Abstract
Introduction
Holmium laser enucleation of the prostate (HoLEP) combined with mechanical morcellation represents the latest refinement of holmium:YAG surgical treatment for benign prostatic hyperplasia (BPH). Utilizing this technique, even the largest of glands can be effectively treated with minimal morbidity. The learning curve remains an obstacle, preventing more widespread adoption of this procedure. This paper provides an outline of the HoLEP technique as is currently used at two centers in hopes of easing the initial learning curve.
Technical considerations
Detailed descriptions of the major steps of the HoLEP procedure are provided with attention to critical steps such as identification of the surgical capsule, median and lateral lobe enucleation, and morcellation of enucleated tissue.
Conclusions
HoLEP is a promising alternative for the surgical treatment of BPH which allows complete removal of intact lobes of the prostate. Obstruction is relieved immediately with superior hemostasis, no risk of TUR syndrome, and a minimal hospital stay.
Keywords: Benign prostatic hyperplasia, Holmium, Lasers
Introduction
Benign prostatic hyperplasia (BPH), which is prevalent in up to 43% of men over the age of 60[1], continues to be a leading cause of voiding difficulties. Symptoms generally worsen with age, with men experiencing a mean increase of the American Urological Association (AUA) symptom score of 0.18 points per year of follow-up[2] Although symptoms can often be handled adequately with medical therapy, patients who continue to suffer significant voiding problems will eventually require surgical intervention to avoid or treat long-term sequelae such as urinary retention, urinary tract infections, and bladder calculi.
When surgical treatment is considered, transurethral resection of the prostate (TURP) is still the standard to which all other modalities are compared. Although the morbidity associated with TURP can be low when performed by experienced surgeons, complications such as TUR syndrome and bleeding can still occur. For this reason, many alternative energy modalities have been developed in an attempt to not only provide an effective surgical treatment for BPH, but also to minimize the risk of complications and the length of hospital stays.
One such technological advance involves the holmium:YAG laser. This laser has found widespread urologic applications, especially in the treatment of urolithiasis[3] Because the laser's wavelength is highly absorbed by water[4], it is also an effective tool for the ablation and cutting of soft tissue. These characteristics naturally extend into applications for BPH.
Use of the holmium:YAG laser in the treatment of BPH has changed significantly over the past five years. As techniques have been refined and equipment improved, the spectrum of treatment has progressed from simple vaporization of tissue to the complete removal, or enucleation, of intact lobes of prostatic adenoma. At the present time, holmium laser enucleation of the prostate (HoLEP) combined with mechanical morcellation represents the latest refinement of the resection technique. By utilizing this procedure, even extremely large glands which previously would have required an open simple prostatectomy can be treated effectively, with only an overnight stay for the patient. In our experience, the largest gland we have treated successfully with HoLEP had a specimen weight of 376 grams after morcellation (unpublished data).
Although HoLEP is a promising alternative for the surgical treatment of BPH, the learning curve for the procedure remains a significant obstacle. The purpose of this paper is to review the development of HoLEP and present a stepwise outline of the procedure, with a complete list of necessary equipment and attention to critical points during the enucleation process. It is hoped that this description of HoLEP will help to flatten the learning curve for urologists interested in learning this procedure.
Background
Historical development of HoLEP
The holmium:YAG laser (2140 nm) acts through a predominantly photothermal mechanism.[5] Because it is capable of vaporizing soft tissue, application of the holmium laser for BPH first focused on sequential ablation of tissue in order to enlarge a channel through the prostate. Initially, treatment with the holmium laser was performed in association with the neodymium (Nd:YAG) laser, in a technique known as combination endoscopic laser ablation of the prostate (CELAP)[6] It was hoped that the holmium laser would be able to reduce the short-term sequelae of Nd:YAG coagulation prostatectomy, which included prolonged catheterization and delayed symptomatic improvement [7]
With CELAP, a circumferential coagulation of prostatic tissue was first performed using the Nd:YAG laser, followed by vaporization of an adequately sized channel using holmium energy. Unfortunately, this combination procedure was still associated with significant indwelling catheterization times postoperatively, ranging from 4.1 to 7.1 days.[6,8] In addition, many patients required re-catheterization for urinary retention and also had irritative voiding symptoms[4,9]
As more clinical experience with the holmium laser was gained, it became apparent that the laser had sufficient hemostatic effects when used to vaporize soft tissue on its own, without the addition of the Nd:YAG component[4] Further evidence of the effectiveness of the holmium laser on prostate tissue was provided by canine and human studies by Kabalin, who showed that hemostatic vaporization occurred using high powers ranging from 50 to 80 watts[10,11] As a result, holmium laser ablation of the prostate, termed HoLAP, was developed, which eliminated many of the short-term voiding problems associated with use of the Nd:YAG laser.
The main advantage of HoLAP was the ease in learning the procedure. One disadvantage, however, was the lack of tissue availability for pathologic analysis. In addition, the procedure was considered cumbersome, mainly because of the slow process of tissue vaporization. In a randomized comparison of holmium vaporization versus TURP, Mottet, et al, showed that the laser procedure required a significantly longer time (75 vs. 56 minutes) to achieve prostatic defects comparable to those in the TURP patients[12] For this reason, HoLAP was considered useful only for smaller glands. In order to achieve more efficiency, attention then turned to developing a method of removing larger portions of adenoma.
The first description of holmium laser resection of the prostate (HoLRP) was reported by Gilling, et al in 1996[13] This technique was the first to utilize the cutting properties of the holmium laser to detach large sections of adenoma tissue. A major advantage is that normal saline could be used as the irrigation fluid, which eliminates the risk of TUR syndrome during resection. During a HoLRP, the median lobe is resected first, followed by the lateral lobes. Because tissue needs to be retrieved endoscopically, the lobes are divided into manageable pieces while being freed from the surgical capsule. Larger fragments are then recovered from within the bladder using a modified resectoscope loop that engages and holds the tissue against the resectoscope sheath, while smaller fragments can be removed using an Ellik evacuator.
Many centers have published HoLRP series with favorable results [13-20] In all of these series, no patients required transfusions or developed dilutional hyponatremia. In a randomized trial comparing HoLRP to TURP with two year follow-up data, there were no significant differences in AUA symptom scores, quality of life scores, peak flow rates, or complications; however, TURP patients had significantly longer mean catheterization times and hospital stays[15]
Operative time remains an issue for HoLRP, with TURP being completed at significantly faster rates[16] One issue contributing to the overall length of the procedure is the retrieval of the resected tissue. First of all, the size of the tissue fragments is limited by the urethral caliber. In addition, the resectoscope must be removed in its entirety each time a fragment is removed, thus slowing the process. Questions have also been raised about the ability to obtain accurate pathologic diagnoses with HoLRP, citing an abundance of cauterization artifact from analyzed specimens[21]
In order to address the previous concerns with HoLRP, a prototype transurethral tissue morcellator was devised by Fraundorfer and Gilling.[22] By morcellating tissue within the bladder, the resection technique could be modified to allow complete enucleation of the median and lateral lobes of the bladder, without the time-consuming process of tailoring the lobes into manageable fragments. This enables the surgeon to achieve a degree of tissue removal approximating that of an open simple prostatectomy while maintaining a minimally invasive nature. As such, the latest modification of the holmium resection technique is now known as holmium laser enucleation of the prostate (HoLEP), combined with mechanical morcellation. HoLEP can be utilized for virtually any BPH configuration or size. Further, concomitant bladder calculi, if present, are easily vaporized by the holmium laser at the time of HoLEP. Using this technique, even the largest of glands can be safely enucleated, with superior results when compared to the alternative approach of open simple prostatectomy[23]. A summary of the published HoLEP results are detailed in table 1.
Table 1.
Summary of HoLEP Results
| Number of patients | Mean patient age (years) | Mean operative time* (min) | Mean enucleated tissue weight (grams) | Mean length of hospital stay (days) | Mean pre-op Qmax (cc/sec) | Mean post-op Qmax (cc/sec) | Mean pre-op AUA SS | Mean post-op AUA SS | |
| Fraundorfer, et al[22] | 14 | 72.0 | 98 | 37.5 | 1.1 | 7.0 | 25.2 | 21.2 | 7.2 |
| Gilling, et al[25] | 64 | 70.2 | 59.2 | 35.5 | 1.3 | 8.9 | 23.4 | 23.0 | 8.6 |
| Moody, et al[26] | 61 | 71.3 | 117 | 48.0 | 1.2 | 7.7 | - | 20.4 | 6.7 |
| Gilling, et al. [27]# | 43 | 73.8 | 82.5 | 61.8 | 1.2 | 9.0 | 24.8 | 23.5 | 2.8 |
| Moody, et al. [23]# | 10 | 74.8 | 197 | 151.0 | 2.1 | - | - | 19.0 | 6.3 |
| Kuntz and Lehrich[28]# | 60 | 69.2 | 135.9 | 83.9 | 2.9 | 3.8 | 27.6 | 22.1 | 3.3 |
* Includes enucleation and morcellation time # Patients with > 100 gram prostates
Currently, we use HoLEP exclusively in our respective institutions for the surgical treatment of BPH. The following is a description of our HoLEP technique, detailing the pre-operative work-up, necessary equipment, and stepwise breakdown of the procedure.
HoLEP Technique
Pre-operative work-up
As with any surgical procedure, the HoLEP pre-operative work-up consists of a detailed history and physical, which should include an AUA symptom score and also a flow rate and post-void residual to objectively document the severity of obstruction. Laboratory tests consist of a complete blood count, serum chemistries, PSA level, and urinalysis with urine culture. Any PSA elevation should be followed by appropriate prostate biopsies before proceeding with HoLEP. A coagulation profile is not routinely obtained at our institutions unless a history of bleeding diathesis is present.
Because size estimates from digital rectal exams can be inaccurate, obtaining a transrectal ultrasound (TRUS) prostate volume pre-operatively is recommended. This is an important data point, as one should aim to start with smaller glands (i.e. < 40 cc) when first learning the procedure. Further, TRUS provides a clearer estimation of the time required for the procedure when arranging operative schedules. In addition, flexible cystoscopy is performed on all patients as part of their workup in order to exclude other forms of obstruction such as urethral strictures.
Lastly, informed consent must be obtained from the patient. Particular emphasis must be placed on the possibility of retrograde ejaculation after the procedure, which had previously been documented to occur in up to 96% of HoLRP patients[16] and in our experience, occurs in most HoLEP patients.
Contraindications
The only absolute contraindications to the HoLEP procedure are a lack of surgical clearance from a cardiopulmonary standpoint or an inability to position the patient in the dorsolithotomy position. In general, bleeding diatheses are not a contraindication to HoLEP; however, we do not recommend treating patients with uncorrected bleeding diatheses during the initial learning curve. In general, if patients are on anti-coagulants (i.e. warfarin) for entities where the medication can be safely stopped (deep venous thrombosis or atrial fibrillation), they should be taken off their medication 3–5 days prior to surgery. The medication can be restarted post-operatively at the normal dose. When anticoagulation cannot be held (i.e. cardiac valves), HoLEP can be performed on fully anticoagulated patients. A list of important equipment required for HoLEP is detailed in table 2.
Table 2.
Essential equipment list
| Video tower and camera |
| Holmium laser unit (must have 80–100 watt maximum power) |
| 550 μ holmium laser fiber |
| 550 μ laser fiber stripper |
| 26 to 28 F continuous flow resectoscope sheath with modified inner sheath (containing laser fiber stabilizing bridge) |
| 30 degree cystoscope lens |
| 7 F laser fiber stabilizing catheter (Cook Urological, Incorporated, Spencer, Indiana) |
| Van Buren sounds |
| Ellik evacuator |
| Stone basket, model FG-24SX-1 (Olympus) |
| 11 F Alligator grasper (Karl Storz) |
| Offset rigid nephroscope (Karl Storz) |
| Morcellator (Lumenis) |
| Normal saline irrigant (3 liter bags) |
| 20 F, 2-way Foley catheter (30 cc balloon) |
| Catheter guide |
Procedure
Step 1 – Urethral calibration
The patient is placed in a dorsolithotomy position, with the thighs abducted maximally to allow appropriate manipulation of the resectoscope during apical dissection of the lateral lobes. After sterile preparation and draping is completed, the patient's urethra is calibrated using Van Buren sounds up to 30 F. Only the anterior urethra should be calibrated and care should be taken to avoid traumatizing the prostatic lobes, which can lead to bleeding and an impaired visual field. The urethra must be able to accept calibration up to 30 F easily; if not, urethrotomy should be considered to widen the distal urethra. This minimizes the possibility of urethral stricture from subsequent resectoscope manipulation.
Step 2 – Insertion of the resectoscope
A 26–28 F continuous flow resectoscope is inserted into the urethra with the Timberlake obturator. The inner sheath, with 7 F laser fiber stabilizing catheter in place, is then locked into the resectoscope. Because of the high power (up to 100 W) utilized for HoLEP, a video system is mandatory for the surgeon's optical protection. The camera is placed on the lens, and the handpiece loosened such that the resectoscope can be rotated easily while holding the camera in a stationary position.
Lastly, the 550 μ laser fiber is placed. Approximately 3 to 4 inches of cladding from the distal portion of the fiber should be stripped off. It is helpful to thread the redundant portion of the fiber through mosquito clamps attached to the drape, thus keeping it out of the surgeon's working space. The fiber can then be inserted into the 7 F stabilizing catheter. Normal saline irrigant is then run through TUR tubing into the resectoscope. The outflow valve should be connected to a piece of tubing to allow drainage into the cystoscopy drape trap and left open, to prevent overdistension of the bladder.
Step 3 – Visualization of the anatomy
With the resectoscope in the bladder, the urethral orifices should be located if possible, though an extremely large median lobe may preclude this. The resectoscope is then pulled back into the prostatic fossa and the extent of lobar hypertrophy assessed. Lastly, the location of the verumontanum and external sphincter is confirmed. The initial step of resection depends on the degree of median lobe hypertrophy. If the median lobe is enlarged, it is best to enucleate this structure first, in order to provide more room for the lateral lobe dissection (Step 4A). If the median lobe is not greatly enlarged, one can begin enucleating a lateral lobe initially (Step 4B). The laser should be set at an energy of 2 J and frequency of 50 Hz.
Step 4A – Enucleation of the median lobe
In the case of trilobar hypertrophy, the surgeon will be able to visualize two sulci at approximately the 5 and 7 o'clock positions, formed by the junctions of the lateral and median lobes (Figure 1). Beginning at the level of the bladder neck at the 7 o'clock position, a groove is formed by cutting with the laser along the sulcus, to a point just lateral and proximal to the verumontanum. This groove is deepened to the level of the surgical capsule. As the groove is developed, it should also be undermined and widened to allow separation of the right lateral lobe and median lobe. The process is then repeated to create a groove at the 5 o'clock position.
Figure 1.
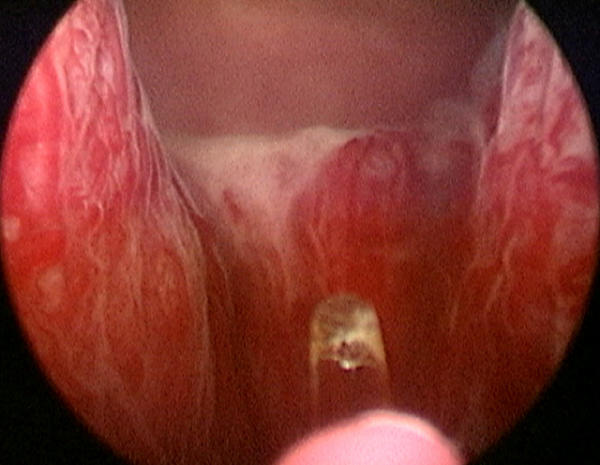
Sulci between median and lateral lobes (at 5 and 7 o'clock)
During the initial learning curve, it is often difficult to discern the level of the plane between the adenoma and surgical capsule when deepening the groove. To facilitate the development of the correct plane, one should first attempt to deepen the groove proximally, as the surgical capsule in this region is more easily defined by its characteristic white circular fibers (Figure 2). As the incision progresses along the sulcus to the mid-portion of the prostatic fossa, the level of the capsule can be more difficult to define. The groove should end just laterally to the verumontanum. If the surgeon is unsure about the depth of the more distal portion of the groove, an initial undermining of the floor of the lateral lobe just lateral to the verumontanum can help to define the proper plane. To begin this dissection, one should guide the laser fiber in a transverse fashion laterally from the verumontanum. The beak of the resectoscope can then be angled under the lateral lobe as the plane between the adenoma and the surgical capsule develops. The appearance of the capsule here is a smooth, glistening surface with occasional diaphanous, cobweb-like attachments to the adenomatous tissue. At times, the capsule may be thinned to the point where fat is visible. The appearance of fat should alert the surgeon to continue developing a plane just superficial to this level. However, these areas seal post-operatively without sequela and are not an indication to abort or shorten the procedure, as in the case of TURP.
Figure 2.
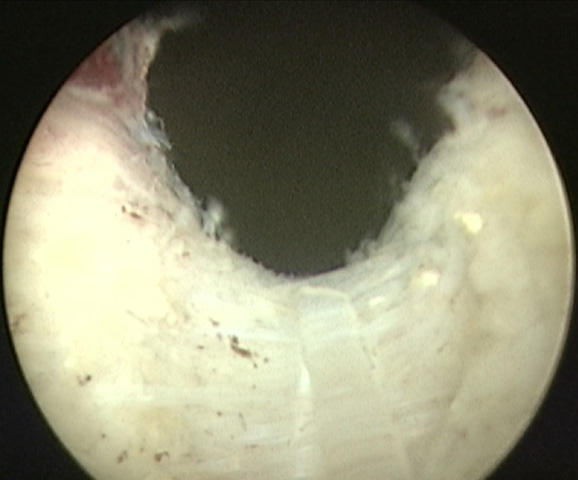
Appearance of proximal surgical capsule
At this point, undermining of the median lobe is begun just proximal to the verumontanum. The laser fiber is swept in a transverse fashion between the two grooves, staying in the plane between the surgical capsule and the adenoma. As the distal portion of the median lobe begins to separate away from the capsule, the beak of the resectoscope should be used as a leverage point to assist in lifting the median lobe upward. This helps define the plane between the adenoma and the capsule. Again, the capsule tends to be smooth and shining, with cobweb-like attachments to the adenoma. The surgeon should take care to follow the capsule as it slopes gently anteriorly toward the region of the bladder neck, to prevent undermining of the trigone. Once the level of the bladder neck is reached, the proximal attachments of the median lobe are freed, and the entire median lobe is pushed into the bladder.
Step 4B – Separation of the right lobe from the capsular floor
If the median lobe does not have significant hypertrophy, a midline groove can be created at the 6 o'clock position beginning at the bladder neck and ending just proximal to the verumontanum. Again, the groove should be defined initially with the tip of the laser fiber and then widened and deepened down to the level of the surgical capsule. Dissection of the right lobe can then be performed by proceeding lateral to the verumontanum in a transverse fashion. Once the initial plane is developed under the right lobe, the surgeon continues in a proximal direction, freeing the lateral lobe from the capsular floor by moving the laser fiber in a transverse, side-to-side motion while using the beak of the resectoscope to leverage the lobe upwards. It is important to avoid rotating the resectoscope too early, as this will cause the fiber tip to arc into the adenoma prematurely and potentially cause loss of plane definition.
Step 5 – Right apical and lateral dissection (from floor)
The plane between the capsular floor and right lobe should be developed proximally toward the bladder. At some point, the surgeon will notice that leverage under the lateral lobe is impaired by the lateral attachments of the lobe, making further proximal progression difficult. Dissection should then proceed to detach the lateral attachments near the apex of the right lateral lobe.
The laser settings are adjusted to a power of 2 J and frequency of 40 Hz during the process of apical dissection, to prevent thermal injury to the sphincter. The resectoscope is retracted distally, and the location of the external urethral sphincter confirmed by visualizing the area where urethral coaptation occurs. After moving the resectoscope proximally to the sphincter, the most lateral point of the previously dissected plane between the floor and right lobe is located. Dissection in this plane is continued, this time staying between the apical tissue of the right lobe and the capsule by rotating the resectoscope in a clockwise direction during cutting. There is limited space for the tip of the resectoscope during this portion of the dissection, restricting the field of view. Therefore, the tip of the laser fiber should be retracted to the point where it is barely visible. The arcing motion of the fiber tip should follow the curve of the surgical capsule as it progresses laterally. It is important to begin the dissection at a point slightly proximal to the apex, in order to leave behind an "apical pad" of tissue that helps protect the integrity of the sphincter (Figure 3).
Figure 3.
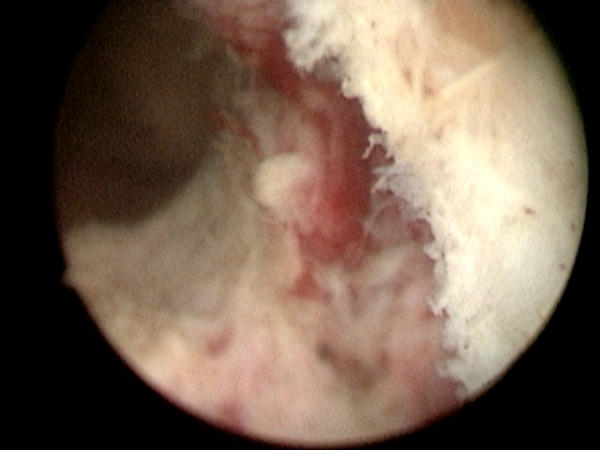
Appearance of left apical pad
Once definition of the apical tissue has begun, the beak of the resectoscope is again guided under the lip of the apex to leverage the adenoma away during dissection. The lateral dissection should proceed up to approximately the 9 to 10 o'clock position, with smaller glands allowing more lateral freedom. The lateral wall of the right lobe should then be freed from the surgical capsule by proceeding proximally along the already defined plane. At this point, the surgeon should be rotating the resectoscope in alternating clockwise and counterclockwise directions, which causes the tip of the laser fiber to proceed in a smooth arc back and forth within the established plane. The dissection should be continued until difficulty is encountered with exposure of the plane proximally. As much of the lateral lobe is dissected away from the bladder neck area as possible, to facilitate the final steps of the enucleation.
Step 6 – Creation of anterior groove
Attention is turned to the 12 o'clock region of the prostatic fossa, where the anterior commisure (midline groove between lateral lobes) is located. The resectoscope is turned 180° to allow the laser fiber to be positioned correctly. Laser settings should be adjusted back to 2 J and 50 Hz. Beginning at the bladder neck and proceeding distally, a groove is cut along the anterior commisure. This groove should span from the level of the bladder neck to the level of the verumontanum. The surgeon should occasionally reconfirm the location of the verumontanum by looking toward the floor, to ensure that the groove is not extended too far distally. Once the groove is created, it is again widened and deepened to the level of the surgical capsule along its entire extent.
Step 7 – Separation of right lobe from roof of capsule
The tip of the laser fiber should be angled toward the plane between the adenoma and the capsule. Initially, the fiber should be directed along the plane proximally and distally in a sequential fashion to begin dividing the anterior attachments of the right lobe. It is helpful for the dissection to begin at the bladder neck, where capsular definition is much easier (Figure 4). The attachments between the anterior portion of the lobe and the capsule are taken down sequentially until the lateral extent of the lobe is reached.
Figure 4.
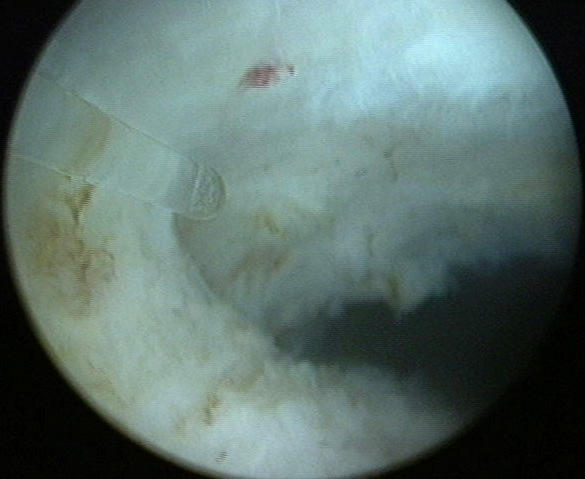
Dissection of plane between anterior surgical capsule and adenoma
Step 8 – Dissection and release of lateral portion of right lobe
It is crucial at this stage to identify the extent of the remaining lateral attachments near the apex of the lobe. This process can be difficult, as the planes of dissection between the adenoma and capsule from the anterior and posterior vectors may not join at the same level laterally. It is helpful to gain perspective by guiding the resectoscope first along the anterior plane and then the posterior plane, to fully visualize the lateral extent of each. Once the junction between the planes is defined, usually by a mucosal strip or bridge (Figure 5), it is cut by the laser to join the planes. Laser settings are 2 J and 40 Hz during division of the mucosal strip. The surgeon can then continue the dissection proximally, at 2 J and 50 Hz, to free the lateral extent of the lobe.
Figure 5.
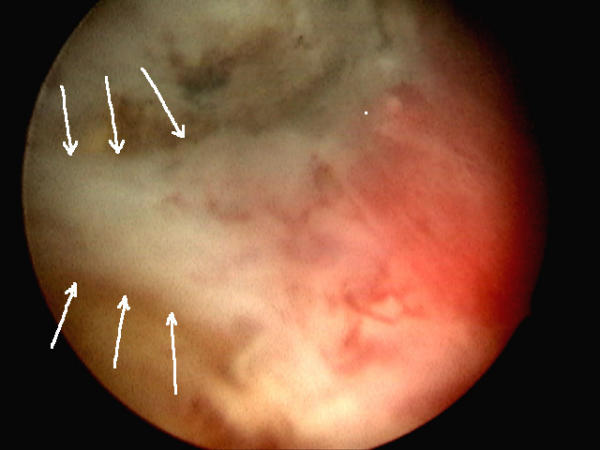
Appearance of mucosal strip (to left)
Step 9 – Completion of right lobe enucleation
At this point, the right lobe will be held only by attachments at the bladder neck level. The anterior attachments at the bladder neck should already have been divided. By following this plane further laterally, any remaining attachments in this area can be freed. Finally, one should proceed under the lobe and finish dividing attachments at the floor of the capsule and the posterior bladder neck. The lobe can then be pushed into the bladder by leveraging upward with the beak of the resectoscope (Figure 6). Care should be taken to avoid injuring the bladder neck during this process, which can occur if the resectoscope beak is inadvertently pushed too posteriorly.
Figure 6.
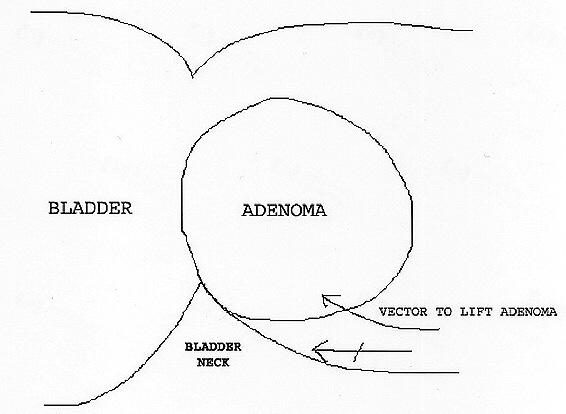
Use of resectoscope beak to leverage adenoma upward
Step 10 – Enucleation of the left lobe
The left lateral side of the 5 o'clock groove (or 6 o'clock groove in cases where the median lobe did not require enucleation) is undermined, beginning at the bladder neck level and progressing distally. This begins separation of the posterior portion of the lateral lobe from the capsular floor. The plane between the left lobe and the floor of the surgical capsule is further defined by cutting laterally from the verumontanum in a transverse fashion. The beak of the resectoscope can then be used to provide leverage under the lateral lobe while division of the posterior attachments is continued. Dissection of the left lobe apex, anterior portion of the lobe, connection of the planes laterally, and division of the remaining attachments at the bladder neck are done as previously described.
Step 11 – Inspection of the prostatic fossa
Once all lobes have been dissected free and pushed into the bladder, the laser settings should be changed to 2.5 J and 40 Hz (for large bleeders) or 1.5 J and 30 Hz (for smaller bleeders and near apex) to achieve optimal coagulation. The capsular surface is then inspected carefully. Any capsular bleeders should be coagulated by first defocusing the laser (positioning tip of the fiber 2–3 mm away from the surface) and then activating it until hemostasis is achieved. A dry fossa is essential before beginning morcellation to optimize visualization and minimize the risk of bladder injury. If any residual portions of adenoma remain along the surface of the capsule, these can be easily vaporized or enucleated with the laser.
Step 12 – Morcellation
The inner sheath of the resectoscope along with the laser fiber and stabilizing catheter are removed. The rigid offset nephroscope is then inserted into the outer resectoscope sheath. We utilize a commercially available morcellator (VersaCut, Lumenis Incorporated, Santa Clara, CA) consisting of a set of hollow reciprocating blades (5 mm diameter shaft) attached to a handle apparatus (Figure 7). Offset nephroscopes with a 5 mm working channel (Karl Storz, Olympus) are required to accommodate the morcellator. Olympus is the only manufacturer with a purpose-built device for HoLEP and morcellation. A vacuum pump is connected to the morcellator handle to provide suction through the blades, and device activation controlled through a foot pedal. Inflow tubing with normal saline should be connected to both inflow ports (outer resectoscope sheath, rigid nephroscope) to ensure that the bladder remains distended during the morcellation process. This is crucial, as collapse of the bladder can lead to significant injury to the bladder wall.
Figure 7.
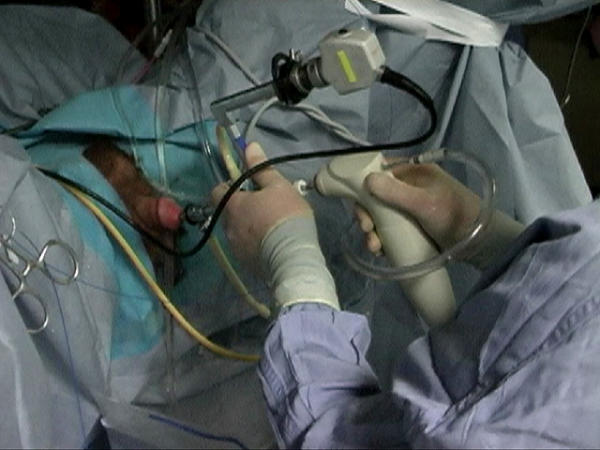
Morcellator inserted into offset nephroscope
Under direct visualization, the tip of the morcellator is inserted into the bladder and guided beneath a portion of adenoma. The pedal is then depressed partially to trigger the suction. Once a portion of adenoma is engaged by the morcellator, the pedal is fully depressed to activate the blades. It is important to keep the tip of the morcellator anteriorly within the bladder and within the visual field at all times. The morcellator shaft should be moved in and out of the working channel in small increments and rotated at times, to optimize engagement of tissue. When the remaining tissue pieces are small, the surgeon should engage them with suction alone and pull them into the prostatic fossa, where morcellation can be done safely by pinning the tissue against the capsule. If difficulty is encountered in engaging these smaller pieces, the Olympus stone basket (Olympus America Incorporated, Melville, NY) or 11F Alligator forceps can be used to facilitate their removal. Lastly, the Ellik evacuator is utilized to remove any remaining pieces of adenoma or clot. The patient is then given 20 mg of furosemide intravenously to promote urine flow during the immediate post-operative period. If the procedure has been long or the capsule is thin, it is advisable to give another 20 mg of furosemide intraoperatively to avoid fluid overload. On occasion, a smooth surfaced portion of adenoma fails to engage in the morcellator. If it is too large to be removed with the basket or forceps, the adenoma should be moved into the prostatic fossa. It can then be incised at different points with the laser fiber, providing more surface area for morcellator engagement without the risk of bladder injury.
As a option to morcellation, other investigators have described a modified tissue retrieval technique where an enucleated prostate lobe is initially left attached at the bladder neck by a small pedicle[24] A TUR electrocautery loop is subsequently utilized to resect the lobe into pieces retrievable with an evacuator. While this alternative obviates the need for a mechanical morcellator, the median prostate volume of the patient cohort treated with this technique was only 38 ml. Overall, we believe that the morcellator remains the most efficient method of tissue removal following enucleation. By employing the safety measures described earlier, morcellation can be performed in a safe manner with little chance of morbidity.
A 20 F, 3-way Foley catheter is placed at the conclusion of the case with the aid of a catheter guide. As continuous bladder irrigation is seldom required, the third port is plugged; however, it remains available should the need for irrigation arise. Currently, patients have their catheters removed early the following morning. HoLEP can be performed as an outpatient or overnight (i.e. short stay) procedure, depending on patient preference.
Conclusions
Worldwide experience with the HoLEP technique has shown the procedure to be a safe and effective alternative for the surgical treatment of BPH. Although enucleation of prostate adenoma can be performed by a variety of energy sources, only the holmium laser possesses the ideal combination of cutting and coagulation. As a result, the risk of dilutional hyponatremia and the need for transfusion are essentially eliminated. Hospital stays are minimal, and almost all patients are able to be discharged catheter-free. Although the learning curve for this procedure remains steep, the subsequent patient advantages should continue to expand the appeal and usage of the HoLEP technique within the urologic community. This technique has the potential to become the standard for surgical management of BPH.
Competing Interests
JL has a financial interest and/or other relationship with the Lumenis Corporation
Authors' Contributions
RK – Study conception and drafting of manuscript
RP, SK, TS – Critical revision
ME, JL – Critical revision and supervision
Contributor Information
Ramsay L Kuo, Email: rkuo@iupui.edu.
Ryan F Paterson, Email: rpaterson@clarian.org.
Samuel C Kim, Email: samckim@iupui.edu.
Tibério M Siqueira Jr, Email: tiberiojr@uol.com.br.
Mostafa M Elhilali, Email: mostafa.elhilali@muhc.mcgill.ca.
James E Lingeman, Email: jlingeman@clarian.com.
References
- Kirby RS. The natural history of benign prostatic hyperplasia: what have we learned in the last decade? Urology. 2000;56:3–6. doi: 10.1016/S0090-4295(00)00747-0. [DOI] [PubMed] [Google Scholar]
- Jacobsen SJ, Girman CJ, Guess HA, Rhodes T, Oesterling JE, Lieber MM. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol. 1996;155:595–600. doi: 10.1097/00005392-199602000-00053. [DOI] [PubMed] [Google Scholar]
- Grasso M. Experience with the holmium laser as an endoscopic lithotrite. Urology. 1996;48:199–206. doi: 10.1016/S0090-4295(96)00158-6. [DOI] [PubMed] [Google Scholar]
- Gilling PJ, Fraundorfer MR. Holmium laser prostatectomy: a technique in evolution. Clin Opin Urol. 1998;8:11–15. doi: 10.1097/00042307-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Chan KF, Vassar GJ, Pfefer TJ, Teichman JM, Glickman RD, Weintraub ST, Welch AJ. Holmium:YAG laser lithotripsy: A dominant photothermal ablative mechanism with chemical decomposition of urinary calculi. Lasers Surg Med. 1999;25:22–37. doi: 10.1002/(SICI)1096-9101(1999)25:1<22::AID-LSM4>3.3.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Gilling PJ, Cass CB, Malcolm AR, Fraundorfer MR. Combination holmium and Nd:YAG laser ablation of the prostate: initial clinical experience. J Endourol. 1995;9:151–153. doi: 10.1089/end.1995.9.151. [DOI] [PubMed] [Google Scholar]
- Kabalin JN, Gilling PJ, Fraundorfer MR. Holmium:yttrium-aluminum-garnet laser prostatectomy. Mayo Clin Proc. 1998;73:792–797. doi: 10.4065/73.8.792. [DOI] [PubMed] [Google Scholar]
- Chun SS, Razvi HA, Denstedt JD. Laser prostatectomy with the holmium: YAG laser. Tech Urol. 1995;1:217–221. [PubMed] [Google Scholar]
- Gilling PJ, Cass CB, Cresswell MD, Malcolm AR, Fraundorfer MR. The use of the holmium laser in the treatment of benign prostatic hyperplasia. J Endourol. 1996;10:459–461. doi: 10.1089/end.1996.10.459. [DOI] [PubMed] [Google Scholar]
- Kabalin JN. Holmium:YAG laser prostatectomy: results of U.S. pilot study. J Endourol. 1996;10:453–457. doi: 10.1089/end.1996.10.453. [DOI] [PubMed] [Google Scholar]
- Kabalin JN. Holmium: YAG laser prostatectomy canine feasibility study. Lasers Surg Med. 1996;18:221–224. doi: 10.1002/(SICI)1096-9101(1996)18:3<221::AID-LSM1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Mottet N, Anidjar M, Bourdon O, Louis JF, Teillac P, Costa P, Le Duc A. Randomized comparison of transurethral electroresection and holmium: YAG laser vaporization for symptomatic benign prostatic hyperplasia. J Endourol. 1999;13:127–130. doi: 10.1089/end.1999.13.127. [DOI] [PubMed] [Google Scholar]
- Gilling PJ, Cass CB, Cresswell MD, Fraundorfer MR. Holmium laser resection of the prostate: preliminary results of a new method for the treatment of benign prostatic hyperplasia. Urology. 1996;47:48–51. doi: 10.1016/S0090-4295(99)80381-1. [DOI] [PubMed] [Google Scholar]
- Cresswell MD, Cass CB, Fraundorfer MR, Gilling PJ. Holmium:YAG laser resection of the prostate: preliminary experience with the first 400 cases. N Z Med J. 1997;110:76–78. [PubMed] [Google Scholar]
- Gilling PJ, Kennett KM, Fraundorfer MR. Holmium laser resection v transurethral resection of the prostate: results of a randomized trial with 2 years of follow-up. J Endourol. 2000;14:757–760. doi: 10.1089/end.2000.14.757. [DOI] [PubMed] [Google Scholar]
- Gilling PJ, Mackey M, Cresswell M, Kennett K, Kabalin JN, Fraundorfer MR. Holmium laser versus transurethral resection of the prostate: a randomized prospective trial with 1-year followup. J Urol. 1999;162:1640–1644. doi: 10.1097/00005392-199911000-00018. [DOI] [PubMed] [Google Scholar]
- Kabalin JN, Mackey MJ, Cresswell MD, Fraundorfer MR, Gilling PJ. Holmium: YAG laser resection of prostate (HoLRP) for patients in urinary retention. J Endourol. 1997;11:291–293. doi: 10.1089/end.1997.11.291. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Iida S, Tomiyasu K, Shimada A, Noda S. Transurethral holmium laser resection of the prostate. J Urol. 2000;163:515–518. [PubMed] [Google Scholar]
- Matsuoka K, Iida S, Tomiyasu K, Shimada A, Suekane S, Noda S. Holmium laser resection of the prostate. J Endourol. 1998;12:279–282. doi: 10.1089/end.1998.12.279. [DOI] [PubMed] [Google Scholar]
- Mackey MJ, Chilton CP, Gilling PJ, Fraundorfer M, Cresswell MD. The results of holmium laser resection of the prostate. Br J Urol. 1998;81:518–519. doi: 10.1046/j.1464-410x.1998.00618.x. [DOI] [PubMed] [Google Scholar]
- Gan E, Costello A, Slavin J, Stillwell RG. Pitfalls in the diagnosis of prostate adenocarcinoma from holmium resection of the prostate. Tech Urol. 2000;6:185–188. [PubMed] [Google Scholar]
- Fraundorfer MR, Gilling PJ. Holmium:YAG laser enucleation of the prostate combined with mechanical morcellation: preliminary results. Eur Urol. 1998;33:69–72. doi: 10.1159/000019535. [DOI] [PubMed] [Google Scholar]
- Moody JA, Lingeman JE. Holmium laser enucleation for prostate adenoma greater than 100 gm.: comparison to open prostatectomy. J Urol. 2001;165:459–462. doi: 10.1097/00005392-200102000-00025. [DOI] [PubMed] [Google Scholar]
- Hochreiter WW, Thalmann GN, Burkhard FC, Studer UE. Holmium laser enucleation of the prostate combined with electrocautery resection: the mushroom technique. J Urol. 2002;168:1470–1474. doi: 10.1097/00005392-200210010-00040. [DOI] [PubMed] [Google Scholar]
- Gilling PJ, Kennett K, Das AK, Thompson D, Fraundorfer MR. Holmium laser enucleation of the prostate (HoLEP) combined with transurethral tissue morcellation: an update on the early clinical experience. J Endourol. 1998;12:457–459. doi: 10.1089/end.1998.12.457. [DOI] [PubMed] [Google Scholar]
- Moody JA, Lingeman JE. Holmium laser enucleation of the prostate with tissue morcellation: initial United States experience. J Endourol. 2000;14:219–223. doi: 10.1089/end.2000.14.219. [DOI] [PubMed] [Google Scholar]
- Gilling PJ, Kennett KM, Fraundorfer MR. Holmium laser enucleation of the prostate for glands larger than 100 g: an endourologic alternative to open prostatectomy. J Endourol. 2000;14:529–531. doi: 10.1089/end.2000.14.529. [DOI] [PubMed] [Google Scholar]
- Kuntz RM, Lehrich K. Transurethral holmium laser enucleation versus transvesical open enucleation for prostate adenoma greater than 100 gm.:: a randomized prospective trial of 120 patients. J Urol. 2002;168:1465–1469. doi: 10.1097/00005392-200210010-00039. [DOI] [PubMed] [Google Scholar]


