Abstract
Background
Various causes of malabsorption syndrome (MAS) are associated with intestinal stasis that may cause small intestinal bacterial overgrowth (SIBO). Frequency, nature and antibiotic sensitivity of SIBO in patients with MAS are not well understood.
Methods
Jejunal aspirates of 50 consecutive patients with MAS were cultured for bacteria and colony counts and antibiotic sensitivity were performed. Twelve patients with irritable bowel syndrome were studied as controls.
Results
Culture revealed growth of bacteria in 34/50 (68%) patients with MAS and 3/12 controls (p < 0.05). Colony counts ranged from 3 × 102 to 1015 (median 105) in MAS and 100 to 1000 (median 700) CFU/ml in controls (p 0.003). 21/50 (42%) patients had counts ≥105 CFU/ml in MAS and none of controls (p < 0.05). Aerobes were isolated in 34/34 and anaerobe in 1/34. Commonest Gram positive and negative bacteria were Streptococcus species and Escherichia coli respectively. The isolated bacteria were more often sensitive to quinolones than to tetracycline (ciprofloxacin: 39/47 and norfloxacin: 34/47 vs. tetracycline 19/47, <0.01), ampicillin, erythromycin and co-trimoxazole (21/44, 14/22 and 24/47 respectively vs. tetracycline, p = ns).
Conclusions
SIBO is common in patients with MAS due to various causes and quinolones may be the preferred treatment. This needs to be proved further by a randomized controlled trial.
Keywords: Small bowel bacterial overgrowth, jejunal aspirate culture, gut flora, India, antibiogram, tropical sprue
Background
Small intestinal bacterial overgrowth syndrome (SIBO) is defined as overgrowth of ≥105 colony forming unit (CFU) per ml of bacteria in the proximal small bowel [1]. Some authors considered a diagnosis of SIBO even with a lower colony count (≥ 103 CFU/ml) if the species of bacteria isolated in jejunal aspirate were those which, colonize large bowel [2,3]. Various anatomical lesions of small bowel and slowing of its motility may lead to bacterial overgrowth [4]. Several specific diseases e.g. celiac disease, tropical sprue (TS) and parasitic infestations have been shown to reduce intestinal motility [5-7]. Classic radiological findings of 'segmentation', 'flocculation' and dilatation of small bowel in barium series in patients with malabsorption are secondary to intestinal stasis [8]. Therefore, patients with specific causes of MAS like TS, celiac disease, parasitic infestations are also prone to SIBO. Frequency, nature and clinical significance of SIBO in patients without any other known cause of malabsorption syndrome (MAS, e.g. TS, celiac disease) are well established. Despite description of small bowel stasis in patients with various known causes of MAS, reports on frequency, nature and antibiotic sensitivity of SIBO in these patients are scant. We hypothesize that patients with MAS, irrespective of etiology, might have SIBO.
Studies on bacterial population contaminating upper gut and their antibiotic sensitivity pattern from developed countries are sparse [9,10]. Sensitivity of these bacteria to currently available antibiotics has been reported only once [9]. There is no study on spectrum of bacteria and their sensitivity pattern to various antibiotics in patients with MAS from developing countries. SIBO is often treated with repeated courses of antibiotics. Tetracycline was the mainstay of therapy for SIBO in past [11]. With the availability of several newer safe, effective and non-absorbable antibiotics, studying in vitro sensitivity of bacteria isolated from small bowel of these patients to such antibiotics appears worthwhile. Accordingly, we undertook this study prospectively.
Methods
Patients
50 patients (age 44 ± 8.5 years, 31 males) with chronic diarrhea, weight loss and anemia diagnosed as having MAS attending Luminal Gastroenterology Clinic of our department were studied. Diagnosis of MAS was established by abnormal D-xylose test (< 1 g/5 g/5 h) with or without abnormal 24-h fecal fat estimation (≥ 7 g/d) while on fat loading by Van de Kamer's technique [12]. The clinical details of these patients have been reported elsewhere [6,13,14]. No patient received antibiotics within 8 weeks preceding the study. The criteria used for diagnosis of various causes of MAS were as follows: Celiac disease, a) presence of anti-endomysial antibody, b) suggestive histology and c) response to gluten free diet; giardiasis, demonstration of trophozoite form of the organism in stool and/or duodenal biopsy; intestinal tuberculosis, a) demonstration of acid fast bacilli in intestine or extra-intestinal site and b) response to therapy; strongyloidiasis, demonstration of the parasite in intestinal biopsy and/or stool microscopy; intestinal lymphangiectasia, dilated lymphatic channels in subepithelial location in intestinal biopsy; acquired immunodeficiency syndrome by serology; hypogammaglobulinemia by serum immunoglobulin estimation; TS, a) no specific cause for MAS and, b) persistent response to tetracycline and folic acid.
The diagnoses of patients included in this study were as follows, tropical sprue (16), celiac disease (5), intestinal tuberculosis (3), panhypogammaglobulinemia (2) one of whom had strogyloidiasis, selective IgA deficiency (1), acquired immunodeficiency syndrome (1), giardiasis (2) and intestinal lymphangiectasia (1). Seven patients had SIBO secondary to either structural lesions or motility abnormality of gut (ileocolic anastomosis in 4, diverticulosis in 1, intestinal hypomotility due to scleroderma in 1 and diabetic autonomic neuropathy and hypothyroidism in 1). In 12/50 patients cause of MAS could not be ascertained. The protocol of the study was approved by the ethics committee of the institute and patients gave consent for inclusion into the study.
Controls
12 patients with constipation predominant irritable bowel syndrome (IBS) diagnosed by Rome criteria [15] were used as controls. Clinical details of these patients have been reported elsewhere [6]. No patient with IBS had biochemical evidence of MAS [normal D-xylose test (≥ 1/g/5 g/5 h) and fecal fat estimation by Sudan III stain of spot stool specimen (< 10 droplets/high power field)]. Endoscopic jejunal biopsy was normal in them. None of them had received antibiotics, pro or anti-motility and anti-secretory drugs within 8 weeks preceding the study.
Jejunal aspiration
Jejunal aspirate was collected during jejunoscopy with a pediatric colonoscope and a catheter described earlier [13]. Briefly, the catheter assembly had an inner tube that was 3 cm longer than the outer tube. A rubber obturator blocked the mouth of the outer tube (Fig. 1). The assembly was sterilized by autoclaving. On reaching the jejunum (confirmed by fluoroscopic examination in initial few patients and by length of endoscope inserted), the catheter assembly was introduced through the biopsy channel of a sterilized pediatric colonoscope. The inner tube was then pushed beyond the tip of the outer tube once the outer tube was 4–5 cm ahead of the tip of the endoscope. This led to dislodgement of the rubber obturator from the tip of the outer tube. Jejunal aspirate was collected through the inner tube with a sterile syringe, which was used for aerobic and anaerobic bacterial culture (transported in Robertson's cooked meat medium for the latter), colony count and drug sensitivity pattern.
Figure 1.
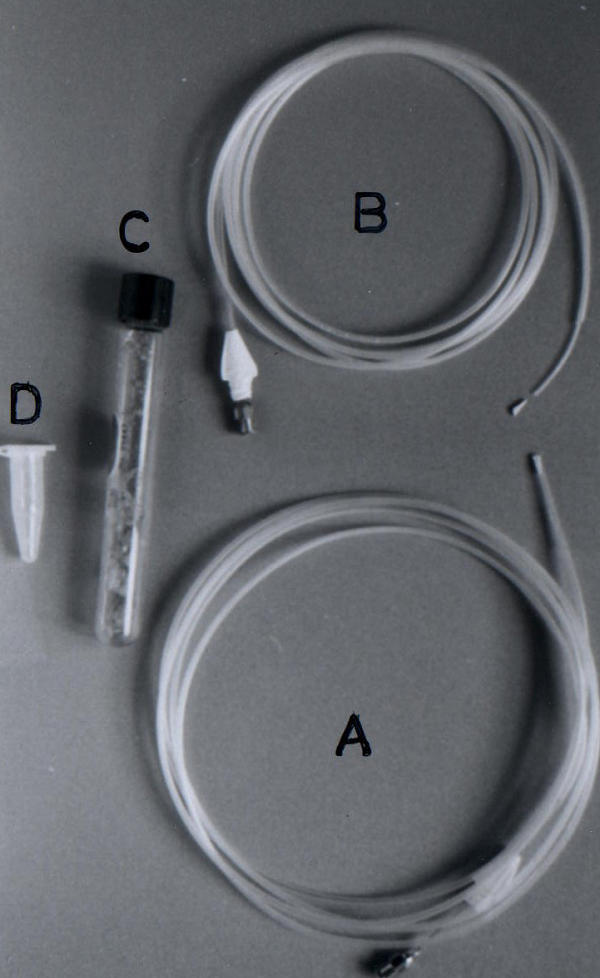
Assembly used for jejunal aspiration. (a) jejunal aspiration catheter with inner tube housed inside the outer tube. The tip of the outer tube is closed with a rubber obturator. (b) Note that the rubber obturator has been dislodged after inner tube has been pushed to come out from the tip of the outer tube. (c) Robertson's cooked meat media used to transport jejunal aspirate for anaerobic culture. (d) Sterile tube used to transport jejunal aspirate for aerobic culture.
Bacteriological studies
Smears were prepared from jejunal aspirates, fixed, Gram stained and examined for presence of Gram positive and negative organisms. Bacterial species were cultured and isolated using standard techniques [16]. Briefly, for aerobic culture, samples were homogenized by vortexing and then diluted serially with sterile distilled water. Dilutions from 5 × 10-1 to 5 × 10-4 were prepared. Aliquots of non-diluted sample and each dilution (100 μl each) were plated on blood agar and MacConkey agar. After 24 to 48-h incubation at 37°C, colonies were counted and bacterial species identified using standard techniques [16,17]. Dilutions were made in Robertson's cooked meat broth for anaerobic culture. Undiluted sample and each dilution (100 μl each) were subcultured on Wilkins-Chalgren agar. For anaerobic culture anaerobic jars (McIntosh) were used. For gassing the culture evacuation replacement system (Anoxomate, Mart-Netherland) was used. The standard anaerobic recipe has 3 evacuation replacement cycles. In the first evacuation phase of the standard anaerobic culture 80% of the jar volume was evacuated. The anoxomate achieves this with high accuracy due to high precision pressure sensor. In the successive replacement, the jar was filled with oxygen free gas mixture (Nitrogen). The jar was finally filled with a gas mixture containing 80%-90% nitrogen, 5%-10% hydrogen and carbon dioxide. Strains of Clostridium difficile, Clostridium perfringens, Bacteroides fragilis and gram negative cocci were used as positive control with each batch. Pseudomonas aeruginosa was used as negative control. Cultures were examined for bacterial growth after incubation for 48-h and if negative, after 5 days at 37°C in an anaerobic chamber. In case of bacterial growth, colonies were counted and bacterial species identified using standard techniques. Rogosa agar was used [16] for lactobacilli and was incubated in anaerobic conditions. Bacterial counts were expressed as logarithm of colony forming units per ml of jejunal fluid. Total bacterial colonies and count of each species were obtained.
Antibiotic sensitivity
All bacterial strains were subjected to in vitro antibiotic sensitivity test. Antibiotic discs (Himedia, Mumbai, India), namely ampicillin (10 μg), ampicillin and salbactum (10 μg each), tetracycline (30 μg), co-trimoxazole (trimethoprim / sulfamethoxazole 1.25 / 23.75 μg), erythromycin (15 μg), norfloxacin (10 μg), ciprofloxacin (5 μg) and cephalexin (30 μg) were used. We chose these antibiotics as these are orally administered and therefore, are likely to be preferred by the clinicians in the treatment of SIBO. The antibiotic discs were stored at 4°C before use. Antibiotic sensitivity test was performed by modified Stoke's method [17]. Briefly, Muller Hinton's agar was prepared and its pH was adjusted to 7.3. It was poured to a depth of 4 mm on flat-bottomed 9-cm petri dishes. These were dried before use. The area of petri dish was arbitrarily divided into three parts, central area for control organisms and two areas on either side of it, for test organisms. The control organisms consisted of Escherichia coli [National Collection of Type Culture (NCTC) 10418], Staphylococcus aureus (NCTC 6571) and Pseudomonas aeruginosa (NCTC 10662) for coliforms, Staphylococcus and pseudomonads respectively. An inoculum of the test organism was prepared by emulsifying part of the growth from each of 5 similar colonies in saline. Turbidity of the suspension was adjusted to 0.5 McFarland standard spectrophotometrically at 530 nm wavelength. Overnight incubation of this inoculum produced semi-confluent growth. Sterile cotton swabs were impregnated with the test and control organisms separately. These swabs were used to inoculate the specified areas of the petri dishes with test and control organisms. A gap of 2–3 mm was left between test and control strains. Antibiotic discs were applied with light pressure on the agar surface using flamed forceps after the inoculum had dried. The petri dishes were incubated at 37°C for 16 to 18-h.
The radial width of the zones outside the antibiotic discs was measured in mm. The zones of inhibition of the test organisms were compared with those of control strains. The results were interpreted based on measurement of zone of inhibition (mm) in test organisms as compared with the controls as follows: (a) sensitive: equal to, greater than or not less than 3-mm as compared with controls, (b) intermediate: ≥ 2-mm but smaller than controls by >3-mm, (c) resistant: ≤ 2-mm.
Statistical analysis
Nominal variables were analyzed by Chi-square test with Yates' correction as applicable. Numerical variables were analyzed by Mann-Whitney U test as the data were not expected to have normal distribution. A p value of < 0.05 was considered as significant.
Results
Gram staining
Gram positive cocci were found in 8/50, Gram negative bacilli in 13/50 and both Gram positive cocci and negative bacilli in 7/50 patients with MAS. In other 22 patients Gram staining did not show any bacteria.
Frequency, nature of the bacteria and colony count
Culture revealed growth of bacteria in 34/50 (68%) patients with MAS and 3/12 patients with IBS (p < 0.05). Total colony count ranged from 3 × 102 to 1 × 1015 colony forming unit (CFU)/ml (median 105) in MAS and 100–1000 (median 700) CFU/ml in patients with IBS (p 0.003); 21/50 (42%) patients had with MAS and none of 12 with IBS had colony count ≥105 CFU/ml (p < 0.05). In patients with MAS, aerobes were isolated in 34/34 and anaerobe in 1/34 with positive culture. Two bacterial species were grown in jejunal aspirate of 13 patients and three bacterial species in one patient. In the remaining 20 patients, growth of a single bacterial species was obtained. Frequency of isolation of different bacteria and colony count of each species of bacteria in patients with MAS are shown in table 1. ≥105 CFU/mL bacteria grew in 6/16 patients with TS, 2/5 with celiac disease, 1/3 with intestinal tuberculosis, 1/1 with panhypogammaglobulinemia and Strongyloidiasis, 1/1 with selective IgA deficiency, all seven with SIBO secondary to structural and motility abnormality and 3/12 with MAS without a known cause.
Table 1.
Frequency of isolation and colony counts of different bacteria in patients with malabsorption syndrome.
| Name of bacteria | Frequency of isolation* | Median colony count (CFU/ml) | Range (CFU/ml) |
| Aerobes | |||
| Escherichia coli | 12 | 105 | 103 to >105 |
| Streptococcus species (Non-A, non-B, non-D) | 12 | >105 | 1.6 × 103 to >105 |
| Klebsiella pneumoniae | 5 | 8 × 104 | 6 × 102 to >105 |
| Enterococcus fecalis | 3 | 105 | 1.6 × 103 to >105 |
| Enterococcus fecium | 1 | >105 | - |
| Staphylococcus aureus | 5 | >105 | 3 × 102 to >105 |
| Pseudomonas aeruginosa | 3 | 105 | 3.6 × 103 to 105 |
| Acinetobacter baumanii | 3 | 6 × 102 | 4 × 102 to >105 |
| Citrobacter freundii | 1 | 104 | - |
| Proteus mirabilis | 1 | 103 | - |
| Anaerobes | |||
| Bacteroides melaninogenicus | 1 | >105 | - |
*Jejunal aspirate of 20 patients grew one, 13 two and one three bacterial species.
Sensitivity testing
In vitro sensitivity to commonly used orally absorbed antibiotics showed (table 2) that bacteria isolated from small bowel were more often sensitive to quinolones (ciprofloxacin and norfloxacin) than to tetracycline (39/47 and 34/47 vs 19/47, p < 0.0001 and <0.01, respectively); it is important to note that tetracycline is commonly used to treat chronic diarrhoea and malabsorption associated with bacterial contamination of small bowel [10,11]. There was no difference in sensitivity to ampicillin, erythromycin, co-trimoxazole with that of tetracycline (21/44, 14/22 and 24/47 vs. 19/47, p = ns). 30/44 and 25/40 bacterial species were sensitive to ampicillin and sulbactam combination and cephalexin respectively.
Table 2.
Sensitivity of the bacteria isolated from jejunal aspirate to various antibiotics.
| Bacteria | Sensitive to | |||||||
| Ampicillin | Ampicillin Sulbactam | Tetracyclin | Cotrimox | Erythro | Norflox | Ciproflox | Cephalexin | |
| E. coli (n = 12) | 3 | 5 | 4 | 5 | NT | 7 | 9 | 5 |
| Streptococcus sp. (n = 12) | 9 | 11 | 6 | 5 | 9 | 11 | 12 | 9 |
| E. fecalis (n = 3) | 3 | 3 | 1 | 0 | 1 | 1 | 1 | NT |
| E. fecium (n = 1) | 1 | 1 | 0 | 0 | 1 | 0 | 0 | NT |
| S. aureus (n = 5) | 3 | 4 | 2 | 5 | 3 | 4 | 5 | 3 |
| C. freundi (n = 1) | 0 | 1 | 0 | 1 | NT | 1 | 1 | 1 |
| K. pneumoniae (n = 5) | 1 | 3 | 2 | 3 | NT | 3 | 4 | 3 |
| P. mirabilis (n = 1) | 1 | 1 | 0 | 0 | NT | 1 | 1 | 1 |
| A. baumanii (n = 3) | 0 | 0 | 2 | 2 | NT | 3 | 3 | 3 |
| P. aeruginosa (n = 3) | NT | NT | 1 | 2 | NT | 3 | 3 | NT |
| B. melaninogenicus (n = 1) | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Total (n = 47) | 21/44 (48) | 30/44 * (68) | 19/47 * (40) | 24/47 (51) | 14/22 (64) | 34/47* (72) | 39/47$ | 25/40 (62.5) |
Figures within parenthesis indicate percentages. NT: not tested. *p < 0.01; $p < 0.0001 (Chi-squared test)
Discussion
In this study we found that the spectrum of bacteria isolated from upper small bowel in patients with malabsorption syndrome from the tropics are somewhat similar to that reported from the west [9]; these bacteria are often resistant to tetracycline, co-trimoxazole, ampicillin and sensitive to quinolones. Therefore, our data suggest the need for undertaking randomized controlled trial of these orally administered antibiotics in the treatment of SIBO and contaminated small bowel syndrome in the tropics.
We have shown that SIBO was common in MAS due to any cause (e.g. TS, celiac disease, parasitic infestation, immunodeficiency states) and resulted from both Gram positive and Gram negative bacteria. SIBO was diagnosed using standard definition (≥105 CFU/ml bacteria in jejunal aspirate) [1]. Predominant Gram positive organism was streptococcus species and the predominant Gram negative organism was Escherichia coli. This is accordance with the earlier report from the west [9]. Anaerobes were infrequently found in our study. This might be related to the fastidious nature of anaerobes and failure to grow even on exposure to small quantity of oxygen [18]. Other reasons could be related to differences in patient population included in our study. We studied patients with MAS due to other primary causes; in contrast, earlier studies included patients with SIBO syndrome [9]. Lower colony counts of aerobes could be the other reason for lower frequency of anaerobes as aerobes utilize oxygen and help in growth of anaerobes [19]. Several isolated studies documented SIBO either using microbiological techniques or glucose hydrogen breath test in patients with celiac disease [20], TS [6], parasitic infestation of small bowel [7,13] and hypogammaglobulinemia [21]. In this study we documented frequent occurrence of SIBO in patients with various causes of MAS as a group. Such secondary SIBO in patients with another cause of MAS may have clinical significance as it can further compound malabsorption of nutrients in addition to the primary disease contributing to it. Studies from Burma suggested SIBO as a cause of malnutrition in pediatric population [22,23]. Secondly, at times a transient response to antibiotics resulting from eradication of such secondary SIBO may mislead the clinician trying to establish the diagnosis of etiology of MAS.
We found frequent resistance to the drugs that are often used in the treatment of SIBO syndrome. Over half of bacteria were resistant to tetracycline, the drug that has been considered as the first line antibiotic in the treatment of SIBO when not guided by antibiogram [11], a quite common practice. Most strains were also resistant to the combination of trimethoprim-sulfamethoxazole. This is in contrast to the earlier western report showing infrequent resistance to most antibiotics [9]. These discrepancies might be explained by differing practice of antibiotic use in different countries. Frequent resistance to commonly used antibiotics in India might owe to over the counter availability and frequent unnecessary use of these antibiotics. In the present study, we deliberately chose to report sensitivity patterns of orally used antibiotics as parenteral antibiotics are rarely used in treatment of SIBO. Most of bacteria isolated were sensitive to both ciprofloxacin and norfloxacin. However, we believe that norfloxacin may be preferred over ciprofloxacin, since norfloxacin has low systemic absorption [24] and therefore, may have infrequent side effects and may be safe particularly during pregnancy. Patients with SIBO often need long-term and repeated courses of antibiotics [25] and therefore, those with lesser toxicity and lower systemic absorption would be preferred. However, this needs to be proved by randomized controlled trial. A recent randomized cross over study on ten patients with SIBO from developed countries documented efficacy of norfloxacin in reducing diarrhoea and breath hydrogen [26].
In conclusion, SIBO is common in patients with malabsorption syndrome due various causes in the tropics and norfloxacin may be considered in its treatment. Our in vitro data also serve as a starting point for further randomized controlled trial of norfloxacin in treatment of SIBO.
Competing interest
None declared.
Authors' contributions
Ujjala Ghoshal conceived the study, performed all microbiological works and analyzed and drafted the manuscript. Uday C Ghoshal designed the study, participated in clinical and endoscopic works and helped the first author in analyzing the data and in drafting the manuscript. Piyush Ranjan participated in clinical works. SR Naik supervised planning, execution and drafting the manuscript. Archana Ayyagari supervised the microbiological works. All authors read and approved the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgement
The authors thank the endoscopy staff of the Department of Gastroenterology and laboratory staff of the Department of Microbiology at their institution for technical help.
Contributor Information
Ujjala Ghoshal, Email: ujjala@sgpgi.ac.in.
Uday C Ghoshal, Email: ghoshal@sgpgi.ac.in.
Piyush Ranjan, Email: pranjan@sgpgi.ac.in.
Subhash R Naik, Email: ghoshal@sgpgi.ac.in.
Archana Ayyagari, Email: archana@sgpgi.ac.in.
References
- Toskes PP. Bacterial overgrowth of the gastrointestinal tract. Adv Int Med. 1993;38:387–407. [PubMed] [Google Scholar]
- Simon GL, Gorbach SL. The human intestinal microflora. Dig Dis Sci. 1986;31:147–62. doi: 10.1007/BF01295996. [DOI] [PubMed] [Google Scholar]
- Justensen T, Haagen Nielsen O, Jacobson E, et al. The normal cultivable microflora in upper jejunal fluid in healthy adults. Scand J Gastroneterol. 1984;19:279–82. [PubMed] [Google Scholar]
- Ballen LS, Russel RM. Bacterial overgrowth syndrome. Clinical Perspectives in Gastroenterology. 2000. pp. 225–31.
- Chiarioni G, Bassotti G, Germani U, et al. Gluten-free diet normalizes mouth-to-cecum transit of a caloric meal in adult patients with celiac disease. Dig Dis Sci. 1997;42:2100–5. doi: 10.1023/A:1018878703699. [DOI] [PubMed] [Google Scholar]
- Ghoshal UC, Ghoshal U, Ayyagari A, Ranjan P, Krishnani N, Misra A, Aggarwal R, Naik S, Naik SR. Tropical sprue is associated with contamination of small bowel with aerobic bacteria and reversible prolongation of orocaecal transit time. J Gastroenterol Hepatol. 2003;18:540–7. doi: 10.1046/j.1440-1746.2003.03006.x. [DOI] [PubMed] [Google Scholar]
- Dwinell MB, Bass P, Schaefer DM, Oaks JA. Tapeworm infection decreases intestinal transit and enteric aerobic bacterial population. Am J Physiol. 1997;273:G480–5. doi: 10.1152/ajpgi.1997.273.2.G480. [DOI] [PubMed] [Google Scholar]
- Eisenberg RL. Gastrointestinal radiology: A pattern approach. Philadelphia: JB Lippincott Company. 1990. pp. 441–51.
- Bouhnik Y, Alain S, Attar A, Flourie B, Raskine L, Sanson-Le Pors MJ, Rambaud JC. Bacterial populations contaminating upper gut in patients with small intestinal bacterial overgrowth syndrome. Am J Gastroenterol. 1999;94:1327–31. doi: 10.1016/S0002-9270(99)00071-4. [DOI] [PubMed] [Google Scholar]
- Isaacs PET, Kim YS. The contaminated small bowel syndrome. Am J Med. 1979;67:1049–56. doi: 10.1016/0002-9343(79)90647-8. [DOI] [PubMed] [Google Scholar]
- Tabacquali S. The pathophysiological role of small intestinal bacterial flora. Scand J Gastroenterol. 1970;5:139–63. [PubMed] [Google Scholar]
- Raffensperger EC, D'Agostino F, Manfredo H, Ramirez M, Brooks FP, O'Neill F. Fecal fat excretion: an analysis of four years' experience. Arch Intern Med. 1967;119:573–6. doi: 10.1001/archinte.119.6.573. [DOI] [PubMed] [Google Scholar]
- Ghoshal UC, Ghoshal U, Kumar A, Jain A, Aggarwal R, Misra A, Ayyagari A, Naik SR. Strongyloides stercoralis infestation associated with septicemia due to intestinal transmural migration of bacteria. J Gastroenterol Heptol. 2002;17:1331–3. doi: 10.1046/j.1440-1746.2002.02750.x. [DOI] [PubMed] [Google Scholar]
- Ranjan P, Ghoshal UC, Ghoshal U, Aggarwal R, Ayyagari A, Naik S, Naik SR. A prospective study of etiological spectrum of malabsorption syndrome in North India. Indian J Gastroenterol. 2001;20:A19. [Google Scholar]
- Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- Collee JG, Miles RS, Walt B. Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editor. Mackie and McCartney Practical Medical Microbiology. New York: Churchill Livingstone; 1996. pp. 131–49. [Google Scholar]
- Anonymous . Processing clinical specimens for anaerobic bacteria: Isolation and identification procedures. In: Baron EJ, Peterson LR, Finegold SM, editor. Bailey and Scott's Diagnostic Microbiology. Philadelphia: Mosby; 1994. pp. 474–503. [Google Scholar]
- Ananthanarayan R, Panikar CKJ. Textbook of microbiology. Chennai: Orient Longman. 1999. pp. 244–9.
- Levitt MD. Oxygen tension in the gut. N Engl J Med. 1970;282:1039–40. doi: 10.1056/NEJM197004302821814. [DOI] [PubMed] [Google Scholar]
- Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: Results of a systemic approach. Am J Gastroenterol. 2002;97:2016–21. doi: 10.1016/S0002-9270(02)04274-0. [DOI] [PubMed] [Google Scholar]
- Nussinson E, Lahav M, Berebi A, Estrov Z, Zur S, Resnitzky P. Secretory piece and IgA deficiency in a patient with Waldenstrom's macroglobulinemia. Am J Gastroenterol. 1986;81:995–8. [PubMed] [Google Scholar]
- Pereira SP, Khin-Maung U, Bolin TD, Duncombe VM, Nyunt-Nyunt-Wai , Myo-Khin , Linklater JM. A pattern of breath hydrogen excretion suggesting small bowel bacterial overgrowth in Burmese village children. J Pediatr Gastroenterol Nutr. 1991;13:32–38. doi: 10.1097/00005176-199107000-00006. [DOI] [PubMed] [Google Scholar]
- Khin-Maung U, Bolin TD, Pereira SP, Duncombe VM, Myo-Khin , Nyunt-Nyunt-Wai , Linklater JM. Epidemiology of small bowel bacterial overgrowth and rice malabsorption in Burmese (Myanmar) village children. Am J Trop Med Hyg. 1992;47:298–304. doi: 10.4269/ajtmh.1992.47.298. [DOI] [PubMed] [Google Scholar]
- Archar GL, Polk KE. Approach to therapy for bacterial diseases. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson LR, editor. Harrison's Principles of Internal Medicine. McGraw-Hill, Inc; 2001. pp. 867–82. [Google Scholar]
- King CE, Toskes PP. Small intestinal bacterial overgrowth. Gastroenterology. 1979;76:1035–55. [PubMed] [Google Scholar]
- Attar A, Flourie B, Rambaud JC, Franchisseur C, Ruszniewski P, Bouhnik Y. Antibiotic efficacy in small intestinal bacterial overgrowth-related diarrhoea: a crossover, randomized trial. Gastroenterology. 1999;117:794–7. doi: 10.1016/s0016-5085(99)70336-7. [DOI] [PubMed] [Google Scholar]


