Abstract
Background
We aimed at studying the seasonal changes in mood and behaviour, the distribution of hospital admissions by season, and the persistence of the circadian type in twins with bipolar disorder and their healthy co-twins.
Methods
All Finnish like-sex twins born from 1940 to 1969 were screened for a diagnosis of bipolar type I disorder. The diagnosis was assessed with a structured research interview, and the study subjects (n = 67) filled in the Seasonal Pattern Assessment Questionnaire (SPAQ) and the Morningness-Eveningness Questionnaire (MEQ). For studying the persistence of the habitual sleep length and circadian type, we used data derived from the Finnish Twin Cohort Questionnaire (FTCQ). Bipolar twins were compared with their healthy co-twins.
Results
Bipolar twins had greater seasonal changes in sleep length (p = 0.01) and mood (p = 0.01), and higher global seasonality scores (p = 0.03) as compared with their co-twins with no mental disorder. Sunny days (p = 0.03) had a greater positive effect on wellbeing in the bipolar than healthy co-twins.
Conclusions
Our results support the view that bipolar disorder is sensitive to the environmental influence in general and to the seasonal effect in specific. Exposure to natural light appears to have a substantial effect on wellbeing in twins with bipolar disorder.
Keywords: Bipolar disorder, Circadian type, Mood, Season, Twin, Weather
Background
Approximately 10% of all affective disorders show a seasonal pattern of recurrence, half of these being recurrent depressive disorders and the other half bipolar disorders [1]. Studies that have considered the onset of depressive episodes usually report two peaks of incidence: one in spring, and another in autumn [2]. In patients with bipolar disorder, admissions for manic and depressive episodes frequently follow a seasonal pattern with the peaks during either autumn or winter, or autumn and spring [3,4]. However, the lack of seasonal variation in admissions due to bipolar disorder has also been reported [5].
Patients suffering from seasonal changes in mood and behaviour are frequently recognised as having affective disorder, but other primary diagnoses are also common [6]. In particular, seasonal affective disorder is a form of recurrent major depressive or bipolar disorder with a seasonal pattern for which light therapy has been established as the first-line treatment option [7,8]. It usually takes the course with winter depressive episodes that are followed by summer remissions, although in some cases the pattern may be reversed [9].
Recently, it has been noticed that not only depressed but also bipolar patients staying in sunny hospital rooms seem to recover more rapidly than those in dull rooms [10,11]. Low levels of illumination appear to be linked to the emergence of atypical depressive symptoms even among healthy people [12]. To sum up, there is marked seasonal variation in affective disorders, and they seem to be responsive to a number of environmental stimuli, such as exposure to light.
There may also be substantial changes in sleep length by season. Disruptions in the sleep-wake cycle can be a trigger of manic episodes in bipolar disorder [13]. Twin studies suggest that there is genetic susceptibility to seasonal changes [14,15]. Therefore, it seems likely that patients with bipolar disorder have greater seasonal changes in mood and behaviour and those in sleep length than subjects with no mental disorder.
Morningness or eveningness, an endogenous component of the circadian clock, is characterised by inter-individual differences in circadian phase and requires a specific timing of complex behaviours. A subjective report of the preference for morning or evening activities is predictive of individually preferred bedtimes, and there is a strong link between the preferred sleep-wake schedules and peak body temperature times indicative of the intrinsic period [16-19]. Depressed patients have more frequently the preference of evening activities as compared with healthy individuals [20], but there are no such data concerning bipolar disorder.
Study aims
We aimed at studying the seasonal variation in hospital admissions and self-reports of wellbeing in twins with bipolar disorder. We predicted that twins with bipolar disorder would present more pronounced changes in the latter than their healthy co-twins. Another aim was to study the effect of natural light exposure. We predicted that exposure to natural light would have positive effects on wellbeing in twins with bipolar disorder. We also aimed at assessing the circadian preference, sleep length and seasonal pattern in mood and behaviour among twins with bipolar disorder.
Methods
Subjects
We started the search for study participants with the National Hospital Discharge Register of Finland in order to identify all patients with at least one of the following: ICD-8 codes 296.10 or 296.30 in 1969–86 [21], or DSM-III-R codes 296.4, 296.5 or 296.6 in 1987–91 [22]. Dates for each episode were recorded. The National Population Register was thereafter used to locate twins born between 1940 and 1969. We also checked the Finnish Twin Cohorts to identify any additional twins [23]. One twin-pair with no history of mental illness was included to increase the number of healthy controls. All the twins identified were sent an invitation to participate with their co-twin in the study. 67 twins filled in the Seasonal Pattern Assessment Questionnaire (SPAQ) as part of the research interviews that took place during the period of November 1998 to December 1999. Six twins refused from any further contacts, and finally 61 twins were sent the Morningness-Eveningness Questionnaire (MEQ) in January 2002. The response rate was 62% (36 out of 61).
Diagnostic assessment
All the probands and co-twins were interviewed by one of the authors using the structured clinical interview for DSM-IV diagnoses [24]. The interviews were made blind to zygosity. The diagnostic procedure has been described in detail elsewhere [25]. The zygosity determination was based on genetic marker analysis and on the questionnaire on resemblance during childhood [26].
Assessment of seasonal changes and circadian type
The SPAQ was used for the assessment of seasonal variation in the length of sleep, social activity, mood, weight, appetite, and energy level [7]. The sum of these six scales yields the Global Seasonality Score (GSS), ranging from 0 to 24. The SPAQ also investigated the experienced changes in wellbeing attributed to local weather conditions. The sum of these ten scales yields a global score, designated here as the Global Weather Score (GWS), ranging from -30 to + 30. Negative scores indicate negative effects and positive scores indicate positive effects on wellbeing. In addition, two new variables were coded and thereafter analysed separately: bright light exposure (the sum of sunny days plus long days) and dim light exposure (the sum of grey cloudy days plus foggy or smoggy days plus short days).
The MEQ is a self-report questionnaire that has been used for the assessment of preferred timing of complex behaviours [27]. The MEQ includes 19 items, and the sum yields a global score, designated here as the Morningness-Eveningness Score (MES), ranging from 16 to 86. The highest scores indicate definite morningness, and the lowest ones definite eveningness. The Finnish Twin Cohort Questionnaire (FTCQ) included one question concerning the circadian type (item #19 of the MEQ). We used the data on this item for studying the stability of circadian preference.
Sleep length
To study the sleep length and subjective feelings of sleep debt, we used the data derived from the FTCQ administered in 1975, 1981 and 1990. The overall response rates were 89%, 84% and 77%, respectively. The FTCQ included two questions about sleep length ("How many hours you usually sleep at night?" and "How many hours you estimate to need to sleep, to feel perky next day?"). A new variable, sleep debt, was calculated from these two questions by subtraction.
Statistics
To take into account the correlated nature of twin data, we used adjusted Pearson F-statistics and Wald tests for clustered data to compare bipolar with healthy twins [28]. This method caters for the fact that there were pairs in which both twins had bipolar disorder. Survey estimation prevalence was applied for the assessment of the domain and sum variables derived from the questionnaires [29]. The Wilcoxon signed ranks tests were computed for the analysis of differences in these scores within the discordant twin-pairs, i.e. in which one twin had bipolar disorder and the other did not. Intra-pair correlations were calculated for the GSS and GWS, and partial correlation coefficients to estimate the association between the MES and the GSS. Chi-square and Fisher's exact tests were used for the analysis of categorical variables.
Ethics
The Ministry of Social Affairs and Health, and the Ethics Committee of the National Public Health Institute, Finland, approved the study protocol. Written informed consent was obtained from all subjects after they had received a complete description of the study protocol.
Results
The sample consisted of 67 study subjects (Table 1). Of them, 39 had bipolar disorder, with the mean age of 44.3 years (ranging from 29 to 57), and 20 were assessed as healthy (no mental disorder), with the mean age of 44.7 years (ranging from 33 to 57). The number of discordant pairs was 15. In addition, 8 subjects had mental disorder other than bipolar disorder, and they were excluded from subsequent analysis.
Table 1.
The study sample of bipolar disorder twins and co-twins.
| Affection status | Zygosity | Sex |
| Both twins participated, n = 54 | ||
| BP*-Healthy* | DZ | FF |
| BP*-Healthy* | DZ | FF |
| BP*-Healthy* | DZ | FF |
| BP*-Healthy* | DZ | MM |
| BP*-Healthy | DZ | MF |
| BP-Healthy* | DZ | MF |
| BP-Healthy* | DZ | FF |
| BP-Healthy* | DZ | FF |
| BP*-Healthy | DZ | FF |
| BP*-Healthy | MZ | FF |
| BP-Healthy* | DZ | FF |
| BP-Healthy* | DZ | FF |
| BP*-Healthy | DZ | FF |
| BP-Healthy | DZ | MM |
| BP-Healthy | MZ | MM |
| BP-Alcohol abuse | DZ | MM |
| BP-Alcohol abuse* | DZ | FF |
| BP*-MDD | DZ | FM |
| BP*-Schizophrenia* | MZ | MM |
| BP*-MDD* | DZ | FF |
| BP*-MDD* | DZ | FF |
| BP*-BP* | MZ | FF |
| BP*-BP* | DZ | FF |
| BP*-BP | MZ | MM |
| BP*-BP | DZ | MM |
| BP-BP | MZ | MM |
| Healthy-Healthy | MZ | FF |
| One twin participated, n = 13 (Co-twin did not participate, n = 13) | ||
| BP*-(Schizophrenia) | DZ | MM |
| BP*-(BP) | DZ | MM |
| BP*-(Healthy) | DZ | MM |
| BP-(Alcohol abuse) | DZ | MM |
| Healthy-(BP) | DZ | MM |
| Healthy-(BP) | DZ | MM |
| BP-(Healthy) | DZ | MM |
| Healthy-(BP) | DZ | MM |
| BP-(BP) | DZ | MM |
| MDD-(BP) | MZ | FF |
| Post-partum depression-(BP) | DZ | FM |
| BP-(Schizophrenia) | DZ | FM |
| BP-(BP) | DZ | MM |
*Subjects who filled in the MEQ in 2002, n = 36. Abbreviations: BP = bipolar disorder, MDD = major depressive disorder, DZ = dizygotic, MZ = monozygotic, M = male, F = female.
There was a greater proportion of men among the bipolar than healthy twins (F [1,37] = 4.75, p = 0.04), but there was no difference in the affection status by zygosity.
Seasonal variation in hospital admission
Twins with bipolar disorder (n = 39) had most (31 %) of they hospital admissions during autumn (Table 2). The distribution of depressive and manic episodes did not differ significantly by season (χ2 = 7.36, df = 3, p = 0.06), although the depressive episodes were most common in the autumn and winter, and manic episodes in the autumn and summer. We also counted the most frequent season of admission for each individual with recurrent episodes. Most of these admissions occurred during autumn (34% of all episodes, 33 patients), admissions due to depressives episodes being most frequent in the autumn and winter (67 % of all episodes, 11 patients) and admissions due to manic episodes in the autumn, spring, or summer (86% of all episodes, 29 patients).
Table 2.
The seasonal pattern of episodes in twins with bipolar disorder.
| Season | Episodes | ||
| depressive | manic | total | |
| Autumn | 20 | 42 | 62 |
| Winter | 13 | 29 | 42 |
| Spring | 7 | 36 | 43 |
| Summer | 8 | 46 | 54 |
| Total | 48 | 153 | 201 |
The seasonal distribution of hospital admissions did not match with the self-reports of feeling worst, as assessed with the SPAQ (χ2 = 2.29, df = 1, p = 0.13). Similarly, we analysed the self-reported length of sleep. The period of sleeping most did coincide with the admissions, whereas the period of sleeping least did not.
Seasonal changes in mood and behaviour
There were significant differences in the extent of seasonal changes in mood (mean = 1.66, 95% CI of 1.35 to 1.97, and mean = 1.10, 95% CI = 0.73 to 1.47; F [1,36] = 6.24, p = 0.02), weight (mean = 0.97, 95%CI of 0.65 to 1.29, and mean = 0.45, 95% CI of 0.13 to 0.77; F [1,37] = 5.88, p = 0.02), appetite (mean = 0.90, 95% CI of 0.57 to 1.23, and mean = 0.40, 95% CI of 0.08 to 0.72; F [1,37] = 4.76, p = 0.04), and levels of energy (mean = 1.64, 95% CI of 1.27 to 2.01, and mean = 1.10, 95% CI of 0.73 to 1.47; F [1,37] = 5.98, p = 0.02), as well as in the GSS (mean = 8.21, 95% CI of 6.82 to 9.59, and mean = 5.20, 95% CI of 3.49 to 6.91; F [1,37] = 9.73, p = 0.004) between the bipolar and healthy twins, respectively. The changes were of greater extent in the bipolar twins.
Weather associated changes in wellbeing
There was a significant difference in the effect of dry days on feelings of wellbeing (mean = 1.47, 95% CI of 0.97 to 1.97, and mean = 0.68, 95% CI of 0.20 to 1.17; F [1,34] = 4.34, p = 0.04) between the bipolar and healthy twins, respectively. Dry days induced a more positive effect on wellbeing in the bipolar twins (in 23 of 30, 77%). Interestingly, short days had only a negative effect on wellbeing in the monozygotic twins, whereas the response was diverse among the dizygotic twins (χ2 = 7.92, df = 2, p = 0.02).
Analysis of discordant pairs
There were greater seasonal changes in sleep length (mean = 1.86, 95% CI of 1.22 to 2.49, and mean = 1.07, 95% CI = 0.46 to 1.68; Z = -2.58, p = 0.01), and mood (mean = 2.00, 95% CI of 1.45 to 2.55, and mean = 1.07, 95% CI of 0.62 to 1.51; Z = -2.50, p = 0.01), and the GSS (mean = 8.73, 95% CI = 6.41 to 11.06, and mean = 5.33, 95% CI of 3.09 to 7.57; Z = -2.23, p = 0.03) in the bipolar twins compared with their healthy co-twins, respectively. In addition, sunny days (Z = -2.15, p = 0.03) had a greater positive effect on wellbeing in the bipolar than healthy co-twins. The mean difference (95% CI) between the bipolar and healthy twins in the GSS was 3.40 (0.65 to 6.15), in sleep length 1.00 (0.36 to 1.64), in social activity 0.80 (0.01 to 1.59), in mood 0.93 (0.29 to 1.58), and in sunny days 1.00 (0.15 to 1.85).
Analysis of intra-pair correlations
The intra-pair correlation of the GSS was 0.16 for the seven monozygotic twin-pairs (p = 0.73), and 0.21 for the twenty dizygotic twin-pairs (p = 0.38). The intra-pair correlation of the GWS was -0.16 for the monozygotic twins (p = 0.76), and 0.04 for the dizygotic twins (p = 0.88).
Sleep length and debt
The bipolar twins slept longer, as assessed with the FTCQ in 1990, compared with healthy twins (F [1,18] = 7.47, p = 0.01; see Table 3). At follow-up, the mean sleep length was 8.21 hours for the morning types and 7.66 hours for the evening types, as assessed with the SPAQ in 1999. When we compared data on sleep length derived from the SPAQ with those from the FTCQ, healthy twins slept less in 1999 than in 1981 (Table 4). The morning types with bipolar disorder slept more in 1999 than in 1981. The longest sleep length was 8.59 hours among the morning types (in winter), and the shortest one was 6.70 among the evening types (in summer).
Table 3.
Sleep length and debt, wellbeing and circadian preference by affection status.
| Bipolar disorder | No mental disorder | |||||
| n | mean | 95% CI | n | mean | 95% CI | |
| FTCQ | ||||||
| sleep length (h) | ||||||
| in 1975 | 21 | 7.86 | 7.61 to 8.11 | 9 | 7.83 | 7.56 to 8.11 |
| in 1981 | 22 | 7.77 | 7.38 to 8.17 | 12 | 7.79 | 7.28 to 8.31 |
| in 1990 | 18 | 7.92 | 7.48 to 8.35 | 10 | 7.15 | 6.81 to 7.49 |
| sleep debt (h) | ||||||
| in 1981 | 22 | -0.34 | -0.74 to 0.06 | 12 | 0.04 | -0.24 to 0.33 |
| in 1990 | 18 | -0.25 | -0.85 to 0.35 | 10 | 0 | -0.24 to 0.24 |
| SPAQ | ||||||
| GSS | 39 | 8.21 | 6.82 to 9.59 | 20 | 5.20 | 3.49 to 6.91 |
| GWS | 37 | 2.43 | 0.52 to 4.34 | 19 | 1.32 | -0.05 to 2.68 |
| MES | 21 | 53.29 | 50.2 to 59.2 | 11 | 54.64 | 50.2 to 59.1 |
Abbreviations: FTCQ = The Finnish Twin Cohort Questionnaire, SPAQ = The Seasonal Pattern Assessment Questionnaire, GSS = Global Seasonality Score, GWS = Global Weather Score, MES = Morningness-Eveningness Score.
Table 4.
Sleep length in the morning and evening types by affection status.
| Sleep length (h) | Bipolar disorder | No mental disorder | ||||
| n | mean | 95% CI | n | mean | 95% CI | |
| Morning type | ||||||
| in 1981 from FTCQ | 6 | 7.58 | 6.62 to 8.55 | 6 | 7.67 | 6.53 to 8.80 |
| in 1999 from SPAQ | 9 | 8.72 | 7.76 to 9.69 | 6 | 7.25 | 6.63 to 7.87 |
| Evening type | ||||||
| in 1981 from FTCQ | 10 | 7.70 | 7.06 to 8.34 | 6 | 7.92 | 7.40 to 8.43 |
| in 1999 from SPAQ | 12 | 7.77 | 6.99 to 8.56 | 6 | 7.38 | 6.28 to 8.47 |
Circadian type
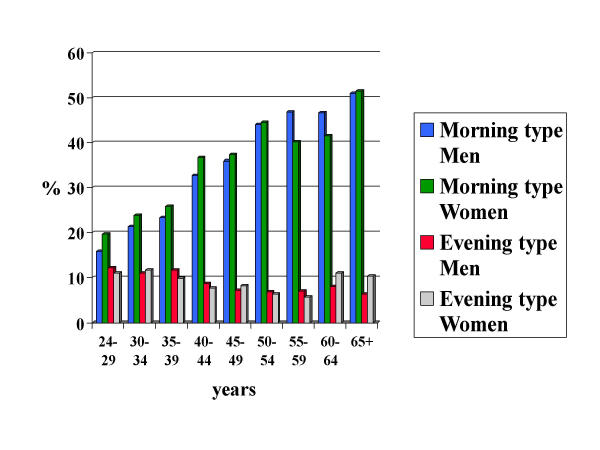
Among in the whole twin cohort (assessed with the FTCQ), the stability of circadian preference remained good from 1981 to 1986 (χ2 = 134.4, df = 9, p < 0.0001). Preference for morning activities tended to become slightly more prevalent with ageing in the whole cohort of 11,404 men and 12,445 women (Figure 1), as in our study sample. There were no significant differences in the MES (F [1,34] = 0.17, p = 0.68), or the circadian type preferences (F [1,35] = 0.02, p = 0.88) between the bipolar and healthy twins (Table 3). The MES did not differ between monozygotic and dizygotic twins, or between men and women.
Figure 1.
Morning and evening types among the twin cohort.
Preference for evening activities was associated with a higher GSS (r= -0.045, p < 0.01), after controlling for the sex, age, zygosity, and affection status. This association was seen only for the total score, and there were no significant associations between the circadian type and the seasonal changes in length of sleep, weight, or appetite. Most often, the changes in weight as well as those in appetite were reported to be greater in the winter and smaller in the summer. The most frequent change in weight (in 51% of the individuals) was a gain of 2 to 3.5 kg during the year, and in 32% of the individuals there was a change in appetite by season.
Discussion
The autumn was generally the worst time to patients with depressive and manic episodes, especially for those with recurrent episodes. Therefore, the negative influence of autumn on mental wellbeing needs to be noticed with more care in clinical work. Surprisingly, the self-reports of feeling worst did not match with the temporal distribution of depressed and manic episodes, instead the period of sleeping most appeared to coincide with admissions due to a depressive episode. Our results agree with previous reports in that the increased length of sleep was indeed associated with compromised wellbeing [30,31].
Our main finding was that the seasonal changes in sleep length and those in mood were greater in twins with bipolar disorder compared with their co-twins with no mental disorder. Interestingly, sunny days had a greater positive effect on wellbeing in the bipolar twins, whom in addition had a higher global seasonality score than their healthy co-twins. These findings support the view that bipolar disorder is associated with marked seasonal variation and the course of illness is susceptible to the perceived light exposure from the habitat. Recently, it has also been shown that light therapy is likely to add on the efficacy of total sleep deprivation for the treatment of depressed patients with bipolar disorder [29].
Our findings suggest that the subjectively reported seasonal changes are related rather to the phenotype of bipolar disorder than to the genetic susceptibility to seasonal variation. Here, our results did not agree with previous reports [14,15] in that there would be a marked genetic impact on the seasonal changes in mood and behaviour. Our study population was small, but however consisted of bipolar patients and their siblings. While high scores on the GSS are correlated with bipolar disorder, it may be that the genetic component cannot be detected in this kind of study sample. For the GWS, either, there was no evidence of genetic influence in this sample, but specific environmental factors had a significant effect on changes in wellbeing related to local weather conditions such as hours of sunshine. Therefore, our findings need to be considered as preliminary and interesting, but not of sound evidence.
Recently, the sex difference in circadian preference has been reported [32,33]. In our study, there was no such difference. In addition, preference for evening activities has been associated with a greater need for sleep [34]. In our study, the evening types had a greater sleep debt than the morning types. This finding agrees with the previous data showing that the evening types do experience a need for longer sleep. During the follow-up of 1981 to 1999, the length of sleep shortened among the healthy twins. The shortage of sleep has been a common phenomenon in western societies. In contrast, bipolar twins of our sample slept even more in 1999.
These changes in circadian type preferences and the increasing need for sleep might reflect specific factors underlying the circadian vulnerability in bipolar disorder, being different from the one proposed for major depressive disorder. The dual vulnerability hypothesis claims that affective disorder with the seasonal pattern is a combination of a seasonal factor and a depression factor and consequently represents the extreme end of one trait. Subjects with a stronger seasonal factor primarily have vegetative symptoms (e.g. hypersomnia), without marked cognitive symptoms. At the opposite end of the trait, subjects with a stronger depression factor tend to have cognitive symptoms (e.g. insomnia), without marked vegetative symptoms [13,35]. Previous studies have reported that individuals with insomnia are more likely to have a major depressive disorder, and longitudinal studies have in addition shown that the persistence of insomnia tends to be associated with the appearance of a new depressive episode [33].
Limitations
Our study was a study of twins, which as such can limit the generalisation of our results. Both the seasonal variation in mood and behaviour and the changes in wellbeing attributed to local weather conditions were retrospectively assessed using a self-rating scale. Scores on the SPAQ tend to vary by season, but not by day length [36]. However, this is not a true limitation in our study, because the SPAQ was administered with the research interviews that were distributed evenly over the year.
While depressed patients tend to show a greater preference for evening activities as compared with healthy individuals [6], our bipolar patients were not more often of the evening type than their healthy co-twins. We also found that the eveningness was associated with a higher global seasonality score. It is therefore likely that the mechanisms of action that affect the phenotypes of circadian type and those of the seasonal pattern are shared, or at least closely related to each other. For the assessment of circadian type, the sample size was small, but derived from an extensive population-based sample using the National Population Register and the Finnish Twin Cohort. The sample has been collected from Finland, a country residing at far northern latitudes, which can limit the generalisation of our results as far as the globe is concerned.
There is no previous report of the differences in the circadian type in patients with bipolar disorder. Our results of changes in circadian type preferences and increased sleep length during the follow-up in bipolar twins could be consequences of the illness itself, or of treatments used. Unfortunately, we were not able to evaluate these effects more profoundly.
Conclusions
Our results support the view that there is seasonal variation in bipolar disorder and the exposure to natural light appears to have a substantial effect on wellbeing. Patients with bipolar disorder were not more often the evening types.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This research was supported in part by Academy of Finland (40747 and 201097).
Contributor Information
Reeta Hakkarainen, Email: reeta.hakkarainen@ktl.fi.
Carolina Johansson, Email: carolina.johansson@cmm.ki.se.
Tuula Kieseppä, Email: tuula.kieseppa@ktl.fi.
Timo Partonen, Email: timo.partonen@ktl.fi.
Markku Koskenvuo, Email: markku.koskenvuo@pp.inet.fi.
Jaakko Kaprio, Email: jaakko.kaprio@helsinki.fi.
Jouko Lönnqvist, Email: jouko.lonnqvist@ktl.fi.
References
- Faedda GL, Tondo L, Teicher MH, Baldessarini RJ, Gelbard HA, Floris GF. Seasonal mood disorders. Patterns of seasonal recurrence in mania and depression. Arch Gen Psychiatry. 1993;50:17–23. doi: 10.1001/archpsyc.1993.01820130019004. [DOI] [PubMed] [Google Scholar]
- Fossey E, Shapiro CM. Seasonality in psychiatry – a review. Can J Psychiatry. 1992;37:299–308. doi: 10.1177/070674379203700503. [DOI] [PubMed] [Google Scholar]
- Parker G, Walter S. Seasonal variation in depressive disorders and suicidal deaths in New South Wales. Br J Psychiatry. 1982;140:626–632. doi: 10.1192/bjp.140.6.626. [DOI] [PubMed] [Google Scholar]
- Silverstone T, Romans S, Hunt N, McPherson H. Is there a seasonal pattern of relapse in bipolar affective disorders? A dual northern and southern hemisphere cohort study. Br J Psychiatry. 1995;167:58–60. doi: 10.1192/bjp.167.1.58. [DOI] [PubMed] [Google Scholar]
- Partonen T, Lönnqvist J. Seasonal variation in bipolar disorder. Br J Psychiatry. 1996;169:641–646. doi: 10.1192/bjp.169.5.641. [DOI] [PubMed] [Google Scholar]
- Partonen T, Piiroinen P, Lönnqvist J. Season-dependent symptoms in consultant-liaison patients. International journal of psychiatry in clinical practise. 2000;4:151–154. doi: 10.1080/13651500050518343. [DOI] [PubMed] [Google Scholar]
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, Mueller PS, Newsome DA, Wehr TA. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- Partonen T, Lönnqvist J. Seasonal affective disorder. Lancet. 1998;352:1369–1374. doi: 10.1016/S0140-6736(98)01015-0. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Sack DA, Rosenthal NE. Seasonal affective disorder with summer depression and winter hypomania. Am J Psychiatry. 1987;144:1602–1603. doi: 10.1176/ajp.144.12.1602. [DOI] [PubMed] [Google Scholar]
- Beauchemin KM, Hays P. Sunny hospital rooms expedite recovery from severe and refractory depressions. J Affect Disord. 1996;40:49–51. doi: 10.1016/0165-0327(96)00040-7. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colombo C, Barbini B, Campori E, Smeraldi E. Morning sunlight reduces length of hospitalization in bipolar depression. J Affect Disord. 2001;62:221–223. doi: 10.1016/S0165-0327(00)00149-X. [DOI] [PubMed] [Google Scholar]
- Espiritu RC, Kripke DF, Ancoli-Israel S, Mowen MA, Mason WJ, Fell RL, Klauber MR, Kaplan OJ. Low illumination experienced by San Diego adults: association with atypical depressive symptoms. Biol Psychiatry. 1994;35:403–407. doi: 10.1016/0006-3223(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Sack DA, Rosenthal NE. Sleep reduction as a final common pathway in the genesis of mania. Am J Psychiatry. 1987;144:201–204. doi: 10.1176/ajp.144.2.201. [DOI] [PubMed] [Google Scholar]
- Madden PA, Heath AC, Rosenthal NE, Martin NG. Seasonal changes in mood and behavior. The role of genetic factors. Arch Gen Psychiatry. 1996;53:47–55. doi: 10.1001/archpsyc.1996.01830010049008. [DOI] [PubMed] [Google Scholar]
- Jang KL, Lam RW, Livesley WJ, Vernon PA. Gender differences in the heritability of seasonal mood change. Psychiatry Res. 1997;70:145–154. doi: 10.1016/S0165-1781(97)00030-9. [DOI] [PubMed] [Google Scholar]
- Östberg O. Circadian rhythms of food intake and oral temperature in "morning" and "evening" groups of individuals. Ergonomics. 1973;16:203–209. doi: 10.1080/00140137308924497. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Miyasita A, Inugami M, Fukuda K, Miyata Y. Differences in sleep-wake habits and EEG sleep variables between active morning and evening subjects. Sleep. 1987;10:330–342. doi: 10.1093/sleep/10.4.330. [DOI] [PubMed] [Google Scholar]
- Kerkhof GA, Van Dongen HP. Morning-type and evening-type individuals differ in the phase position of their endogenous circadian oscillator. Neurosci Lett. 1996;218:153–156. doi: 10.1016/S0304-3940(96)13140-2. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Drennan MD, Klauber MR, Kripke DF, Goyette LM. The effects of depression and age on the Horne-Ostberg morningness-eveningness score. J Affect Disord. 1991;23:93–98. doi: 10.1016/0165-0327(91)90096-B. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Manual of the international statistical classification of diseases, injuries, and causes of death. 8. Geneve: World Health Organization; 1967. [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- Kaprio J, Koskenvuo M, Rose RJ. Population-based twin registries: illustrative applications in genetic epidemiology and behavioral genetics from the Finnish Twin Cohort Study. Acta Genet Med Gemellol. 1990;39:427–439. doi: 10.1017/s0001566000003652. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Gibbon M, Williams JBW. The structured Clinical Interview for DSM-IV Axis I and II Disorders (SCID I and II) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Kieseppä T, Partonen T, Kaprio J, Lönnqvist J. Accuracy of register and record-based bipolar I diagnoses in Finland a study of twins. Acta Neuropsychiatrica. 2000;12:106–109. doi: 10.1017/S0924270800035535. [DOI] [PubMed] [Google Scholar]
- Sarna S, Kaprio J, Sistonen P, Koskenvuo M. Diagnosis of twin zygosity by mailed questionnaire. Hum Hered. 1978;28:241–254. doi: 10.1159/000152964. [DOI] [PubMed] [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1975;4:97–110. [PubMed] [Google Scholar]
- STATA Corporation STATA Statistics/Data Analysis 70 College Station: TX:STATA Corporation. 2001.
- Colombo C, Lucca A, Benedetti F, Barbini B, Campori E, Smeraldi E. Total sleep deprivation combined with lithium and light therapy in the treatment of bipolar depression: replication of main effects and interaction. Psychiatry Res. 2000;95:43–53. doi: 10.1016/S0165-1781(00)00164-5. [DOI] [PubMed] [Google Scholar]
- Jean-Louis G, Kripke DF, Ancoli-Israel S. Sleep and quality of well-being. Sleep. 2000;23:1115–1121. [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- Natale V, Adan A, Chotai J. Further results on the association between morningness-eveningness preference and the season of birth in human adults. Neuropsychobiology. 2002;46:209–214. doi: 10.1159/000067803. [DOI] [PubMed] [Google Scholar]
- Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiol Int. 2002;19:709–720. doi: 10.1081/CBI-120005390. [DOI] [PubMed] [Google Scholar]
- Taillard J, Philip P, Bioulac B. Morningness/eveningness and the need for sleep. J Sleep Res. 1999;8:291–295. doi: 10.1046/j.1365-2869.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- Lam RW, Tam EM, Yatham LN, Shiah IS, Zis AP. Seasonal depression: the dual vulnerability hypothesis revisited. J Affect Disord. 2001;63:123–132. doi: 10.1016/S0165-0327(00)00196-8. [DOI] [PubMed] [Google Scholar]
- Lund E, Hansen V. Responses to the Seasonal Pattern Assessment Questionnaire in different seasons. Am J Psychiatry. 2001;158:316–318. doi: 10.1176/appi.ajp.158.2.316. [DOI] [PubMed] [Google Scholar]