Abstract
Enforced expression of growth factor independent 1 (Gfi-1), a transcription repressor induced by T cell activation and IL-4/Stat6 signaling, strikingly enhances Th2 cell expansion. Using conditionally Gfi1-deficient mice prepared for this study, we show that in vitro or in vivo deletion of this factor dramatically reduces Th2, but not Th1, cell expansion in response to IL-2. Both increased cell apoptosis and reduced cell proliferation resulted from Gfi1 deletion. IL-2-Stat5 signaling was partially reduced in Gfi1-deficient Th2 cells, but overexpression of Stat5 failed to restore normal Th2 expansion in these cells, suggesting that Gfi-1 also functioned downstream of, or in parallel with, Stat5 signaling. Reduced Th2 cell expansion in the absence of Gfi-1 was confirmed by the diminished frequency of IL-4-producing cells when these mice were infected with Schistosoma mansoni.
Keywords: cytokine, GATA-3, Schistosome, T cell differentiation
Upon antigen stimulation, naïve CD4 T cells can differentiate into distinct cell types, including the type 1 and type 2 forms of T helper cells (Th1 and Th2) that play important roles in adaptive immunity (1–5). Th1 cells produce IFN-γ and are involved in immunity against intracellular pathogens. Th2 cells produce IL-4, IL-5, and IL-13 and are involved in humoral immunity against extracellular pathogens, particularly helminths. Th1 and Th2 cells are also involved in the induction or maintenance of autoimmune and immunopathologic diseases. Thus, Th1 cells are linked to many organ-specific autoimmune diseases, and Th2 cells play critical roles in asthma and other allergic diseases. Although peptide affinity for T cell antigen receptor, concentration of peptide, and the particular costimulatory molecules involved play important roles in determining the T cell fate, the cytokine milieu is the most important factor for this process. Thus, IL-12 and IFN-γ induce Th1 differentiation (6–12); IL-4 and IL-2 induce Th2 differentiation (13–18).
During Th2 differentiation, the expression levels of GATA-3, the Th2 master regulator (19–23), are enhanced as a result of T cell antigen receptor-mediated signaling and/or activation of the IL-4-Stat6 pathway (20, 24, 25). This process was initially reported to be fully instructive (26). Our previous report that growth factor independent 1 (Gfi-1), induced by IL-4 signaling in activated T cells through Stat6, favors the growth of GATA-3hi cells in IL-2 suggests that there is also a selective component involving Gfi-1 during the Th2 differentiation process (27).
The effects of Gfi-1 seen in overexpression studies do not necessarily mean that Gfi-1 has such functions under physiological conditions. Although Gfi1 knockout mice had been made by two groups (28, 29), these animals display many abnormalities including neutropenia (28, 29), a T cell development defect (29, 30), a hematopoietic stem cell defect (31, 32), as well as defects in dendritic cell development and function (33). Because of their striking defect in T cell development, a large proportion of the peripheral CD4 T cells found in the Gfi1−/− mice display a memory-like phenotype, which may result from lymphopenia-driven cell proliferation, precluding an analysis of the role played by Gfi-1 in the differentiation and/or growth of normal mature CD4 T cells.
To test the physiological function of Gfi-1 in peripheral T cells, we prepared a Gfi1-conditional knockout mouse strain using the Cre-loxP system. Deletion of Gfi1 caused reduced Th2 cell expansion both in vitro and in vivo.
Results
Generation and Characterization of Gfi1−/−, Gfi1fl/fl, and Gfi1fl/fl-CD4Cre Mice.
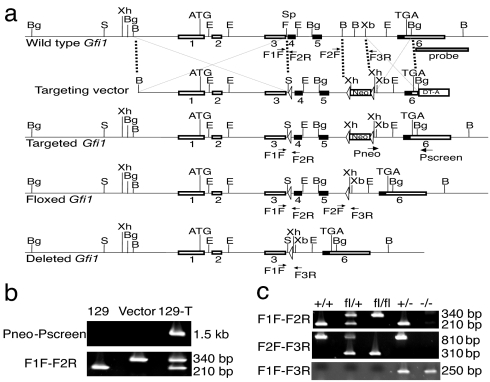
Germ-line Gfi1 knockout mice displayed multiple defects including blockages at different stages of CD4 T cell development. To study Gfi-1 function in mature CD4 T cells, we generated a conditional Gfi1 mutant allele using the Cre-loxP system. Exon 4 and exon 5 of the Gfi1 gene encode four N-terminal zinc fingers important for DNA binding (34) and thus for its function. A construct consisting of a DNA fragment including exon 4 and exon 5 flanked by two loxP sites was prepared. Deletion of these two exons should also introduce a reading frame shift that would prevent the expression of exon 6. The targeting strategy is illustrated in Fig. 1a.
Fig. 1.
Generation and screening of floxed and deleted Gfi1 alleles. (a) Schematic view of the wild-type Gfi1 allele, of the targeting vector containing an expression element for diphtheria toxin A chain (DT-A), of the targeted allele after homologous recombination, and of the floxed or deleted Gfi1 alleles after Cre-mediated excision. Some landmark restriction enzyme sites (B, BamHI; Bg, BglII; E, EcoRI; F, FspI; S, SalI; Sp, SphI; Xb, XbaI; Xh, XhoI) and PCR primers (F1F, F2R, F2F, F3R, Pneo, Pscreen) are shown. (b) The targeted Gfi1 allele was identified after homologous recombination. PCR results using DNA from 129 ES cells (129), from the targeting vector (vector), and targeted ES cells (129-T) as templates are shown. (c) After Cre-mediated excision, floxed and deleted Gfi1 alleles were identified by PCR. Samples from wild-type (+/+), heterozygous floxed (fl/+), homozygous floxed (fl/fl), heterozygous deleted (+/−), and homozygous deleted (−/−) mice are shown.
Upon homologous recombination, a Gfi1 allele containing three loxP sites was generated and identified by PCR (Fig. 1 a and b). PCR results were confirmed by Southern blot analysis (data not shown). Mice carrying the germ-line-transmitted Gfi1 mutant were then bred to EIIa-Cre transgenic mice (35). Among their offspring, mice that had deleted just the neo cassette (floxed allele, fl) or all deleteable elements (deleted allele, −) were obtained (Fig. 1 a and c). Gfi1+/− mice were intercrossed to generate Gfi1−/− mice. Gfi1fl/+ mice lacking the EIIa-Cre transgene were backcrossed to C57BL/6 mice for 5–9 generations and then intercrossed to obtain mice for experimental purposes.
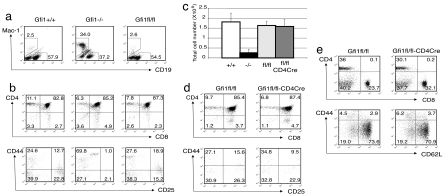
Consistent with previous reports (28, 29), Gfi1−/− mice usually have small body size (data not shown). A high percentage of an abnormal myeloid cell population (34% of total splenocytes), positive for CD11b (Mac-1), was detected in Gfi1−/− mice (Fig. 2a). Surprisingly, ≈10–20% of Gfi1fl/fl mice displayed partial phenotypes of Gfi1−/− mice, i.e., slightly reduced body size and an elevated percentage of CD11b+ cells in the spleen. This set of Gfi1fl/fl mice was excluded from the experiments thereafter. The majority of the Gfi1fl/fl mice appeared healthy and had no obvious phenotypic differences from wild-type (+/+) littermates. The abnormal CD11b+ population was absent from the spleens of these Gfi1fl/fl mice (Fig. 2a). A modest reduction in the percentage of B cells in spleen was noticeable in Gfi1−/− but not in “healthy” Gfi1fl/fl mice. Most importantly, a thymocyte developmental blockage from double negative (DN)1 (CD44hiCD25loCD4−CD8− cells) to DN2 (CD44intCD25hiCD4−CD8− cells), which presumably results in the small thymus of the germ-line-deficient mice, was observed in Gfi1−/− but not in Gfi1fl/fl mice (Fig. 2b). Thus, the cellularity of thymuses from Gfi1−/− mice was reduced to ≈14% of those from wild-type mice in agreement with previous reports (29, 30); healthy Gfi1fl/fl mice had normal-sized thymuses with CD4/CD8 profiles similar to those of wild-type mice (Fig. 2 b and c).
Fig. 2.
Characterization of conditional and germ-line Gfi1 knockout mice. (a) A flow cytometric analysis of splenocytes after cell surface staining for B cells and atypical myeloid cells. (b and d) Cell surface staining with antibodies to CD4 and CD8 on thymocytes. Dot plots for CD44/CD25 were gated on CD4−CD8− (DN) cells. (c) Total cell number counts of thymocytes from 4- to 6-week-old wild-type (+/+, n = 8), germ-line Gfi1 knockout (−/−, n = 7), homozygous floxed Gfi1 (fl/fl, n = 8), and T cell-specific Gfi1 knockout (fl/fl-CD4Cre, n = 4) mice. (e) Cell surface staining of lymph node T cells with antibodies to CD4 and CD8. Dot plots for CD44/CD62L were gated on CD4 cells. Numbers indicate percentage of cells in each quadrant or gate.
Gfi1fl/fl mice were then bred to CD4Cre transgenic mice (36). In the Gfi1fl/fl-CD4Cre mice (T cell-specific Gfi1-deficient mice), although the ratio of CD4 single positive to CD8 single positive thymocytes was somewhat reduced, the size of the thymus was within the normal range and no DN1-DN2 developmental blockage was noted (Fig. 2 c and d). No abnormal myeloid cells were detected in the spleens of Gfi1fl/fl-CD4Cre mice (data not shown). In the lymph nodes of Gfi1fl/fl-CD4Cre mice, CD4 T cells were only slightly lower in percentage compared with that in Gfi1fl/fl mice (30% versus 36%; Fig. 2e). Most importantly, among those CD4 cells, the percentage of naïve cells (CD62LhiCD44lo) in Gfi1fl/fl-CD4Cre lymph nodes was similar to that in Gfi1fl/fl lymph nodes (71% versus 74%). The deletion efficiency of Gfi1 in both purified CD4 and CD8 T cells from Gfi1fl/fl-CD4Cre mice was >99% judged by real-time PCR results (data not shown).
Deletion of Gfi1 Impairs Th2 Cell Proliferation in Vitro.
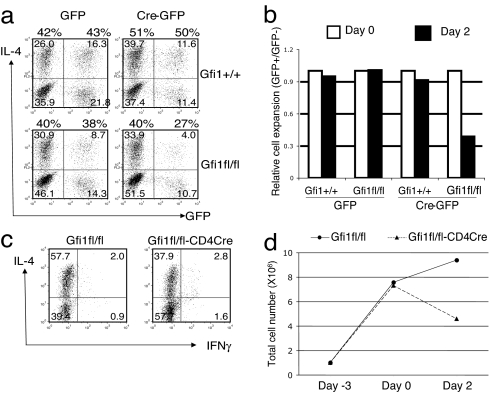
CD4 T cells from Gfi1fl/fl or Gfi1+/+ mice were activated for 24 h with anti-CD3/anti-CD28 under Th2 priming conditions (IL-2, IL-4, anti-IL-12, and anti-IFN-γ) and infected with murine stem cell virus (MSCV)-Cre (37), a retrovirus coexpressing Cre and GFP. Th2 priming was continued for 4 additional days; the cells were restimulated, and intracellular staining for IL-4 was carried out. Cre− and Cre+ cells were identified on the basis of expression of GFP. Infection of Gfi1fl/fl cells with the MSCV-Cre, resulting in the deletion of Gfi1, had limited effect on Th2 cell polarization. Thus, the percentage of IL-4-producing cells was only modestly decreased, from 40% in the Gfi1fl/fl GFP− population to 27% in the Gfi1fl/fl GFP+ population (Fig. 3a). After priming, the relative capacity of the Gfi-1− and Gfi-1+ cells to expand in IL-2 was determined by the monitoring ratio of GFP+/GFP− cells. Deletion of Gfi1 reduced the relative cell expansion in IL-2 over a 2-day period by a factor of 3 (Fig. 3b).
Fig. 3.
Th2 cell expansion in IL-2 medium is impaired in the absence of Gfi-1. CD4 T cells were activated under Th2 conditions for 24 h, infected with the MSCV-Cre followed by an additional 4-day culture under continuous Th2 conditions (a and b). CD4 T cells were activated under Th2 conditions for 3 days (c and d). Cells were washed and then cultured in IL-2-containing medium. After stimulation with phorbol 12-myristate 13-acetate and ionomycin for 4 h in the presence of monensin, cells were fixed, permeabilized, and stained for IL-4, IFN-γ, CD4, and/or GFP expression (a and c). Numbers indicate percentage of cells in each quadrant. Relative cell expansion was determined by measuring the ratio of GFP/GFP− cells at day 2 of culture in IL-2-containing medium compared with the ratio at end of the priming (day 0), which was set to 1 (b). Total cell numbers before priming (day −3), after priming (day 0), and after 2 days of culture in IL-2-containing medium (day 2) (d). Data are representative of at least five (a and b) and three (c and d) similar independent experiments.
Because Gfi-1 is induced during the initial 24 h of culture under Th2 priming conditions (27), before the deletion by Cre occurs, the defect in Th2 cell expansion might be even greater if Gfi1 were deleted earlier. Thus, CD4 T cells were purified from Gfi1fl/fl-CD4Cre mice and Gfi1fl/fl mice. After 3-day priming under Th2 conditions and restimulation for 4 h, a decrease in the percentage of IL-4-producing cells was observed (37.9% for Gfi1fl/fl-CD4Cre versus 57.7% for Gfi1fl/fl; Fig. 3c), similar to the results from MSCV-Cre experiments. Surprisingly, Gfi1-deficient cells (Gfi1fl/fl-CD4Cre) expanded to the same extent as wild-type cells (Gfi1fl/fl) during the 3 days of T cell receptor stimulation (Fig. 3d). However, when these cells were placed into IL-2, the expansion of wild-type Th2 cells continued, whereas the total cell number of Gfi1-deficient Th2 cells fell (Fig. 3d). The expansion of Th1 cells in IL-2 in the absence of Gfi-1 was only slightly affected in some experiments or not at all affected in others (data not shown). The percentage of IFN-γ-producing cells as well as their mean fluorescent intensity among Th1 cells was usually enhanced when Gfi-1 was absent (Fig. 6a, which is published as supporting information on the PNAS web site).
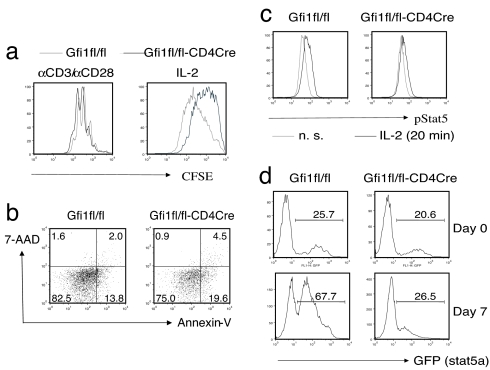
To determine whether Gfi-1 affects cell proliferation, cells were labeled with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) before or after Th2 priming. When cells were labeled with CFSE at the beginning of the Th2 priming process and analyzed by flow cytometry 3 days later, Gfi1-deficient cells diluted CFSE as efficiently as did wild-type cells (Fig. 4a). However, if cells were labeled with CFSE after Th2 priming and then cultured in IL-2 for 1 week, Gfi1-deficient cells divided at a slower rate than did wild-type cells as judged by CFSE dilution. These results, consistent with the cell yield at different stages as shown in Fig. 3d, suggest that Gfi-1 plays an important role in cytokine-mediated but not T cell antigen receptor-driven T cell proliferation. To address whether Gfi1 deletion also affects cell apoptosis, annexin-V staining was performed on day 2 of culture in IL-2. The percentage of annexin-V-positive cells in Gfi1fl/fl-CD4Cre Th2 cells (24.1%) was greater than that in Gfi1fl/fl Th2 cells (15.8%), suggesting that Gfi-1 also diminishes cell apoptosis (Fig. 4b).
Fig. 4.
Gfi1 deletion in Th2 cells led to reduced cell proliferation as well as enhanced cell apoptosis in response to IL-2/Stat5. CD4 T cells were activated under Th2 conditions for 3 days, washed, and then cultured in IL-2-containing medium. (a) CD4 T cells were labeled with CFSE either before priming (αCD3/αCD28) or after priming (IL-2), and CFSE dilution was measured 3 or 7 days after labeling. Annexin-V staining (b) or phospho-Stat5 staining (c) was carried out on day 2 on cells cultured in IL-2 after Th2 priming. (d) CD4 T cells were activated and infected with the Stat5a-GFP-retrovirus as described in Fig. 3a. The percentage of GFP-positive cells was measured by flow cytometry before (day 0) or after culture in IL-2-containing medium for 7 days (day 7). Data are representative of two independent experiments.
Gfi-1 and IL-2-Stat5 Signaling in Th2 Cells.
We previously reported that enforced Gfi-1 expression caused increased Stat5 signaling in response to IL-2 (27). Indeed, in response to IL-2, Gfi1fl/fl-CD4Cre Th2 cells displayed less Stat5 tyrosine phosphorylation than did Gfi1fl/fl Th2 cells (Fig. 4c). To address whether the decreased Stat5 signaling in Gfi1-deficient Th2 cells can explain the decreased cell expansion in such cells, a Stat5-GFP-retrovirus (38) was introduced during Th2 priming. Before cells were cultured in IL-2 medium (day 0) and after 7 days of culture in IL-2 (day 7), the percentage of GFP+ cells was assessed by flow cytometry. Interestingly, enhanced Stat5 signaling as a result of overexpression of Stat5 dramatically promoted cell expansion in wild-type Th2 cells but had little effect in Gfi1-deficient cells (Fig. 4d). In addition, in the absence of added cytokines, overexpression of Gfi-1 further promotes the expansion of Th2 cells that express a constitutively active form of Stat5 (Fig. 6b). These data imply that although Gfi-1 can regulate IL-2-Stat5 signaling, it also functions downstream of, or in parallel with, Stat5 signaling to achieve optimal Th2 cell expansion under physiological conditions.
Deletion of Gfi1 Diminishes Th2 Responses in Vivo.
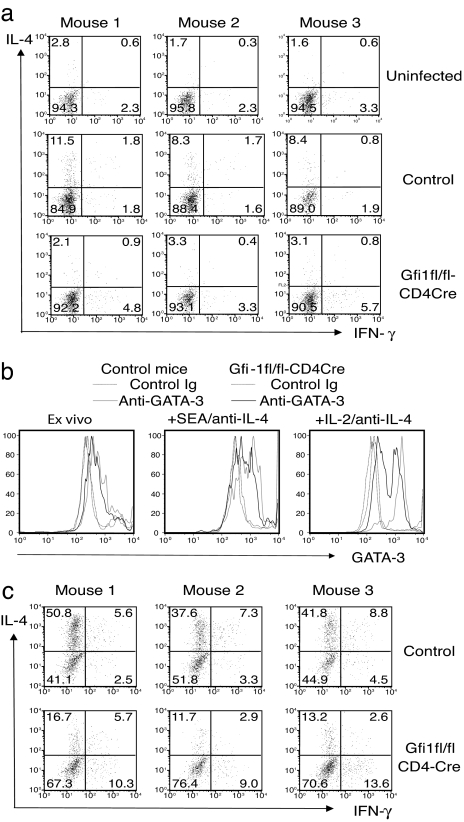
Schistosoma mansoni is a helminthic parasite that triggers a highly polarized Th2 response beginning at 6–8 weeks after infection (39). To test whether Gfi-1 is important for in vivo Th2 responses, Gfi1fl/fl-CD4Cre mice were infected with S. mansoni. Eight weeks later, cytokine production by mesenteric lymph node cells was measured after ex vivo stimulation. Fewer mesenteric lymph node CD4+CD44hi cells from Gfi1fl/fl-CD4Cre mice made IL-4 (3–4%) than did cells from infected control mice (9–13%, Fig. 5a). In addition, the proportion of cells expressing large amounts of GATA-3, as determined by intracellular staining, was less than that among the control Gfi1-sufficient group (Fig. 5b). In vitro stimulation of such cells with schistosoma egg antigen (SEA) for 5 days in the presence of anti-IL-4 significantly increased the percentage of GATA-3hi cells in both groups; however, there were still fewer GATA-3hi cells in the Gfi1-deficient population. Furthermore, after another 3 days of culture in IL-2 medium, most of the cells in control group were GATA-3hi, whereas >50% of the cells in Gfi1-deficient group were GATA-3lo. Accordingly, when IL-4 production was assessed by intracellular staining after SEA stimulation, ≈3-fold fewer Gfi1-deficient cells made IL-4 than did wild-type cells (Fig. 5c). The production of other Th2 cytokines including IL-5, IL-10, and IL-13 was also reduced by ≈3-fold in Gfi1-deficient cells when analyzed by ELISA (data not shown). In vivo Th1 responses to Toxoplasma gondii in Gfi1fl/fl-CD4Cre mice were slightly higher than those in control mice (Fig. 6c). These results indicate that Gfi-1 play an important role in regulating Th2 responses in vivo, possibly by providing GATA-3hi cells with a growth advantage in response to cytokines.
Fig. 5.
Gfi-1 plays an important role in in vivo Th2 responses. Mice were percutaneously infected with 35 S. mansoni cercariae (Naval Medical Research Institute strain) and killed 8 weeks later. Mesenteric lymph node cells were either stimulated with plate-bound anti-CD3 and anti-CD28 for 6 h to assess cytokine production (a) or with a soluble extract of schistosome eggs (SEA) in the presence of anti-IL-4, anti-IFN-γ, and anti-IL-12 for 5 days. Cells restimulated with SEA were then washed and cultured in IL-2-containing medium for additional 3 days. Cells were stained for GATA-3 expression at different stages (b) or restimulated by plate-bound anti-CD3 and anti-CD28 to assess the cytokine production after SEA stimulation (c). Data are representative of three independent experiments.
Discussion
We previously reported that Gfi-1 was induced by IL-4/Stat6 signaling in activated T cells and that, when its expression was prolonged, it substantially enhanced Th2 but not Th1 cell expansion in response to IL-2 (27). However, the physiological role of Gfi-1 during Th2 responses, particularly during in vivo Th2 responses, was unclear. Multiple defects, including defects in neutrophil development (28, 29), T cell development (30), dendritic cell maturation (33), as well as in haematopoietic stem cell renewal (31, 32), make it difficult to use germ-line knockout mice to assess Gfi-1's role in mature T cells during immune responses. Therefore, we generated a conditional Gfi1 knockout mouse strain using the Cre-loxP system. In the majority of the homozygous “floxed” mice, in which both Gfi1 alleles were flanked by loxP sites, no abnormalities in the development of myeloid lineage or lymphoid lineage cells were found, whereas germ-line deletion of Gfi1 resulted in abnormal development of both lineages, as previously reported. By crossing floxed Gfi1 mice to CD4Cre transgenic mice, we obtained mice in which Gfi1 is only deleted in mature T cells. Using such mice, we found that deletion of Gfi1 impaired expansion of normal Th2 cells in vitro and in vivo, resulting in a lower percentage of GATA-3hi cells among the Th2 population. On the basis of these results and previous data that forced expression of Gfi-1 results in a proliferative advantage for GATA-3hi cells grown in IL-2, leading to the dominance of the population of Gfi-1+GATA-3hi cells, we conclude that Gfi-1 plays an important role in Th2 cell expansion.
Th1 cell expansion in response to IL-2 seems less dependent on, or independent of, Gfi-1. Because Gfi-1 is expressed at lower levels in Th1 cells, such low levels of expression may be insufficient to activate this growth pathway. Furthermore, overexpression of Gfi-1 by retroviral infection in Th1 cells did not convey a growth advantage to such cells (27). Therefore, it is unlikely that Th1 cells use the pathway mediated by Gfi-1 for cell expansion. The fact that Gfi-1 collaborates with GATA-3 in cell expansion in response to IL-2 further indicates that Gfi-1-mediated growth is Th2 specific (27). These results imply that Gfi-1 plays an important physiologic role in Th2 cell expansion and that, by acting only on Th2 cells, its expression helps to explain how Th2 cells may come to dominate cell populations responding to given stimuli.
On the other hand, Gfi-1 does have some functions in regulating Th1 responses despite its low expression levels. The frequency of IFN-γ-producing cells and the amount of IFN-γ per cell were consistently greater in Gfi1-deficient Th1 cells compared with that in wild-type Th1 cells (Fig. 6a). IFN-γ mRNA in Gfi1-deficient Th2 cells was also substantially increased (data not shown). These results suggest that Gfi-1 may directly inhibit IFN-γ production.
Gfi-1 was first discovered in assays that measure conversion of a cell line from IL-2 dependence to IL-2 independence (40), thus its name “growth factor independent-1.” However, for Gfi-1-driven Th2 cell expansion, Gfi-1 depends on cytokine signaling (27). Stat5 activation induced by cytokines including IL-2 plays an important role in T cell proliferation and survival (41, 42). Initially, we thought that Gfi-1 promoted Th2 cell proliferation through its capacity to enhance IL-2/Stat5 signaling. Thus, Stat5 phosphorylation induced by IL-2 as well as CD25 expression in Gfi-1 overexpressing Th2 cells was greatly increased or prolonged (27). The members of the suppressor of cytokine signaling (SOCS) family, induced by cytokines, negatively regulate cytokine signaling (43). In Gfi-1-infected Th2 cells, the expression levels of SOCS-1 and SOCS-3 were decreased, suggesting a mechanism for enhancing IL-2/Stat5 signaling (Fig. 6d). Indeed, SOCS-1 and SOCS-3 have been reported as repression targets of Gfi-1B (44), the other member of Gfi-1 family (34, 45, 46). Conversely, in Gfi1-deficient Th2 cells, IL-2-induced Stat5 phosphorylation was reduced, which correlated with a modest increase in the expression levels of SOCS-1 (Fig. 6e). However, overexpression of Stat5a in Gfi1-deficient Th2 cells failed to rescue the reduction in cell expansion in response to IL-2. In addition, Th2 cells expressing a constitutively active form of Stat5a as a result of retroviral infection, which can expand in the absence of added cytokines, showed further enhancement in cell growth upon overexpression of Gfi-1 (Fig. 6b). These results imply that even in the presence of excess Stat5 or when Stat5 signaling is unregulated, Gfi-1 still plays a role in cell growth. Thus, although Gfi-1 does regulate Stat5 signaling, possibly through inhibition of SOCS family protein expression, it appears to also work in parallel with, or downstream of, Stat5 signaling for Th2 cell expansion.
Materials and Methods
Generation of Mice Carrying Floxed Gfi1 Alleles and Cre Transgenes.
A targeting vector was constructed by cloning Gfi1 fragments digested from a BAC clone [obtained from Incyte Genomics, Inc., Palo Alto, CA; clone address: 250(C3)] into the pL2-Neo vector (a gift from Hua Gu, Columbia University, New York, NY), as shown in Fig. 1a. The SphI/BamHI fragment (≈1.8 kb) containing Gfi1 exons 4 and 5 was flanked by two loxP sites. A neo expression cassette (from pMC1-neo; Stratagene, La Jolla, CA), also flanked by loxP sites, was present (≈1.4 kb) and located 3′ of the SphI/BamHI fragment. The 5′ Gfi1 gene element (≈4.4 kb) extended from the BamHI site to the FspI site in intron 3 (3 bp 3′ of SphI). The 3′ Gfi1 gene element (≈1.3 kb) was obtained from the XbaI/BglII fragment in intron 5 and exon 6. An expression cassette for diphtheria toxin A chain (DT-A) was placed 3′ of the construct to facilitate negative selection for homologous recombination. Murine 129 embryonic stem cells (line R1) (47) were electroporated with the construct, and neomycin-resistant clones were screened by PCR with the following primer pairs: Pneo/Pscreen (located within the Neo expression cassette and outside of the targeting vector respectively) and F1F/F2R as shown in Fig. 1 a and b (see below for primer sequences). The targeted ES cells were further verified by Southern blotting using a 3′ DNA probe (≈1.5 kb) obtained from the 250(C3) BAC after BglII/BamHI digestion (Fig. 1a and data not shown). Gfi1-targeted mice were obtained and then crossed to C57BL/6-EIIa-Cre (35) transgenic mice. Mice carrying either the Gfi1 floxed allele (fl) or the Gfi1 knockout allele (−) were identified by PCR using the following primer pairs: F1F/F2R, F2F/F3R, and F1F/F3R, as shown in Fig. 1 a and c. Gfi1fl/+ mice not carrying the EIIa-Cre transgene were backcrossed to C57BL/6 for five to nine generations then intercrossed to generate homozygous mice. Some mice were also crossed to CD4Cre. CD4Cre transgenic mice (line 4196), originally generated by C. B. Wilson and colleagues (36), were purchased from Taconic (Germantown, NY). All of the mice were bred and maintained in the National Institute of Allergy and Infectious Diseases specific pathogen-free animal facility, and the experiments were done when mice were at 5–12 weeks of age under an approved protocol according to the National Institute of Allergy and Infectious Diseases guidelines for animal care.
Genotyping by PCR.
Cells or tail samples were lysed and genotyped by PCR as described (22). The sequences of the primers are: 5′-TCTTGCAAAACCACACTGCTCGACAT-3′ (Pneo), 5′-GTAGCAGCTGCCCAGAGTTCATT-3′ (Pscreen), 5′-CAGTCCGTGACCCTCCAGCAT-3′ (F1F), 5′-CTGGGAGTGCACTGCCTTGTGTT-3′ (F2R), 5′-GTTCCCTAACAGTGACAATTCCTTA-3′ (F2F), and 5′-CCATCTCTCCTTGTGCTTAAGAT-3′ (F3R). Cre was genotyped by real-time PCR using the primer/probe set as described (22).
Cell Culture.
Lymph node CD4 T cells were prepared by magnetic-activated cell separation purification using CD4 microbeads (Miltenyi Biotec, Auburn, CA); purity was usually 90–95%. In some experiments, naïve CD4 T cells were obtained by cell sorting for the CD4+CD8−CD62LhiCD44lo cell population, with an ≈98% purity. T cell-depleted antigen-presenting cells (APCs) were prepared by incubating spleen cells with anti-Thy1.2 and rabbit complement (Cedarlane Laboratories Limited, Burlington, NC) at 37°C for 45 min, and then irradiated at 30 Gy (3,000 rad). T cells were cocultured with APCs at a 1:5 ratio, in the presence of anti-CD3 (1 μg/ml) and anti-CD28 (3 μg/ml). Human IL-2 (50 units/ml) was added to all cultures together with different combinations of antibodies and cytokines: for Th1 conditions, anti-IL-4 (10 μg/ml) and IL-12 (10 ng/ml); for Th2 conditions, IL-4 (5,000 units/ml), anti-IFN-γ (10 μg/ml), and anti-IL-12 (10 μg/ml); and for Th-null conditions, anti-IL-4 (10 μg/ml), anti-IFN-γ (10 μg/ml), and anti-IL-12 (10 μg/ml).
Preparation of Retroviral Constructs and Infection.
MSCV-Cre (37) was kindly provided by D. R. Littman (Skirball Institute of Biomolecular Medicine, New York, NY). Stat5a-GFP-retrovirus (38) was kindly provided by W. J. Leonard (National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD). Retroviruses were packaged in the Phoenix-Eco packaging cell line as described (48). Infection was carried out at 24 h after initiation of cultures in which CD4 T cells were activated with anti-CD3/anti-CD28 under Th2 conditions in the presence of APC.
Flow Cytometry Analysis.
The expression of GFP in retrovirally infected cells was measured by flow cytometry. Cell surface staining was carried out in PBS containing 0.1% PBS with different combinations of antibodies. Anti-CD4-FITC, anti-CD19-phycoerythrin (PE), anti-CD25-PE, anti-CD62L-PE, anti-CD8-Cy-Chrome, anti-CD4-APC, anti-Mac-1-APC, and anti-CD44-APC were purchased from BD Pharmingen (San Diego, CA). For intracellular cytokine staining, cells were restimulated with phorbol 12-myristate 13-acetate (10 ng/ml) and ionomycin (1 μM) for 4 h or with plate-bound anti-CD3 and anti-CD28 (3 μg/ml of each) for 6 h in the presence of monensin (2 μM) during last 3 h. For phospho-Stat5 staining, cells were washed three times with Hanks' balanced salt solution and cultured in serum-free medium for 4 h, followed by IL-2 stimulation (500 units/ml) for 20 min. Harvested samples were fixed with 4% formaldehyde, washed, and permeabilized in 0.5% Triton X-100/0.1% BSA in PBS before staining with anti-IL-4-PE and anti-IFN-γ-APC for cytokine production or with anti-pStat5-PE (BD Pharmingen) for Stat5 tyrosine phosphorylation. Protein levels of GATA-3 were assessed by intracellular staining with anti-GATA-3 (Santa Cruz Biotechnology, Santa Cruz, CA; HG3–31) or a control mouse IgG1 followed by Cy5-anti-mouse IgG staining.
Cell Proliferation and Apoptosis Assay.
Cells were labeled with CFSE as described (27). The division status of cells was determined by measuring CFSE fluorescence after certain periods of culture. Apoptosis was measured by annexin-V-PE staining according to the manufacturer's protocol (BD Pharmingen).
S. mansoni Infection and SEA Stimulation.
Mice were percutaneously infected with 35 S. mansoni cercariae (Naval Medical Research Institute strain) and killed 8 weeks later as described (39). Mesenteric lymph node cells were harvested. They were then either stimulated with plate-bound anti-CD3 and anti-CD28 for 6 h to assess the cytokine production or restimulated with a soluble extract of schistosome eggs (SEA) and prepared as described (49) in the presence of anti-IL-4, anti-IFN-γ, and anti-IL-12 for 5 days. Cells were then washed and cultured in IL-2-containing medium for additional 3 days.
Supplementary Material
Acknowledgments
We thank Dr. A. Sher for his helpful suggestions on this project and critical review of this manuscript, Dr. A. Cheever for analyzing mice infected with S. mansoni, and Ms. S. Tanksley for cell sorting. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases.
Abbreviations
- Gfi-1
growth factor independent 1
- MSCV
murine stem cell virus
- Th
T helper
- DN
double negative
- CFSE
5,6-carboxyfluorescein diacetate succinimidyl ester
- SEA
schistosoma egg antigen
- SOCS
suppressor of cytokine signaling
- APC
antigen-presenting cells
- PE
phycoerythrin.
Footnotes
The authors declare no conflict of interest.
References
- 1.Seder RA, Paul WE. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 2.Murphy KM, Reiner SL. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 3.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 4.Mowen KA, Glimcher LH. Immunol Rev. 2004;202:203–222. doi: 10.1111/j.0105-2896.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 5.Ansel KM, Djuretic I, Tanasa B, Rao A. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 6.Seder RA, Gazzinelli R, Sher A, Paul WE. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 8.Wenner CA, Guler ML, Macatonia SE, O'Garra A, Murphy KM. J Immunol. 1996;156:1442–1447. [PubMed] [Google Scholar]
- 9.Bradley LM, Dalton DK, Croft M. J Immunol. 1996;157:1350–1358. [PubMed] [Google Scholar]
- 10.Szabo SJ, Dighe AS, Gubler U, Murphy KM. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O'Shea JJ. Proc Natl Acad Sci USA. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 13.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Sasson SZ, Le Gros G, Conrad DH, Finkelman FD, Paul WE. J Immunol. 1990;145:1127–1136. [PubMed] [Google Scholar]
- 15.Swain SL, Weinberg AD, English M, Huston G. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 16.Seder RA, Germain RN, Linsley PS, Paul WE. J Exp Med. 1994;179:299–304. doi: 10.1084/jem.179.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J, Cote-Sierra J, Guo L, Paul WE. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 18.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Proc Natl Acad Sci USA. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. Cell Res. 2006;16:3–10. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
- 20.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. J Biol Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 21.Zheng W, Flavell RA. Cell. 1997;89:587–596. [Google Scholar]
- 22.Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF, Jr, Guo L, Paul WE. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 23.Pai SY, Truitt ML, Ho IC. Proc Natl Acad Sci USA. 2004;101:1993–1998. doi: 10.1073/pnas.0308697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 25.Ouyang W, Lohning M, Gao Z, Assenmacher M, Ranganath S, Radbruch A, Murphy KM. Immunity. 2000;12:27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 26.Farrar JD, Ouyang W, Lohning M, Assenmacher M, Radbruch A, Kanagawa O, Murphy KM. J Exp Med. 2001;193:643–650. doi: 10.1084/jem.193.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu J, Guo L, Min B, Watson CJ, Hu-Li J, Young HA, Tsichlis PN, Paul WE. Immunity. 2002;16:733–744. doi: 10.1016/s1074-7613(02)00317-5. [DOI] [PubMed] [Google Scholar]
- 28.Karsunky H, Zeng H, Schmidt T, Zevnik B, Kluge R, Schmid KW, Duhrsen U, Moroy T. Nat Genet. 2002;30:295–300. doi: 10.1038/ng831. [DOI] [PubMed] [Google Scholar]
- 29.Hock H, Hamblen MJ, Rooke HM, Traver D, Bronson RT, Cameron S, Orkin SH. Immunity. 2003;18:109–120. doi: 10.1016/s1074-7613(02)00501-0. [DOI] [PubMed] [Google Scholar]
- 30.Yucel R, Karsunky H, Klein-Hitpass L, Moroy T. J Exp Med. 2003;197:831–844. doi: 10.1084/jem.20021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hock H, Hamblen MJ, Rooke HM, Schindler JW, Saleque S, Fujiwara Y, Orkin SH. Nature. 2004;431:1002–1007. doi: 10.1038/nature02994. [DOI] [PubMed] [Google Scholar]
- 32.Zeng H, Yucel R, Kosan C, Klein-Hitpass L, Moroy T. EMBO J. 2004;23:4116–4125. doi: 10.1038/sj.emboj.7600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rathinam C, Geffers R, Yucel R, Buer J, Welte K, Moroy T, Klein C. Immunity. 2005;22:717–728. doi: 10.1016/j.immuni.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Grimes HL, Chan TO, Zweidler-McKay PA, Tong B, Tsichlis PN. Mol Cell Biol. 1996;16:6263–6272. doi: 10.1128/mcb.16.11.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, et al. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 37.Zou YR, Sunshine MJ, Taniuchi I, Hatam F, Killeen N, Littman DR. Nat Genet. 2001;29:332–336. doi: 10.1038/ng750. [DOI] [PubMed] [Google Scholar]
- 38.Xue HH, Fink DW, Jr, Zhang X, Qin J, Turck CW, Leonard WJ. Int Immunol. 2002;14:1263–1271. doi: 10.1093/intimm/dxf101. [DOI] [PubMed] [Google Scholar]
- 39.Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Ward JM, Cheever AW, Paul WE, Sher A. J Immunol. 1999;163:337–342. [PubMed] [Google Scholar]
- 40.Gilks CB, Bear SE, Grimes HL, Tsichlis PN. Mol Cell Biol. 1993;13:1759–1768. doi: 10.1128/mcb.13.3.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin JX, Leonard WJ. Oncogene. 2000;19:2566–2576. doi: 10.1038/sj.onc.1203523. [DOI] [PubMed] [Google Scholar]
- 42.Moriggl R, Topham DJ, Teglund S, Sexl V, McKay C, Wang D, Hoffmeyer A, van Deursen J, Sangster MY, Bunting KD, et al. Immunity. 1999;10:249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- 43.Alexander WS, Hilton DJ. Annu Rev Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 44.Jegalian AG, Wu H. J Biol Chem. 2002;277:2345–2352. doi: 10.1074/jbc.M105575200. [DOI] [PubMed] [Google Scholar]
- 45.Tong B, Grimes HL, Yang TY, Bear SE, Qin Z, Du K, El-Deiry WS, Tsichlis PN. Mol Cell Biol. 1998;18:2462–2473. doi: 10.1128/mcb.18.5.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saleque S, Cameron S, Orkin SH. Genes Dev. 2002;16:301–306. doi: 10.1101/gad.959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu J, Guo L, Watson CJ, Hu-Li J, Paul WE. J Immunol. 2001;166:7276–7281. doi: 10.4049/jimmunol.166.12.7276. [DOI] [PubMed] [Google Scholar]
- 49.Boros DL, Warren KS. J Exp Med. 1970;132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.