). The bound peptides were eluted with 1 μl of 60% acetonitrile/3% formic acid directly into a nanospray capillary and then introduced into an API QSTAR pulsar i hybrid quadrupole-time-of-flight mass spectrometer (MDS Sciex, Ontario, Canada). Product ion scans were carried out in positive ion-mode and MS survey scan for peptides from m/z 600 to 1,500 were measured. Selected ions were fragmented by collision-induced dissociation (CID) with nitrogen in the collision cell and spectra of fragment ions produced were recorded in the time-of-flight mass analyzer.
Identification of modification on IKKα and IKKβ.
Five micrograms of plasmid encoding Flag-tagged IKKα were cotransfected along with 5 μg of either pSFV-YopJ-wt or pSFV-YopJ-C172A into each of three 10-cm tissue culture dishes. Forty-eight hours after transfection, cells were harvested, and Flag-tagged IKKα protein was immunoprecipitated by using EZview Red Anti-Flag M2 affinity gel (Sigma). Immunoprecipitates were resolved on NuPAGE 4–12% Bis-Tris Gels (Invitrogen). Protein bands corresponding to modified and unmodified IKKα were excised from a Coomassie blue-stained gel, washed, alkylated, and in-gel digested with chymotrypsin.
Peptides from the in-gel digest mixtures were separated by nanoscale liquid chromatography (LC Packings, Amsterdam, The Netherlands) on a reverse-phase C18 column (150 × 0.075 mm i.d., flow rate 0.15 μl/min). The eluate was introduced directly into a Q-STAR hybrid tandem mass spectrometer (LC-MS/MS). The spectra were searched against a NCBI nonredundant database with MASCOT MS/MS Ions search (www.matrixscience.com). The modified peptides and acetylation sites were identified by manual inspection of the fragmentation series. The same procedure was used with transfected Flag-IKKβ (in place of IKKα) to identify the site of modification in IKKβ.
Acetyl Transferase Assays.
GST-MEK2 (0.5 μg) (purified by using standard protocols for GST-tagged protein purification) was incubated with varying amounts (0–200 ng) of (His)6-YopJ-wt or (His)6-YopJ-C172A and 80 μM [1-14C]acetyl coenzymeA (56 mCi/mmol; Amersham) (1 Ci = 37 GBq) at 30°C for 40 min. Reaction products were resolved on 4–12% SDS/PAGE gels. Gels were stained with Coomassie blue, destained, soaked in Amplify (Amersham) for 30 min, dried, and subjected to autoradiography for 72 h. For time-course experiments, 0.5 μg of GST-MEK2 was incubated with 80 ng of (His)6-YopJ-wt or (His)6-YopJ-C172A and 80 μM [1-14C]acetyl coenzymeA at 30°C for varying lengths of time. Reactions were stopped by the addition of SDS/PAGE sample buffer and analyzed as described above.
Supplementary Material
Acknowledgments
R.M. thanks Sarada Raghavan, JyotiRanjan Mishra, S. Raghavi, and Veronica Rodrigues for help and cooperation. R.M. acknowledges grants from the British Council, India, and from The Royal Society (International Incoming Fellowship) and is currently supported by the Medical Research Council, U.K. (Career Development Fellowship).
Abbreviations
- ISO
isoproterenol
- amu
atomic mass unit
- IκB
inhibitor of NF-κB
- IKK
IκB kinase
- MEK1/2
mitogen-activated protein kinase kinase 1/2
- Yop
Yersinia outer protein
Note.
While this study was being completed, Mukherjee et al. (30) reported that YopJ acetylates MKK6 in the activation loop in a manner similar to that reported in this study. Our results confirm that YopJ has acetyl transferase activity, and, taken together, our studies show that, in mammalian cells, this activity inhibits the MAP kinase- and NF-κB-signaling pathways by targeting the activation loops of MAP kinase kinases (MKKs) and IKKs.
Footnotes
The authors declare no conflict of interest.
References
- 1.Galan JE, Cossart P. Curr Opin Microbiol. 2005;8:1–3. doi: 10.1016/j.mib.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Aktories K, Barbieri JT. Nat Rev Microbiol. 2005;3:397–410. doi: 10.1038/nrmicro1150. [DOI] [PubMed] [Google Scholar]
- 3.Cornelis GR. Proc Natl Acad Sci USA. 2000;97:8778–8783. doi: 10.1073/pnas.97.16.8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galan JE, Collmer A. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 5.Viboud GI, Bliska JB. Annu Rev Microbiol. 2005;59:69–89. doi: 10.1146/annurev.micro.59.030804.121320. [DOI] [PubMed] [Google Scholar]
- 6.McDonald C, Vacratsis PO, Bliska JB, Dixon JE. J Biol Chem. 2003;278:18514–18523. doi: 10.1074/jbc.M301226200. [DOI] [PubMed] [Google Scholar]
- 7.Monack DM, Mecsas J, Ghori N, Falkow S. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Ting AT, Marcu KB, Bliska JB. J Immunol. 2005;174:7939–7949. doi: 10.4049/jimmunol.174.12.7939. [DOI] [PubMed] [Google Scholar]
- 9.Orth K, Palmer LE, Bao ZQ, Stewart S, Rudolph AE, Bliska JB, Dixon JE. Science. 1999;285:1920–1923. doi: 10.1126/science.285.5435.1920. [DOI] [PubMed] [Google Scholar]
- 10.Orth K, Xu Z, Mudgett MB, Bao ZQ, Palmer LE, Bliska JB, Mangel WF, Staskawicz B, Dixon JE. Science. 2000;290:1594–1597. doi: 10.1126/science.290.5496.1594. [DOI] [PubMed] [Google Scholar]
- 11.Schesser K, Spiik AK, Dukuzumuremyi JM, Neurath MF, Pettersson S, Wolf-Watz H. Mol Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZJ. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, et al. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 14.Karin M, Ben-Neriah Y. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 16.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 17.Zandi E, Chen Y, Karin M. Science. 1998;281:1360–1363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 18.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 19.Kapahi P, Takahashi T, Natoli G, Adams SR, Chen Y, Tsien RY, Karin M. J Biol Chem. 2000;275:36062–36066. doi: 10.1074/jbc.M007204200. [DOI] [PubMed] [Google Scholar]
- 20.Jeon KI, Byun MS, Jue DM. Exp Mol Med. 2003;35:61–66. doi: 10.1038/emm.2003.9. [DOI] [PubMed] [Google Scholar]
- 21.Carter RS, Pennington KN, Ungurait BJ, Arrate P, Ballard DW. J Biol Chem. 2003;278:48903–48906. doi: 10.1074/jbc.M310686200. [DOI] [PubMed] [Google Scholar]
- 22.Collier-Hyams LS, Zeng H, Sun J, Tomlinson AD, Bao ZQ, Chen H, Madara JL, Orth K, Neish AS. J Immunol. 2002;169:2846–2850. doi: 10.4049/jimmunol.169.6.2846. [DOI] [PubMed] [Google Scholar]
- 23.Sun J, Hobert ME, Rao AS, Neish AS, Madara JL. Am J Physiol Gastrointest Liver Physiol. 2004;287:G220–227. doi: 10.1152/ajpgi.00498.2003. [DOI] [PubMed] [Google Scholar]
- 24.Albanese C, Wu K, D'Amico M, Jarrett C, Joyce D, Hughes J, Hulit J, Sakamaki T, Fu M, Ben-Ze'ev A, et al. Mol Biol Cell. 2003;14:585–599. doi: 10.1091/mbc.02-06-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouzarides T. EMBO J. 2000;19:1176–1179. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quivy V, Van Lint C. Biochem Pharmacol. 2004;68:1221–1229. doi: 10.1016/j.bcp.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 27.Tegeder I, Pfeilschifter J, Geisslinger G. FASEB J. 2001;15:2057–2072. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- 28.Gao Z, Zuberi A, Quon MJ, Dong Z, Ye J. J Biol Chem. 2003;278:24944–24950. doi: 10.1074/jbc.M300423200. [DOI] [PubMed] [Google Scholar]
- 29.Shevchenko A, Wilm M, Vorm O, Mann M. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee S, Keitany G, Li Y, Wang Y, Ball HL, Goldsmith EJ, Orth K. Science. 2006;312:1211–1214. doi: 10.1126/science.1126867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.