Abstract
Arthropod and vertebrate limbs develop from secondary embryonic fields. In insects, the wing imaginal disk is subdivided early in development into the wing and notum subfields. The activity of the Wingless protein is fundamental for this subdivision and seems to be the first element of the hierarchy of regulatory genes promoting wing formation. Drosophila epidermal growth factor receptor (DER) signaling has many functions in fly development. Here we show that antagonizing DER signaling during the second larval instar leads to notum to wing transformations and wing mirror-image duplications. DER signaling is necessary for confining the wing subregion in the developing wing disk and for the specification of posterior identity. To do so, DER signaling acts by restricting the expression of Wingless to the dorsal-posterior quadrant of wing discs, suppressing wing-organizing activities, and by cooperating in the maintenance of Engrailed expression in posterior compartment cells.
During development, cellular identities are specified within initially undifferentiated fields of cells (1). This specification is achieved by the progressive determination of cells by particular regulators that increasingly restrict their developmental potential. The growth and patterning of the wing disk of Drosophila is directed from organization centers that are created by the juxtaposition of anterior and posterior (A/P) and dorsal and ventral (D/V) compartments (2–4) generated during the development of the disk. How these axial attributes participate in the specification of both distal (wing) and proximal (notum, hinge) subfields (5) is not well established.
Drosophila epidermal growth factor receptor (DER) signaling has been implicated in the control of cell size, cell proliferation, and the allocation of vein cell fates in the wing disk (reviewed in ref. 6). The expression of vein (vn), a gene coding for a DER ligand, is restricted to a posterior and dorsal domain in second instar wing discs (7, 8) (see Fig. 1H). Hypomorphic alleles of vn show pattern duplications of anterior structures replacing posterior territories in the wing disk (7). Similar phenotypes have been found in some allelic combinations of pointed, a gene that codes for a transcription factor putatively under DER control (9). These findings suggest that the activation of the DER signaling cascade could be involved in assuring the specification of posterior cells in the wing. On the other hand, the specification of the wing field relies on the expression of wingless (wg) at second larval instar stages (10, 11). The ectopic expression of Wg at this stage can induce wing tissue in notum territories (10). However, the ability of wg to activate wing specification is spatially restricted to cells at central positions of the disk, which suggests that a second activity limits the competence of cells to respond to wg and restricts the size and the position of the wing field (10). Here we show that DER signaling is involved in the maintenance of Engrailed (En) expression in posterior compartment cells and limits the expression of Wg to the dorsal-posterior quadrant of wing discs. We conclude that DER represses Wg, confining the wing subregion of the developing wing disk, and cooperates with En in the specification of posterior identity.
Figure 1.
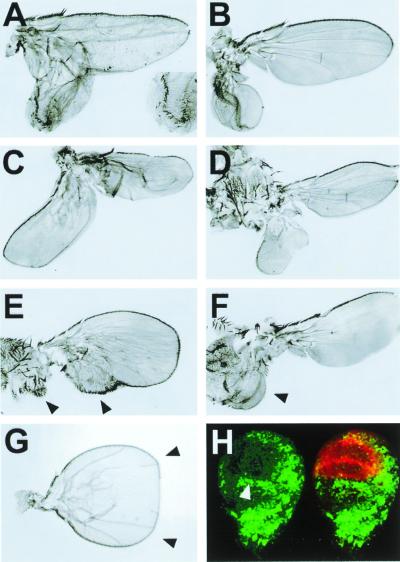
Loss of DER signaling generates cell fate transformations in the wing disk. (A) DNRaf (UAS-DNRaf3.1 expressed ectopically under the control of en-Gal4 throughout the posterior wing compartment (raised at 25°C). (Inset) The posterior to anterior transformation of the wing margin. Similar results were obtained after ectopic expression of UAS-DNRas (a dominant-negative form of Ras1). (B), DERtsla/DERco flies raised at 17°C, transferred to 25°C for 24 h at second instar larval stages, and then returned to 17°C (see Materials and Methods). Wing mirror-image duplication. (C and D) Overexpression of DNRaf3.1 in clones generated by the flip-out technique and induced at 36 ± 12 h after egg laying (see Materials and Methods). (C) Wing mirror-image duplication. (D) Notum to wing transformation. (E) En (UAS-En) expressed ectopically under the control of en-Gal4 throughout the posterior wing compartment interferes with en autoregulation (17). Posterior to anterior transformations of distal (wing) and proximal (notum) structures (arrowheads). Also see ref. 8. (F) Early induced clones of Wg overexpression (36 ± 12 h after egg laying). Notum to wing transformation (arrowhead). No posterior to anterior cell fate changes are induced. Also see ref. 3. (G) cnk mutant showing a wing-mirror-image duplication (arrowheads). (H) Distribution of Wg expression and MAPK activity in early third instar wing discs. MAPK activity, detected with an anti-dpERK antibody (green), is excluded from the wing pouch (arrowhead) and complementary to Wg expression (red).
Materials and Methods
Genetic Strains.
At the DER locus, we used the temperature-sensitive allele DERtsla and the null allele DERco (12); at the locus connector enhancer of ksr (cnk), we used a hypomorph revertant (cnkb9) induced by imprecise excision of the P-element insertion l(2)16314 (13). The decapentaplegic (dpp) LacZ enhancer trap line was dppBS3.0. The apterous (ap) LacZ enhancer trap line was aprk568. The en-Gal4 driver line was used for expression in the wing posterior compartment. The UAS-DNRaf3.1 line was generated in the laboratory (14). UAS-DNRas, UAS-DNWg, and UAS-Wg were provided by N. Perrimon (Harvard Univ., Boston), A. Martínez-Arias (Cambridge Univ., Cambridge, U.K.), and F. Díaz-Benjumea (Consejo Superior de Investigaciones Científicas, Madrid), respectively.
Generation of Mosaics.
Clones of cells expressing Gal4 were induced at 24–48 and 60–72 h after egg laying by 40-min heat shocks at 37°C in flies y,w,FLP1.22; Act5C<FRT yellow+ FRT> Gal4,UAS-GFP over UAS-DNRaf3.1 or double UAS-DNWg + UAS-DNRaf3.1. The flip-out of the <FRT yellow+ FRT> cassette results in expression of the transcriptional activator Gal4 under the control of the Act5C promoter (15). Clones were detected by expression of green fluorescent protein and were analyzed in third instar larvae or adult flies. Only early clones (24–48 h after egg laying) induce proximal to distal and posterior to anterior (P/A) transformations. Clones of cells expressing ectopic Wg were induced at 24–48 h after egg laying by 7-min heat shocks at 37°C in flies of the genotype y,w,FLP1.22; P[abx/Ubx<FRT f+ FRT> Gal4-LacZ]/UAS-Wg (16).
Time Course of Dominant-Negative Raf (DNRaf) Overexpression.
We used the cold sensitivity of Gal4 to minimize the effect of en-Gal4 expression. en-Gal4/UAS-DNRaf3.1 flies only show proximal to distal and A/P transformations at 25°C, but not at 17°C. All flies reared at 25°C and then shifted to 17°C showed these phenotypes if the shift was performed at 68 h after egg laying. This time marks the earliest point at which DER signaling affects proximal to distal and P/A fates. This figure must be corrected for the developmental delay of the en-Gal4/UAS-DNRaf3.1 at 25°C (≈2 days). Thus, transformations do not occur earlier than ≈55 h after egg laying (correction factor 0.81X) in normal growth conditions (flies reared at 25°C). Rearing flies at 17°C and shifting them to 25°C defines the latest time at which transformations arise after interfering with DER signaling. This time point is 120 h at 17°C. Taking into account the developmental delay for flies growing at this temperature (correction factor 0.6X), we conclude that the function of DER regulating Wg and En ends at 72 h after egg laying under normal culture conditions. Similar results were obtained in shift experiments by using the DER temperature-sensitive allele Egftsla.
Immunocytochemistry.
We used rabbit anti-β-galactosidase (Cappel), rabbit anti-Vestigial (Vg) (provided by S. Carroll, Univ. of Wisconsin, Madison, WI), mouse monoclonal anti-Wg (provided by S. Cohen, European Molecular Biology Laboratory, Heidelberg), mouse monoclonal anti-Pdm-1 (17) (provided by F. Díaz-Benjumea), mouse monoclonal anti-En, mouse monoclonal anti-dpERK (activated MAPK) (Sigma), and Rat anti-Cubitus interruptus (Ci) (provided by J. L. Gómez-Skarmeta, Consejo Superior de Investigaciones Científicas). Secondary antibodies were from Jackson ImmunoResearch (used at 1/200 dilution).
Results
To study DER function during early wing development, we reduced DER signaling at different times by using thermosensitive alleles of DER (12) or by overexpression of DNRaf (14). We also analyzed hypomorphic vn and cnk [a regulatory member of the Ras signaling cascade (18)] alleles. Under these conditions, we observed with high frequency posterior to anterior transformations (Fig. 1 A–C and G), proximal (notum) to distal (wing) transformations (Fig. 1D), and a reduction (or absence) of the notum region (see below). When DNRaf is expressed in clones induced during the second instar, we found different kinds of phenotypes. Large clones in the posterior notum/hinge anlage lead to notum to wing transformations (Fig. 1D), whereas large clones covering the posterior of the wing give rise to posterior to anterior transformations (Fig. 1C). These phenotypes were found only after inducing a large amount of confluent clones (see Materials and Methods). Clones of cells overexpressing DNRaf in other regions at this age, or anywhere at later stages, give rise to different defects, such as those previously described on cell proliferation and vein cell fates (6).
Early Ras Signaling Activity Is Necessary for Posterior Cell Fate Specification by En.
Posterior to anterior transformations are associated with mirror-image duplications that are reminiscent of those observed after reducing the expression of en, a gene that confers posterior identity (4, 19–21), in posterior wing cells. En represses ci and limits the expression of dpp (22–24) to anterior compartment cells adjacent to En-expressing cells. Dpp acts as a long-range morphogen emanating from the compartment border and directs the growth and patterning of the wing (25, 26). Local loss of en function is sufficient to generate a complete transformation of posterior cells to anterior, and as a consequence, to induce the ectopic expression of dpp and an ectopic anterior compartment (4).
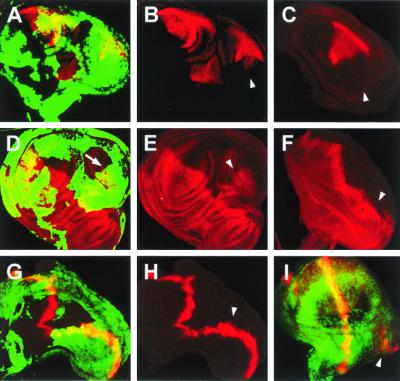
The mirror-image wing duplication resulting from the reduction of DER signaling in the posterior compartment correlates with a down-regulation of En protein expression (Fig. 2 A–C) and the up-regulation of Ci (Fig. 2 D–F). Accordingly, ectopic dpp expression is activated and a new A/P border becomes implemented (Fig. 2 G–I). It bears mention that dpp is activated within the posterior compartment (Fig. 2I), suggesting that the posterior En-nonexpressing cells are not recruited from anterior regions of the wing disk.
Figure 2.
Suppression of DER signaling leads to overgrowth and the misregulation of markers associated with the specification of the A/P boundary. (A, B, D, E, G, and H) large early clones (36 ± 12 h after egg laying) of cells that overexpressed DNRaf3.1 induced by using the flip-out technique (see Materials and Methods) identified by the expression of green fluorescent protein (green). (C and F) cnkb9 mutant wing discs. (A–C) Mirror-image wing pouch showing suppression of En expression (red). En expression is down-regulated on the posterior side of the duplicated wing (arrowheads). (D–F) Mirror-image wing pouch showing ectopic Ci expression (red) on the posterior side of the duplicated wing (arrowheads). Note that in D, the boundary of the clone runs along the new A/P border (arrow). (G and H) Dpp expression (red) defines a new wing margin (arrowhead). (I) Dpp expression (red) appears in a group of cells isolated from the A/P boundary within a DNRaf3.1 expressing clone (arrowhead). This strongly suggests that the expression of Dpp is generated de novo within mutant territory and is not the result of recruitment of anterior cells.
Neighboring cells to those expressing DNRaf in clones are recruited to generate the new A/P border. This is a nonautonomous effect that also has been described for en clones, leading to mirror-image duplications (4). Moreover, when DNRaf is expressed in clones, not all cells within the clone down-regulate En. DER signaling is therefore important for the maintenance of En expression in the posterior cells of the wing pouch and the enactment of posterior cell fates, although its effects appear to be nonautonomous.
Ras Signaling Activity Restricts Wg Expression and Limits the Wing Field Boundary.
Wg is expressed in an anterior-ventral area roughly complementary to vn expression (Fig. 1H), near the interface between the A/P and D/V compartment boundaries (27). We found that reducing DER signaling during the second larval instar resulted in the generation of ectopic wings out from peripheral notal tissue in posterior territories (Fig. 1D). It is worth pointing out that this ectopic wing tissue does not develop at the expense of the notum, which, in many cases, is not affected. These phenotypes are similar to those observed after early Wg overexpression (Fig. 1F). They are never observed in the absence of en, which only promotes posterior to anterior notum and wing transformations (compare Fig. 1D with 1E).
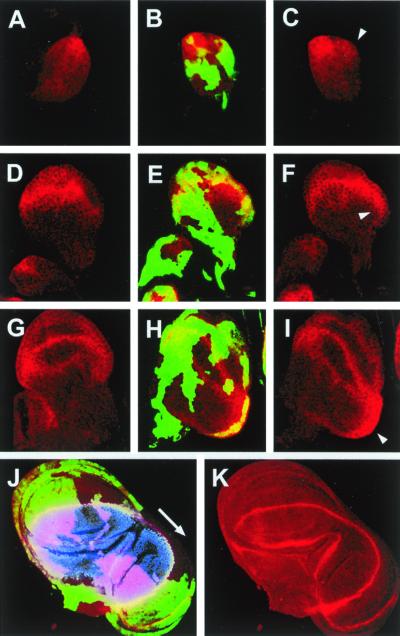
This leads to the proposition that DER signaling would restrict the domain of expression of wg and define the boundaries of the field of cells that is going to undergo a wing developmental program. To examine this possibility, we analyzed the expression of Wg in clones ectopically expressing DNRaf. In large clones covering most of the notum/hinge region, Wg expression is up-regulated from the early second larval instar in the posterior of the wing disk (compare Fig. 3 B and C with Fig. 3A), extending progressively (compare Fig. 3A with Fig. 3I). The expansion of Wg expression drives the enlargement of the D/V axis toward posterior territories [manifested in the expanded field of ap-expressing cells (3) in the duplicated areas (Fig. 3 J and K)], giving rise to a fully developed duplicated wing.
Figure 3.
DER signaling antagonizes Wg expression. DNRaf3.1-expressing cells were identified by the expression of green fluorescent protein (green). Wg expression is shown in red. (A, D, and G) Control wild-type discs. (B, C, E, F, H, and I) Discs with clones of cells that overexpressed DNRaf3.1 under the control of actin-Gal4 (see Materials and Methods). (A–C) Early L2 wing discs. Wg is expressed in the anterior-ventral quadrant. In the absence of DER signaling, Wg expression is induced in a more posterior position (arrowhead). (D–F) Late L2 wing discs. Wg is expressed in the presumptive wing pouch. Wg expression extends to posterior proximal positions (arrowhead) in discs lacking DER signaling. (G–I) Early L3 wing discs. Expression of Wg evolves in the wing margin and wing pouch circles. In the absence of DER signaling, Wg expression expands further to posterior proximal positions (arrowhead). (J and K) Late L3 wing disk. Wg is ectopically expressed in the duplicated wing pouch. Nuclear apterous expression (blue) outlines the expansion of the wing dorsal compartment to the duplicated wing pouch, stretching out the D/V border to more posterior cells.
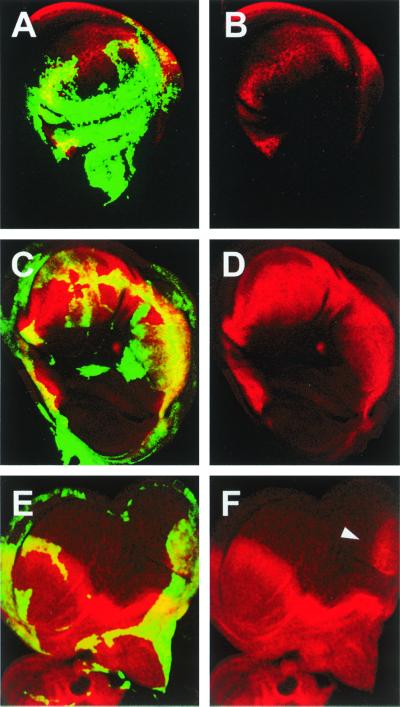
To test whether the ectopic expression of Wg, induced by the down-regulation of DER activity, was sufficient to promote notum to wing transformations, we aimed to interfere with Wg by inducing the expression, in clones, of a dominant-negative form of Wg (DNWg) (28) along with DNRaf. In most cases, double mutant clones covering the whole posterior compartment show a partial reduction in the size of the wing pouch and notum and a suppression of notum to wing transformations or ectopic induction of wing markers [vg expression (11)] (compare Fig. 4 A and B with Fig. 4 C and D). We conclude that the repression of wg expression by DER signaling in the notum, under wild-type conditions, is necessary to set a limit for the wing field. This relationship partly resembles the antagonism between the DER and wg signaling pathways for the specification of the ventral larval cuticle (29), although in this case wg and DER do not counteract each other, but control epidermal differentiation through the opposite transcriptional regulation of downstream genes (30).
Figure 4.
Notum to wing transformations induced by interference with DER signaling are mediated by Wg. Early flip-out clones were identified by the expression of green fluorescent protein (green). (A and B) Flip-out clones that overexpressed DNRaf3.1. Notum to wing transformation showing ectopic Vg expression (red). (C–F) Flip-out clones that overexpressed DNRaf3.1 and DNWg. (C and D) No notum to wing transformations were found (data not shown) and no ectopic Vg expression was detected, despite the fact that the whole posterior compartment expresses both proteins. (E and F) Posterior to anterior transformation in the wing pouch is not abolished. Ci protein (red) is ectopically expressed in posterior territories (arrowhead).
Interestingly, Wg overexpression induces notum to wing transformations (Fig. 1F), but never mirror-image duplications, such as those obtained after reducing DER activity (Fig. 1D). This suggests that wg function mediates notum to wing conversion, but its overexpression is not sufficient for posterior to anterior transformation. Indeed, the overexpression of DNWg is not able to rescue the down-regulation of En, the up-regulation of Ci (Fig. 4 E and F), or wing mirror-image duplications (data not shown), which result from interfering with DER signaling. These data suggest that the effects of DER signaling on en and wg functions are independent, although we cannot discard regulatory interactions between wg and en at this stage.
Discussion
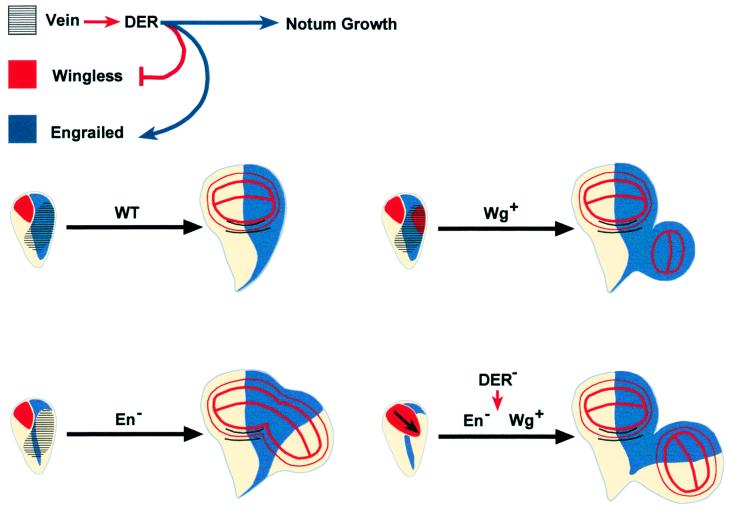
The function of DER controlling en and wg (in the specification of posterior and proximal compartments) is limited to second instar larval stages, and in particular to a period expanding between 55 and 72 h after egg laying (see Materials and Methods). At this stage, the activity of DER is necessary for the reinforcement of en activity; in its absence, the most proximal posterior cells in the wing anlage are transformed toward anterior and a new A/P border becomes implemented (Fig. 5). We only observe posterior to anterior transformations when DNRaf is induced in large territories. This fact and the observed nonautonomy of DER signaling on En expression hint at cell community effects (31) as an important parameter involved in the maintenance of posterior cell fates.
Figure 5.
A model for notum/wing subfield specification. In the second instar larval stage, vn is expressed in a dorsal-posterior position in the wing disk. wg is expressed in the anterior-ventral quadrant of the disk. en is expressed in posterior cells. DER (and MAPK) signaling cooperate with en to define posterior cell identities. In the absence of DER activity, posterior cells are transformed toward anterior fates. DER (and MAPK) activity represses wg expression and defines the notum and wing field border. wg expression is constrained to a ventral-posterior region where its activity implements vg expression and wing development. DER activity in the presumptive notum region is also necessary for notum cell survival in a function independent of wg and en.
DER is defining the posterior limit of wg expression at a time that is coincident with wg early function defining the wing field (10). Interfering with DER signaling allows the expansion of Wg expression, and posterior proximal cells become transformed to wing cell fates (Fig. 5). Interestingly, Wg is not up-regulated in every cell with impaired DER signaling, and other, still undefined, factors should participate in the control of its spatial distribution.
Remarkably, both posterior and anterior and notum to wing transformations are not affected in a dpp mutant background, although their proliferation is impaired (data not shown). We therefore propose that the observed transformations are most likely a result of changes in cell fate specification and independent of disk growth.
DER signaling also appears to have a role during notum development. Indeed, we find strong reductions in notum size, in both vn and cnk alleles, and after antagonizing DER signaling at second instar larval stages (see Fig. 2 and data not shown). This phenotype does not appear to be a result of a change in the fate of notum cells, as they do not acquire specific wing markers such as Pdm-1 (17) (data not shown). Furthermore, the duplications of wing territories are not at the expense of the notum field (see above). Thus, DER appears to be involved in the control of notum growth, independently from its functions controlling en and wg (Fig. 5).
The reiterative use of DER has been demonstrated in the generation of multiple fates in the developing fly eye (32). Here we find a fundamental early function for DER in the underlying patterning system of the wing subfield, controlling the activity of two genes, en and wg. It is significant that fibroblast growth factor [Ras-mitogen-activated protein kinase (MAPK)] activities are implicated in the initiation of the whole program of limb development in vertebrates (reviewed in ref. 33), which is remarkably similar to that of the Drosophila wing. It remains to be seen whether a similar strategy applies to the activities of Ras-MAPK cascades during vertebrate limb bud development.
Acknowledgments
We are grateful to Antonio García-Bellido for his support and advice. We also thank T. Kornberg, J.C. Izpisua-Belmonte, M. Freeman, J.F. de Celis, and C. Extavour for critical reading of the manuscript. A.B. was funded by a Comunidad Autonoma de Madrid postdoctoral Fellowship, F.R. was supported by a grant from the Welcome Trust to M. Akam, and E.M.-B. is a Research Scientist at the Consejo Superior de Investigaciones Científicas. An institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular “Severo Ochoa” is also acknowledged.
Abbreviations
- A/P
anterior and posterior
- D/V
dorsal and ventral
- DER
Drosophila epidermal growth factor receptor
- vn
vein
- wg
wingless
- en
engrailed
- cnk
connector enhancer of ksr
- dpp
decapentaplegic
- vg
vestigial
- ci
cubitus interruptus
- MAPK
mitogen-activated protein kinase
- DN
dominant-negative
- ap
apterous
References
- 1.French V P, Bryant P J, Bryant S V. Science. 1976;193:969–981. doi: 10.1126/science.948762. [DOI] [PubMed] [Google Scholar]
- 2.Blair S S. BioEssays. 1995;17:299–309. doi: 10.1002/bies.950170406. [DOI] [PubMed] [Google Scholar]
- 3.Díaz-Benjumea F, Cohen S M. Cell. 1993;75:741–752. doi: 10.1016/0092-8674(93)90494-b. [DOI] [PubMed] [Google Scholar]
- 4.Tabata T, Schwartz C, Gustavson E, Ali Z, Kornberg T B. Development (Cambridge, UK) 1995;121:3359–3369. doi: 10.1242/dev.121.10.3359. [DOI] [PubMed] [Google Scholar]
- 5.García-Bellido A. In: Cell Patterning. Porter R, Rivers J, editors. Boston: CIBA Symposium; 1975. pp. 161–183. [Google Scholar]
- 6.Schweitzer R, Shilo B Z. Trends Genet. 1997;13:191–196. doi: 10.1016/s0168-9525(97)01091-3. [DOI] [PubMed] [Google Scholar]
- 7.Simcox A A, Grumbling G, Schnepp B, Bennington-Mathias C, Hersperger E, Shearn A. Dev Biol. 1996;177:475–489. doi: 10.1006/dbio.1996.0179. [DOI] [PubMed] [Google Scholar]
- 8.Schnepp B, Grumbling G, Donaldson T, Simcox A. Genes Dev. 1996;10:2302–2313. doi: 10.1101/gad.10.18.2302. [DOI] [PubMed] [Google Scholar]
- 9.Scholz H, Deatrick J, Klaes A, Klambt C. Genetics. 1993;135:455–468. doi: 10.1093/genetics/135.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng M, Díaz-Benjumea F J, Vincent J P, Wu J, Cohen S M. Nature (London) 1996;381:316–318. doi: 10.1038/381316a0. [DOI] [PubMed] [Google Scholar]
- 11.Williams J A, Paddock S W, Carroll S B. Development (Cambridge, UK) 1993;117:571–584. doi: 10.1242/dev.117.2.571. [DOI] [PubMed] [Google Scholar]
- 12.Kumar J P, Tio M, Hsiung F, Akopyan S, Gabay L, Seger R, Shilo B Z, Moses K. Development (Cambridge, UK) 1998;125:3875–3885. doi: 10.1242/dev.125.19.3875. [DOI] [PubMed] [Google Scholar]
- 13.Roch F, Serras F, Cifuentes F J, Corominas M, Alsina B, Amoros M, Lopez-Varea A, Hernandez R, Guerra D, Cavicchi S, et al. Mol Gen Genet. 1998;257:103–112. doi: 10.1007/pl00008620. [DOI] [PubMed] [Google Scholar]
- 14.Martín-Blanco E, Roch F, Noll E, Baonza A, Duffy J B, Perrimon N. Development (Cambridge, UK) 1999;126:5739–5747. doi: 10.1242/dev.126.24.5739. [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. Development (Cambridge, UK) 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- 16.De Celis J F, Bray S. Development (Cambridge, UK) 1997;124:3241–3251. doi: 10.1242/dev.124.17.3241. [DOI] [PubMed] [Google Scholar]
- 17.Ng M, Díaz-Benjumea F J, Cohen S M. Development (Cambridge, UK) 1995;121:589–599. doi: 10.1242/dev.121.2.589. [DOI] [PubMed] [Google Scholar]
- 18.Therrien M, Wong A M, Rubin G M. Cell. 1998;95:343–353. doi: 10.1016/s0092-8674(00)81766-3. [DOI] [PubMed] [Google Scholar]
- 19.Simmonds A J, Brook W J, Cohen S M, Bell J B. Nature (London) 1995;276:424–427. doi: 10.1038/376424a0. [DOI] [PubMed] [Google Scholar]
- 20.García-Bellido A, Santamaría P. Genetics. 1972;72:87–101. doi: 10.1093/genetics/72.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guillen I, Mullor J L, Capdevila J, Sanchez-Herrero E, Morata G, Guerrero I. Development (Cambridge, UK) 1995;121:3447–3456. doi: 10.1242/dev.121.10.3447. [DOI] [PubMed] [Google Scholar]
- 22.Hidalgo A. Curr Biol. 1994;4:1087–1098. doi: 10.1016/s0960-9822(00)00247-5. [DOI] [PubMed] [Google Scholar]
- 23.Sanicola M, Sekelsky J J, Elson S, Gelbart W M. Genetics. 1995;139:745–756. doi: 10.1093/genetics/139.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz C, Locke J, Nishida C, Kornberg T B. Development (Cambridge, UK) 1995;121:1625–1635. doi: 10.1242/dev.121.6.1625. [DOI] [PubMed] [Google Scholar]
- 25.Zecca M, Basler K, Struhl G. Development (Cambridge, UK) 1995;121:2265–2278. doi: 10.1242/dev.121.8.2265. [DOI] [PubMed] [Google Scholar]
- 26.Nellen D, Burke R, Struhl G, Basler K. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 27.Couso J P, Bate M, Martínez-Arias A. Science. 1993;259:484–489. doi: 10.1126/science.8424170. [DOI] [PubMed] [Google Scholar]
- 28.Klein T, Martínez-Arias A. Development (Cambridge, UK) 1999;126:913–925. doi: 10.1242/dev.126.5.913. [DOI] [PubMed] [Google Scholar]
- 29.Szüts D, Freeman M, Bienz M. Development (Cambridge, UK) 1997;124:3209–3219. doi: 10.1242/dev.124.16.3209. [DOI] [PubMed] [Google Scholar]
- 30.Payre F, Vincent A, Carreno S. Nature (London) 1999;400:271–275. doi: 10.1038/22330. [DOI] [PubMed] [Google Scholar]
- 31.Gurdon J B. Nature (London) 1988;336:772–774. doi: 10.1038/336772a0. [DOI] [PubMed] [Google Scholar]
- 32.Freeman M. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- 33.Schwabe J W, Rodríguez-Esteban C, Izpisua Belmonte J C. Trends Genet. 1998;14:229–235. doi: 10.1016/s0168-9525(98)01477-2. [DOI] [PubMed] [Google Scholar]