Abstract
Activation of the glucocorticoid receptor (GR) triggers apoptosis in T cells. However, activation of the T cell antigen receptor (TCR) blocks glucocorticoid-induced apoptosis, implying functional crosstalk between these two distinct signaling systems. By reconstructing or selectively blocking TCR-stimulated signaling pathways, we show here that TCR activation of the mitogen-activated protein kinase kinase/extracellular signal regulated kinase (MEK/ERK) cascade via Ras is necessary and sufficient to inhibit GR-mediated death in immortalized T and thymocyte cell lines and in primary T cells. Moreover, we found that activation of various pathway components (TCR, Ras, MEK1) altered the transcriptional regulatory activity of GR. In contrast, phosphatidylinositol 3-kinase and Akt, which down-regulate other lymphocyte apoptosis pathways, did not inhibit glucocorticoid-induced apoptosis. Our findings, which link signaling from the TCR cell surface receptor to that from the GR intracellular receptor, demonstrate the importance of the integration of signal transduction pathways in defining regulatory circuits. Because the TCR/Ras/MEK pathway has been shown previously to be essential for positive selection of thymocytes, the TCR/Ras/MEK signaling to GR crosstalk described herein may affect T cell development and homeostasis.
Determining how cellular signaling pathways influence each other is essential for understanding the complexities of physiology, pathology, and development. Integration of multiple signals is likely to be particularly important for signal transducers such as the glucocorticoid receptor (GR) that evoke different effects in different cells and physiologic settings (1). GR, an essential and widely expressed transcriptional regulator, is triggered by hormone binding to translocate to the nucleus and associate with genomic glucocorticoid response elements (GREs), from which it activates or represses transcription depending on the cell and gene context (2, 3). Thus, context-specific factors, including signaling crosstalk, play essential roles in GR function.
In the immune system, GR represses cytokine and interleukin gene transcription and triggers programmed cell death (apoptosis) in T cells (4). Glucocorticoid-induced apoptosis may help to eliminate developing T cells or thymocytes that are differentiating improperly (4). Paradoxically, GR also promotes thymocyte survival. Mice with a thymus-specific reduction in GR produce only 10% of the normal numbers of thymocytes (5). This dual effect of GR on thymocyte survival and apoptosis is reminiscent of the actions of the T cell antigen receptor (TCR), which is essential for thymocyte survival (positive selection) but can also cause apoptosis (negative selection; ref. 6). In the periphery, TCR signaling initiates the cellular immune response leading to T cell proliferation, differentiation into effector T cells, and generation of memory T cells (7). Conversely, TCR stimulation of already activated mature T cells can induce apoptosis in a process known as activation-induced cell death (7). Activation-induced cell death can be inhibited by glucocorticoids, indicating that GR can also mediate survival or apoptosis in mature T cells (8).
TCR activation occurs in conjunction with the TCR-associated signaling complex CD3 (9). Stimulation of the TCR/CD3 complex leads to recruitment and activation of Ras and Rac, small GTPases at the nexus of multiple signaling pathways (10). Studies in transgenic mice reveal that dominant-negative mutants of Ras, Raf, and mitogen-activated protein kinase kinase 1 (MEK1) block positive selection but not negative selection, indicating that the two responses to TCR/CD3 signaling must be governed by different pathways (11).
TCR and GR trigger apoptosis via distinct pathways (12). TCR-induced FasL interacts with its receptor, Fas, which activates caspase-8 (13, 14). In contrast, GR-dependent transcription of unknown genes leads to activation of caspase-9 and subsequent apoptosis (15–18), which can be blocked by increased expression of Bcl-2 (19).
Remarkably, simultaneous stimulation of GR and TCR results in T cell survival (4, 8). Notch and CD28, two other T cell surface receptors that affect T cell development and function, also inhibit glucocorticoid-induced apoptosis (20, 21). Calcineurin seems to be involved in inhibiting glucocorticoid-induced apoptosis by signals that mimic TCR/CD3 (22). Because TCR/CD3 signals are essential for the proliferation and function of T cells in the periphery and are pivotal to the critical process of positive selection in the thymus, we were interested in the integration of GR and TCR signaling pathways that confers T cell survival. Others have shown that GR inhibits TCR-induced apoptosis by repressing FasL transcription (13). We investigated the reciprocal pathway, in which TCR inhibits glucocorticoid-induced apoptosis, and its role in T cell survival.
Materials and Methods
Cells, Reagents, and Plasmids.
Cells from the 2B4.11 (Fas-negative) line (from J. Ashwell, National Institutes of Health, Bethesda, MD) were sorted for high CD3 by flow cytometry (FACSorter, Becton Dickinson) with 145-2C11-FITC (PharMingen). Anti-CD3ɛ purified monoclonal antibodies 145-2C11 and 500-A2 were from N. Cacalano (DNAX, Palo Alto, CA). Rabbit anti-Syrian hamster IgG (H + L) was from Jackson ImmunoResearch. Anti-CD8α-FITC was from PharMingen; anti-CD4-PE was from Becton Dickinson. Antibodies specific for extracellular signal regulated kinase (ERK), Akt, and Bad were from New England Biolabs. Bcl-2 antibody (3F11) was from PharMingen. Bcl-XL antibody (5F2) was from C. B. Thompson (Univ. Pennsylvania, Philadelphia). PD98059 was from Calbiochem; U0126 was from Promega. TAT3 luciferase, mouse mammary tumor virus (MMTV) luciferase, and κB3 DLO luciferase have been described (23–25).
Cell Stimulation and 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) Assay.
Cell stimulations were performed as described (26) with modifications as follows. Microtiter plates were coated with 10 μg/ml rabbit anti-hamster/PBS (4°C, overnight). Cells (105 per ml) were incubated for 10 min with 0.5 μg/ml anti-CD3ɛ (2C11 or 500-A2) and plated at 2 × 104 cells per well, and dexamethasone (dex) was added. Kinase inhibitors were added 1 h before anti-CD3 stimulation. Viability was measured by MTT assay (27). Absorbance was measured with a Molecular Devices Microplate reader (OD at 550 nm) with softmax 2.35 software.
Transient Transfections.
Cells were electroporated as described (28) with 15 μg of total DNA including 5 μg of vector or kinase activator expression plasmid, 5 μg of cytomegalovirus (CMV) long terminal repeat–β-galactosidase (β-gal), and 5 μg of luciferase reporter construct or vector. At 12 h after transfection, cells were treated with 0.1 μM dex with or without 0.5 μg/ml anti-CD3 and then harvested at 8 h for luciferase assay or at 24–30 h for β-gal viability assay (see below). Luciferase activity was measured in extracts with a luciferase assay kit from PharMingen, and β-gal activity was assayed as described (24). Luciferase activity was normalized to β-gal activity.
β-Gal Viability Assays.
Treatments were performed at 12–14 h after transfection, and cells were harvested 30 h later or as indicated. β-gal activity was measured in cell extracts or in viable cells. β-gal assays were performed on cell extracts by using a chemiluminescent kit from Tropix (Bedford, MA). In viable cells, β-gal activity was determined by flow cytometry with fluorescein di-β-d-galactopyranoside (FDG, Molecular Probes) and propidium iodide (PI; 30,000 events per sample). Percentage of FDG-positive (transfected)/PI-negative (live) cells was determined by using a FACScalibur (Becton Dickinson) and cellquest software.
Terminal Deoxynucleotidyltransferase-Mediated UTP End Labeling (TUNEL) Apoptosis Assay.
A TUNEL assay was performed as described (29) with the following modifications of T. Liegler (University of California, San Francisco, Gladstone). Cells were stimulated and cultured as above for 24 h. Cultures were centrifuged for 5 min at 188 × g in microtiter plates and washed in 1 mM EDTA/PBS twice and then PBS alone. Cells were fixed in 1% paraformaldehyde for 15 min on ice. Cells were pelleted (for 5 min at 2,000 × g), permeabilized in 200 μl of 80% (vol/vol) ice-cold ethanol for 5 min, and then washed twice in cold PBS. For terminal transferase reaction, cells were resuspended in 50 μl of terminal deoxynucleotidyltransferase (TdT) solution [5 units of TdT (Roche Molecular Biochemicals, no. 220582), TdT reaction buffer (200 mM potassium cacodylate/25 mM Tris⋅HCl/0.25 mg/ml BSA, pH 6.6), 2.5 mM CoCl2, 10 mM DTT in 10 mM sodium acetate (pH 5.2), 10 μM biotin-16-dUTP (Roche Molecular Biochemicals)] and incubated for 45 min at 37°C. Reaction was stopped with 200 μl of rinsing buffer (0.1% Triton X-100/0.5% BSA in PBS), and cells were pelleted. Cells were resuspended in 100 μl of 1:200 (vol/vol) dilution of Avidin-FITC (Roche Molecular Biochemicals, no. 100205) in 4× SSC/0.1% Triton-X100/5% (vol/vol) nonfat dry milk and incubated in the dark for 30 min at room temperature. Cells were washed twice in rinsing buffer, resuspended in 400 μl of PBS, and analyzed in a FACScalibur by using cellquest software.
Primary Cell Cultures.
Splenic cells (2 × 106 cells per 200 μl) from three C57BL/6 female mice aged 4–8 weeks were cultured as described (30) and treated with dex and/or anti-CD3ɛ as described above. After 24 h, cells were stained with PI, anti-mouse CD4-PE (Becton Dickinson), and anti-mouse CD8-FITC (PharMingen) and then analyzed in a FACScalibur by using cellquest software. The PI-negative (live) cells were electronically gated on size to exclude small, preapoptotic, mostly annexin V+ cells. Percentage of viable cells = [percentage of total PI-negative CD4+CD8− or CD8+CD4− cells with dex/percentage of total PI-negative CD4+CD8− or CD8+CD4− cells without dex] × 100.
Immunoblotting.
Whole-cell extracts from 107 cells per treatment were prepared as described (23) with some modifications. Cells were harvested and washed in PBS, and cell pellets were frozen in liquid nitrogen, resuspended in 50 μl of lysis buffer [5% (vol/vol) glycerol/400 mM NaCl/10 mM Hepes, pH 7.9/0.1 mM EGTA/1 mM DTT/1 mM PMSF/1 μg/ml each of leupeptin, pepstatin A, antipain, chymostatin, and aprotinin/25 mM NaF/2 mM β-glycerophosphate/5 mM pyrophosphate] and then incubated for 30 min on ice. After centrifugation (10,000 × g for 5 min at 4°C), supernatant was mixed with 2× SDS-sample buffer and boiled for 3 min. Extracts for Bcl-2, Bcl-XL, and Bad were prepared as described (31). Immunoblots were performed as recommended by the antibody manufacturer. GR immunoblots were performed with BUGR-2 as described (23).
Results
Ras Inhibits GR-Mediated Apoptosis.
To focus on TCR antagonism of glucocorticoid-induced apoptosis, we used a mutant of the murine T cell hybridoma, 2B4.11, that lacks Fas (13). In contrast to the parental 2B4.11 cells, TCR/CD3 stimulation of 2B4.11 (Fas-negative) cells with anti-CD3 antibody failed to induce apoptosis (Fig. 1A). Nonetheless, TCR stimulation by anti-CD3 rescued these cells from GR-mediated apoptosis (Fig. 1A).
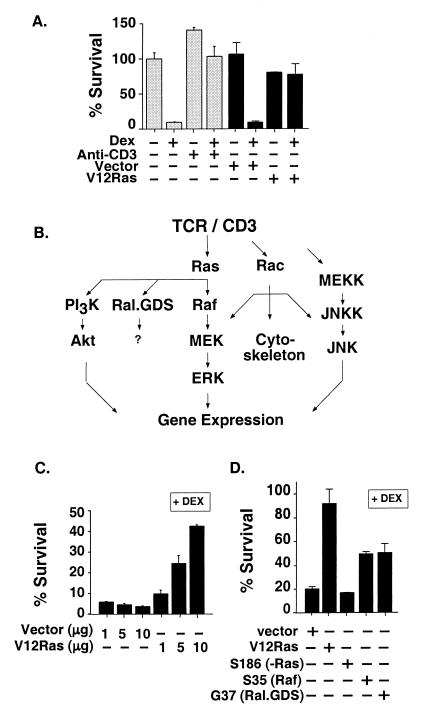
Figure 1.
TCR/CD3, Ras, and Ras-activated kinase pathways prevent dex-induced apoptosis. (A) Viability measured by MTT assay (gray bars) of 2B4.11 (Fas-negative) cells treated with vehicle control (0.001% ethanol), 0.1 μM dex, or anti-CD3ɛ (2C11) for 24 h and expressed as percentage of vehicle-treated cell viability. Survival of cells (black bars) transfected with 5 μg each of RasV12 or vector, pSG5, and CMV-β-gal is shown as measured by flow cytometry as β-gal activity per live cell. Percentage of survival = [percentage of FDG-positive/PI-negative cells with dex/percentage of FDG-positive/PI-negative cells plus vector alone without dex] × 100. (B) TCR/CD3 operates through multiple signaling pathways. Pathways were investigated for their role in TCR/CD3 antagonism of GR-mediated apoptosis by using activated or dominant-negative mutants of pathway components or specific inhibitory compounds. (C) RasV12 rescues S49 murine thymoma cells from dex-induced apoptosis. Survival of cells transfected with 1, 5, or 10 μg each of RasV12 or vector control, pSG5, and CMV-β-gal, treated with 0.1 μM dex for 30 h as measured as β-gal activity in cell extracts is shown. Percentage of survival in the presence of dex = [β-gal units with dex/β-gal units without dex] × 100. (D) RasV12 derivatives that selectively activate Raf or Ral.GDS inhibit dex-induced apoptosis. Cells from the 2B4.11 (Fas-negative) line transfected with 10 μg of vector; RasV12 or the point mutants RasV12 S35 or RasV12 G37; and 5 μg of CMV-β-gal were treated with 0.1 μM dex for 24 h, and β-gal activity was assayed in cell extracts. Data are representative of three independent experiments with triplicates for each data point, and the error bars throughout indicate standard deviation.
Stimulation of TCR/CD3 results in the activation of a network of signaling pathways, some of which are diagrammed in Fig. 1B. We found that an activated mutant of Ras, RasV12 (32), completely abrogated glucocorticoid-induced apoptosis (Fig. 1A). RasV12 also protected S49 cells, a murine thymoma cell line (17, 33) from glucocorticoid-induced apoptosis (Fig. 1C). In contrast, the inactive point mutant RasV12 S186 (34) gave no protection in 2B4.11 (Fas-negative) cells (Fig. 1D). Thus, activated Ras could mimic the TCR/CD3 signal that inhibits glucocorticoid-induced apoptosis.
Ras activates several downstream effector pathways (Fig. 1B). To define the specific Ras-activated pathways that block glucocorticoid-induced apoptosis, we used RasV12 partial loss-of-function mutants that are pathway-selective (35). We found that RasV12 S35, which activates Raf, partially rescued 2B4.11 (Fas-negative) cells from glucocorticoid-induced apoptosis (Fig. 1D). RasV12 G37, which selectively activates Ral.GDS, a GTP exchange factor, also partially rescued 2B4.11 (Fas-negative) cells from glucocorticoid-induced apoptosis (Fig. 1D); we have not yet investigated the pathway involving Ral.GDS. These results suggest that Raf and Ral.GDS may be effectors of the TCR/Ras survival signal.
MEK1 Inhibits GR-Mediated Apoptosis.
To extend our analysis of the Ras/Raf/MEK cascade in blocking glucocorticoid-induced apoptosis, we tested activated mutants of Raf (v-Raf and Raf-CAAX; ref. 36). Unfortunately, overexpression of these mutants was toxic to the 2B4.11 (Fas-negative) cells (data not shown). However, a constitutively active mutant of MEK1, MEK R4F (ΔN3/S218E/S222D; ref. 37), completely protected 2B4.11 (Fas-negative) cells from glucocorticoid-induced apoptosis (Fig. 2A).
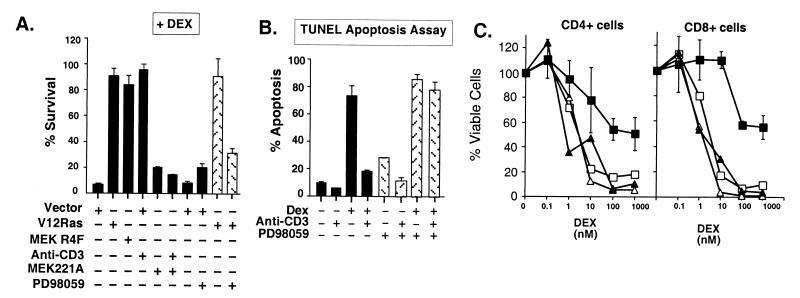
Figure 2.
The MEK/ERK pathway confers protection from dex-induced apoptosis. (A) Constitutively active MEK1 inhibits dex-induced death, and inhibition of MEK1 blocks TCR/CD3 rescue from GR-mediated apoptosis. Viability of 2B4.11 (Fas-negative) cells transfected with CMV-β-gal and vector (pSG5), constitutively active MEK1 (MEK R4F), and dominant-negative MEK221A or RasV12 and then treated as indicated with 0.5 μg/ml anti-CD3ɛ, 0.1 μM dex, 100 μM PD98059, or 0.1% DMSO (vehicle control) for 30 h was assayed by β-gal activity in cell extracts. Results are representative of three experiments with triplicates for each data point. (B) An inhibitor of MEK1 activation, PD98059, blocks TCR/CD3-mediated rescue from glucocorticoid-induced apoptosis. TUNEL assay of 2B4.11 (Fas-negative) cells treated for 24 h with 0.1 μM dex or vehicle control (0.001% ethanol) and 0.5 μg/ml 2C11 (anti-CD3ɛ), 100 μM PD98059 (stippled bars), or vehicle control (0.1% DMSO, black bars). Percentage of apoptosis = percentage of TUNEL-positive cells determined by flow cytometry (30,000 events collected per sample). Data are the average of two independent experiments. (C) TCR/CD3-activated MEK rescues primary mouse T cells from dex-induced apoptosis. Dex dose-response of splenic cell cultures treated with 0.1% DMSO alone (open squares), 100 μM PD98059 alone (open triangles), 1 μg/ml anti-CD3 and DMSO (filled squares), or anti-CD3 and PD98059 (filled triangles) for 24 h. Cells were stained with anti-CD4-PE, anti-CD8-FITC, and PI and analyzed by flow cytometry (30,000 events collected per sample). Percentage of viable cells was determined as described in Materials and Methods. Viability of CD4+CD8− cells (Left) and CD4−CD8+ cells (Right). Data are the average values of two independent experiments.
Complementary with this finding, overexpression of a dominant-negative MEK mutant, MEK221A (38), blocked TCR/CD3-mediated protection against glucocorticoid-induced apoptosis (Fig. 2A). Protection by activated RasV12 was also blocked by the MEK inhibitor PD98059 (ref. 39; Fig. 2A). We analyzed apoptosis with a TUNEL assay and found that TCR/CD3 inhibition of glucocorticoid-induced apoptosis was blocked completely by PD98059 (Fig. 2B). The modest stimulation of apoptosis by PD98059 alone could not account for its effects in blocking TCR/CD3 inhibition of glucocorticoid-induced apoptosis (Fig. 2B). Another MEK1 inhibitor, U0126 (40), also blocked the TCR/CD3 survival signal (data not shown). We conclude from these results that MEK1 activation by TCR/CD3 stimulation and by Ras is necessary and sufficient to prevent glucocorticoid-induced apoptosis.
MEK1 Inhibits GR-Mediated Apoptosis in Primary T Cells.
Primary, unstimulated naïve mouse T cells are sensitive to glucocorticoid-induced apoptosis (41). We tested whether the same TCR–GR crosstalk pathways that operate in immortalized T cells might also rescue primary T cells from glucocorticoid-induced apoptosis. Anti-CD3 stimulation of primary splenic T cells in culture strongly inhibited glucocorticoid-induced apoptosis (Fig. 2C). This result suggested that the same crosstalk pathways may operate in primary T cells and established T cell lines. We investigated the role of the Ras/MEK pathway with the MEK inhibitor PD98059 and found that it abrogated anti-CD3-induced resistance to dex killing in both CD4+ and CD8+ primary T cells (Fig. 2C). We conclude that MEK activation is required for TCR/CD3-mediated inhibition of glucocorticoid-induced apoptosis in naïve, primary T cells.
Phosphatidylinositol 3-Kinase (PI3-Kinase) and Akt Fail to Inhibit GR-Mediated Apoptosis.
Another signaling cascade activated by TCR/CD3 and Ras is the PI3-kinase pathway. PI3-kinase has been shown to protect fibroblasts and neurons from apoptosis, triggered by withdrawal of serum or growth factors, through activation of Akt, a serine/threonine kinase (34, 42, 43). The Ras mutant RasV12 C40, which selectively activates PI3-kinase, protects fibroblasts from apoptosis (34). RasV12C40, however, did not protect 2B4.11 (Fas-negative) cells from glucocorticoid-induced apoptosis (Fig. 3A).
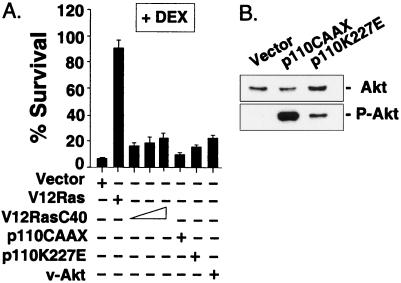
Figure 3.
PI3-kinase and Akt fail to inhibit dex-induced apoptosis. (A) Cells from the 2B4.11 (Fas-negative) line were transfected with CMV-β-gal and one of the following constitutively active mutants: p110CAAX, p110K227E, v-Akt, or 5, 10, or 15 μg of RasV12 C40 (PI3-kinase activator); cells were then treated with 0.1 μM dex or vehicle control for 30 h. β-gal activity was measured in cell extracts. Results are representative of three experiments. (B) Akt is activated in cells expressing constitutively activated PI3-kinase mutants. Phosphorylated (activated) Akt and total Akt protein were analyzed by immunoblot.
We found that activated mutants of the PI3-kinase catalytic subunit, p110, p110K227E, and p110CAAX, failed to inhibit glucocorticoid-induced apoptosis even though they activated Akt (Fig. 3 A and B; refs. 34 and 43). Inhibitors of PI3-kinase, LY294002 and wortmannin, markedly accelerated glucocorticoid-induced apoptosis in the absence of TCR/CD3 stimulation (not shown) and therefore were uninformative. As expected, however, an activated mutant of Akt, v-Akt (34), failed to protect cells from glucocorticoid-induced apoptosis (Fig. 3A). Similarly, expression of c-Akt or activated myristoylated-Akt (43) gave no protection (data not shown). These results indicate that TCR/CD3 inhibition of glucocorticoid-induced apoptosis does not involve PI3-kinase or Akt and is, therefore, distinct from previously identified cellular survival pathways (34, 43).
Endogenous ERK Activity but Not Akt or Bad Correlates with TCR/CD3 Survival Signal.
Using phosphoisoform-specific antibodies in immunoblot analyses, we found that anti-CD3 stimulation of 2B4.11 (Fas-negative) cells activated the mitogen-activated protein kinases ERK1 and ERK2 (Fig. 4), which are substrates of MEK (37). Dex alone did not induce these kinases and did not affect their activation by anti-CD3 (Fig. 4). As expected, PD98059 inhibited anti-CD3 activation of ERK1 and ERK2 (Fig. 4). These results indicate that TCR/CD3-mediated rescue from glucocorticoid-induced apoptosis correlated with activation of ERK1 and ERK2 by endogenous MEK.
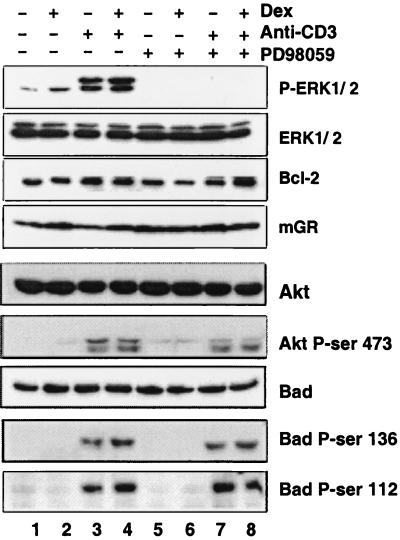
Figure 4.
Phosphorylation changes in ERK but not Akt or Bad correlate with TCR/CD3 inhibition of glucocorticoid-induced apoptosis. Immunoblot analysis was performed on 2B4.11 (Fas-negative) cells treated for 1 h with 0.1% DMSO or 100 μM PD98059 and then for 2 h with 0.1 μM dex and/or 0.5 μg/ml anti-CD3. Immunoblots were performed with antibodies specific for ERK1/2, phospho-ERK1/2, Bcl-2, GR, Akt, phosphoserine 473-Akt, Bad, phosphoserine 136-Bad, and phosphoserine 112-Bad. An 8-h time point is shown for GR.
GR-mediated apoptosis is blocked by increased expression of Bcl-2 and Bcl-XL (19, 41). Conceivably, TCR/CD3 stimulation might affect the expression of these antiapoptotic proteins. However, we found that Bcl-2 and Bcl-XL levels were unaffected by anti-CD3 or dex (Fig. 4 and data not shown).
Finally, we examined the possibility that Akt phosphorylation (and thus inhibition) of the proapoptotic Bcl-2 family member Bad might be involved in the TCR-GR crosstalk pathway (44). We found that steady-state accumulation of Akt and Bad proteins were largely unaffected by any of the treatments (Fig. 4). Interestingly, anti-CD3 treatment induced phosphorylation of Akt and Bad. However, PD98059 inhibition of MEK activation had little or no effect on these modifications. Therefore, phosphorylation of Akt and Bad behaved independently of the CD3-activated antagonism of glucocorticoid-induced apoptosis, which is blocked by PD98059. This result is consistent with the failure of activated PI3-kinase and Akt to prevent GR-mediated apoptosis (see Fig. 3). We conclude that Bad inactivation by Akt does not reside in the TCR/CD3 crosstalk pathway that confers protection from glucocorticoid-induced cell death.
GR Transcription Regulatory Activity Is Altered by TCR/CD3, Ras, and MEK.
Glucocorticoid-induced apoptosis requires GR-mediated changes in gene transcription (18). Hence, TCR/CD3 crosstalk might affect GR directly, altering either its accumulation or its activity. As shown in Fig. 4, TCR/CD3 stimulation did not change GR protein levels, suggesting that TCR/CD3 signaling was not operating on GR expression or turnover.
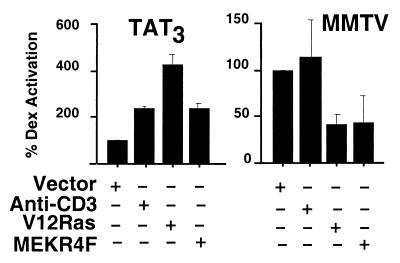
In T cells, glucocorticoids repress the transcription of many genes such as TCR-induced FasL, but the regulation of GR-activated genes in these cells is not well defined. GR-mediated activation of genes is thought to be important for glucocorticoid-induced apoptosis in mice (18, 45), but the GR-regulated genes that confer apoptosis are unknown (17). Therefore, to focus on how TCR/CD3 and its downstream signaling cascades might affect GR transcriptional regulatory activity, we monitored expression of well characterized, “surrogate” reporter constructs. We used reporters associated with two distinct classes of GREs (2). TAT3 is a “simple GRE” (i.e., GR is the sole DNA-bound regulator), whereas the MMTV long terminal repeat contains a “composite GRE” (i.e., GR is one of several regulators that bind and interact; refs. 23 and 24). We transfected reporter plasmids bearing each class of GRE into 2B4.11 (Fas-negative) cells and assessed the effects of TCR/CD3 signaling or cotransfected kinase activators on GR transcriptional activity.
We found that the signaling pathways that inhibit glucocorticoid-induced apoptosis significantly increased the transcriptional activity of GR at the simple GRE, TAT3 (Fig. 5). Anti-CD3, RasV12, and activated MEKR4F all enhanced transcriptional activation of TAT3 by GR compared with treatment with dex alone. In contrast, these same crosstalk pathways had distinct effects at the MMTV composite GRE. RasV12 and MEKR4F suppressed GR activity (Fig. 5). Together, these results demonstrate that crosstalk signaling from TCR/CD3 clearly modulates the endpoint of GR signaling, its transcriptional regulatory activity. Strikingly, the consequence of TCR/CD3 crosstalk signaling is not hard wired but rather depends on the GRE at which the GR is acting.
Figure 5.
TCR/CD3, activated Ras, and MEK1 each alter transcriptional activation by GR. Cells from the 2B4.11 (Fas-negative) line were transfected with CMV-β-gal, GR-dependent luciferase reporter, and pSG5 (vector), RasV12, or MEKR4F and then treated with 0.1 μM dex with or without anti-CD3ɛ for 8 h. Luciferase activity was normalized to β-gal activity. Fold induction with dex alone was 6.7 ± 0.25 for TAT3 luciferase and 26.9 ± 0.80 for (MMTV) long terminal repeat luciferase. Transcription activation with dex alone was set as 100%. Results are representative of three separate experiments.
Discussion
Signaling proteins such as GR and Ras can display either proapoptotic or antiapoptotic activity depending on cell context (4, 34). We show herein that crosstalk between ubiquitous signaling proteins can produce context-specific effects on survival. Thus, a Ras effector pathway could inhibit GR-mediated apoptosis.
More specifically, gain-of-function, loss-of-function, and biochemical approaches were used to establish that TCR activation of the MEK/ERK cascade via Ras was necessary and sufficient to inhibit GR-mediated death in primary and immortalized T cells. In turn, these pathways significantly altered GR transcriptional regulatory activity. Hence, the simplest interpretation of our results is that GR itself is the target of this crosstalk pathway.
Kinase Pathway Modulation of GR Activity.
Consistent with our model, GR (46, 47) and other intracellular receptors (48–51) can be phosphorylated by mitogen-activated protein kinases, and this modification seems to affect receptor transcriptional regulatory activity. As the GR target genes that induce apoptosis have not been identified, we monitored well defined “surrogate” GRE activities to assess the effects of the TCR/CD3 crosstalk pathway on GR activity. It was important to test different classes of response elements, because regulatory effects are strongly context-dependent (2, 45). Indeed, the signaling pathways that inhibit GR-mediated apoptosis significantly and differentially altered GR transcriptional regulatory activity at two distinct classes of GREs. Thus, our study adds a new layer to the multiple determinants of combinatorial control that together impart flexibility and specificity on the actions of GR and other transcriptional regulators. We suggest that the TCR/CD3 crosstalk pathway likely has broad target-selective effects on GR transcriptional regulation.
GR in T Cell Development and Function.
Our results demonstrate that GR can inhibit TCR/CD3 activation-induced death via MEK in primary splenic T cells. Thus, the same crosstalk pathway that we characterized in immortalized cells is also operative in mature, primary cells. In vivo glucocorticoid levels are governed by diurnal cycling and respond strongly to stress. Conceivably, the TCR-GR crosstalk pathway serves to inhibit the glucocorticoid-induced apoptosis of T cells that are combating stresses such as infection or inflammation.
We also found that Ras signaling in a thymoma cell line, S49, prevented glucocorticoid-induced apoptosis, perhaps implying that the same crosstalk pathways operate in thymocytes. Thymocytes undergo selection processes to ensure TCR and coreceptor combinations that are functional but nonresponsive to self-antigens (6). Ashwell and coworkers (4, 52, 53) showed that reduced GR protein in thymocytes can alter the T cell repertoire. Notably, the TCR/Ras/MEK/ERK pathway implicated in positive selection (11) is the same pathway that we have shown to inhibit glucocorticoid-induced apoptosis. It is possible that during T cell development, the TCR/CD3-activated Ras/MEK/ERK pathway confers positive selection at least in part by rescuing cells from glucocorticoid-induced apoptosis. That is, glucocorticoid-induced apoptosis may impose a key restriction that makes TCR signaling via Ras activation of MEK compulsory for thymocyte survival. Confirmation of this hypothesis requires further experimentation.
Glucocorticoids, Homeostasis, and the Immune Repertoire.
The specificity of responses to steroid hormones is likely to be determined by a combination of physiological, cellular, and gene contexts (2, 54). More generally, however, steroids maintain differentiation and homeostasis in many tissues such prostate (testosterone), mammary (estrogens), and endometrial (progesterone) epithelia (55). We suggest that glucocorticoids play a homeostatic role in T cell development and function, balancing the signaling strength of TCR and GR to promote selective cell survival. Thus, fluctuations in glucocorticoid levels that occur in response to diurnal cycling or stress would shift the spectrum of surviving T cells at different times, thereby producing a net expansion in the T cell repertoire.
Our identification of the components of this crosstalk pathway is also of potential clinical value, because it suggests a new layer of regulators that influence hormonal efficacy. For example, dex is initially efficacious for treatment of lymphoma and acute lymphocytic leukemia but progression to dex resistance is commonly encountered (56). Activating N-Ras point mutations have been described in some dex-resistant cases of acute lymphocytic leukemia (57), perhaps implicating the crosstalk pathway that we have characterized. Inhibition of Ras in tumor cells may resensitize them to hormone therapy.
The GR and the TCR are essential and highly sensitive detectors and transducers of extracellular stimuli. Defining the interactions of their pathways will advance our understanding of the signaling crosspoints that determine cell life or death.
Acknowledgments
We thank Drs. N. Ahn, J. Ashwell, A. Bellacosa, J. Downward, N. Hay, P. Rodriguez-Viciana, and D. Stokoe for generously supplying cells and plasmids. We are grateful to Drs. H. Bourne, B. Darimont, A. DeFranco, R. Derynck, D. Hanahan, H. Ingraham, N. Killeen, I. Rogatsky, D. Stokoe, and A. Weiss for critical reading of the manuscript. This work was supported by grants from National Science Foundation and National Institutes of Health to K.R.Y.; C.A.M.J. was a Fellow of the Arthritis Foundation.
Abbreviations
- GR
glucocorticoid receptor
- TCR
T cell antigen receptor
- ERK
extracellular signal regulated kinase
- MEK
mitogen-activated protein kinase kinase
- PI
propidium iodide
- PI3-kinase
phosphatidylinositol 3-kinase
- GRE
glucocorticoid response element
- MMTV
mouse mammary tumor virus
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- dex
dexamethasone
- CMV
cytomegalovirus
- β-gal
β-galactosidase
- FDG
fluorescein di-β-d-galactopyranoside
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP end labeling
References
- 1.Di Croce L, Okret S, Kersten S, Gustafsson J, Parker M, Wahli W, Beato M. EMBO J. 1999;18:6201–6210. doi: 10.1093/emboj/18.22.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamoto K R, Darimont B D, Wagner R L, Iñiguez-Lluhí J A. Cold Spring Harbor Symp Quant Biol. 1998;63:587–598. doi: 10.1101/sqb.1998.63.587. [DOI] [PubMed] [Google Scholar]
- 3.Cole T J, Blendy J A, Monaghan A P, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schütz G. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 4.Tolosa E, Ashwell J D. Neuroimmunomodulation. 1999;6:90–96. doi: 10.1159/000026368. [DOI] [PubMed] [Google Scholar]
- 5.King L B, Vacchio M S, Dixon K, Hunziker R, Margulies D H, Ashwell J D. Immunity. 1995;3:647–656. doi: 10.1016/1074-7613(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 6.Marrack P, Kappler J. Curr Opin Immunol. 1997;9:250–255. doi: 10.1016/s0952-7915(97)80144-6. [DOI] [PubMed] [Google Scholar]
- 7.Osborne B A. Curr Opin Immunol. 1996;8:245–254. doi: 10.1016/s0952-7915(96)80063-x. [DOI] [PubMed] [Google Scholar]
- 8.Iwata M, Ohoka Y, Kuwata T, Asada A. Stem Cells. 1996;14:632–641. doi: 10.1002/stem.140632. [DOI] [PubMed] [Google Scholar]
- 9.Chu D H, Morita C T, Weiss A. Immunol Rev. 1998;165:167–180. doi: 10.1111/j.1600-065x.1998.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 10.Henning S W, Cantrell D A. Curr Opin Immunol. 1998;10:322–329. doi: 10.1016/s0952-7915(98)80171-4. [DOI] [PubMed] [Google Scholar]
- 11.Alberola-Ila J, Takaki S, Kerner J D, Perlmutter R M. Annu Rev Immunol. 1997;15:125–154. doi: 10.1146/annurev.immunol.15.1.125. [DOI] [PubMed] [Google Scholar]
- 12.Zacharchuk C M, Mercep M, Chakraborti P K, Simons S S, Jr, Ashwell J D. J Immunol. 1990;145:4037–4045. [PubMed] [Google Scholar]
- 13.Yang Y, Mercep M, Ware C F, Ashwell J D. J Exp Med. 1995;181:1673–1682. doi: 10.1084/jem.181.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallach D, Varfolomeev E E, Malinin N L, Goltsev Y V, Kovalenko A V, Boldin M P. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 15.Hakem R, Hakem A, Duncan G S, Henderson J T, Woo M, Soengas M S, Elia A, de la Pompa J L, Kagi D, Khoo W, et al. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 16.Kuida K, Haydar T F, Kuan C Y, Gu Y, Taya C, Karasuyama H, Su M S, Rakic P, Flavell R A. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 17.Chapman M S, Askew D J, Kuscuoglu U, Miesfeld R L. Mol Endocrinol. 1996;10:967–978. doi: 10.1210/mend.10.8.8843413. [DOI] [PubMed] [Google Scholar]
- 18.Dieken E S, Miesfeld R L. Mol Cell Biol. 1992;12:589–597. doi: 10.1128/mcb.12.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Memon S A, Moreno M B, Petrak D, Zacharchuk C M. J Immunol. 1995;155:4644–4652. [PubMed] [Google Scholar]
- 20.Deftos M L, He Y W, Ojala E W, Bevan M J. Immunity. 1998;9:777–786. doi: 10.1016/s1074-7613(00)80643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner D H, Jr, Hagman J, Linsley P S, Hodsdon W, Freed J H, Newell M K. J Exp Med. 1996;184:1631–1638. doi: 10.1084/jem.184.5.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y, Tozawa Y, Iseki R, Mukai M, Iwata M. J Immunol. 1995;154:6346–6354. [PubMed] [Google Scholar]
- 23.Vivanco M D, Johnson R, Galante P E, Hanahan D, Yamamoto K R. EMBO J. 1995;14:2217–2228. doi: 10.1002/j.1460-2075.1995.tb07216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iniguez-Lluhi J A, Lou D Y, Yamamoto K R. J Biol Chem. 1997;272:4149–4156. doi: 10.1074/jbc.272.7.4149. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro V S, Truitt K E, Imboden J B, Weiss A. Mol Cell Biol. 1997;17:4051–4058. doi: 10.1128/mcb.17.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamieson C, McCaffrey P G, Rao A, Sen R. J Immunol. 1991;147:416–420. [PubMed] [Google Scholar]
- 27.Mosmann T. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Memon S A, Petrak D, Moreno M B, Zacharchuk C M. J Immunol Methods. 1995;180:15–24. doi: 10.1016/0022-1759(94)00294-7. [DOI] [PubMed] [Google Scholar]
- 29.Gorczyca W, Gong J, Darzynkiewicz Z. Cancer Res. 1993;53:1945–1951. [PubMed] [Google Scholar]
- 30.Killeen N, Stuart S G, Littman D R. EMBO J. 1992;11:4329–4336. doi: 10.1002/j.1460-2075.1992.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H G, Rapp U R, Reed J C. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 32.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 33.Sibley C H, Tomkins G M. Cell. 1974;2:213–220. doi: 10.1016/0092-8674(74)90013-0. [DOI] [PubMed] [Google Scholar]
- 34.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Nature (London) 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 36.Stokoe D, Macdonald S G, Cadwallader K, Symons M, Hancock J F. Science. 1994;264:1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- 37.Resing K A, Ahn N G. Biochemistry. 1998;37:463–475. doi: 10.1021/bi971750x. [DOI] [PubMed] [Google Scholar]
- 38.Cowley S, Paterson H, Kemp P, Marshall C J. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 39.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 40.Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stradley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, et al. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 41.Van Houten N, Blake S F, Li E J, Hallam T A, Chilton D G, Gourley W K, Boise L H, Thompson C B, Thompson E B. Int Immunol. 1997;9:945–953. doi: 10.1093/intimm/9.7.945. [DOI] [PubMed] [Google Scholar]
- 42.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy S G, Wagner A J, Conzen S D, Jordán J, Bellacosa A, Tsichlis P N, Hay N. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 44.Mok C L, Gil-Gómez G, Williams O, Coles M, Taga S, Tolaini M, Norton T, Kioussis D, Brady H J. J Exp Med. 1999;189:575–586. doi: 10.1084/jem.189.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reichardt H M, Kaestner K H, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, et al. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 46.Krstic M D, Rogatsky I, Yamamoto K R, Garabedian M J. Mol Cell Biol. 1997;17:3947–3954. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogatsky I, Logan S K, Garabedian M J. Proc Natl Acad Sci USA. 1998;95:2050–2055. doi: 10.1073/pnas.95.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, et al. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 49.Hammer G D, Krylova I, Zhang Y, Darimont B D, Simpson K, Weigel N L, Ingraham H A. Mol Cell. 1999;3:521–526. doi: 10.1016/s1097-2765(00)80480-3. [DOI] [PubMed] [Google Scholar]
- 50.Tremblay A, Tremblay G B, Labrie F, Giguère V. Mol Cell. 1999;3:513–519. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- 51.Bunone G, Briand P A, Miksicek R J, Picard D. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 52.Tolosa E, King L B, Ashwell J D. Immunity. 1998;8:67–76. doi: 10.1016/s1074-7613(00)80459-8. [DOI] [PubMed] [Google Scholar]
- 53.Lu F W, Yasutomo K, Goodman G B, McHeyzer-Williams L J, McHeyzer-Williams M G, Germain R N, Ashwell J D. Immunity. 2000;12:183–192. doi: 10.1016/s1074-7613(00)80171-5. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto K R. Harvey Lect. 1995;91:1–19. [PubMed] [Google Scholar]
- 55.Isaacs J T. Urol Clin North Am. 1999;26:263–273. doi: 10.1016/s0094-0143(05)70066-5. [DOI] [PubMed] [Google Scholar]
- 56.Klumper E, Pieters R, Veerman A J, Huismans D R, Loonen A H, Hählen K, Kaspers G J, van Wering E R, Hartmann R, Henze G. Blood. 1995;86:3861–3868. [PubMed] [Google Scholar]
- 57.Lübbert M, Mirro J, Jr, Miller C W, Kahan J, Isaac G, Kitchingman G, Mertelsmann R, Herrmann F, McCormick F, Koeffler H P. Blood. 1990;75:1163–1169. [PubMed] [Google Scholar]