Abstract
The hypothalamic-pituitary-adrenal (HPA) axis, including hypothalamic corticotropin-releasing hormone (CRH) and pituitary corticotropin, is one of the first endocrine systems to develop during fetal life, probably because glucocorticoid secretion is necessary for the maturation of many essential fetal organs. Consistent with this, pregnant mice with an inactivating mutation in the Crh gene deliver CRH-deficient offspring that die at birth with dysplastic lungs, which can be prevented by prenatal maternal glucocorticoid treatment. But children lacking the ability to synthesize cortisol (because of various genetic defects in adrenal gland development or steroidogenesis) are not born with respiratory insufficiency or abnormal lung development, suggesting that the transfer of maternal glucocorticoid across the placenta might promote fetal organ maturation in the absence of fetal glucocorticoid production. We used pregnant mice with a normal HPA axis carrying fetuses with CRH deficiency to characterize the relative contributions of the fetal and maternal adrenal to the activity of the fetal HPA axis, and related these findings to fetal lung development. We found that in the presence of fetal adrenal insufficiency, normal fetal lung development is maintained by the transfer of maternal glucocorticoid to the fetus, specifically during the circadian peak in maternal glucocorticoid secretion.
The hypothalamic-pituitary-adrenal (HPA) axis is one of the first endocrine systems to develop during fetal life. In both human and mouse fetuses, pituitary expression of the mRNA encoding proopiomelanocortin [POMC, the precursor of adrenocorticotropin (ACTH)] and the secretion of ACTH precede the synthesis and secretion of other hormones (1, 2). Moreover, among the hypothalamic releasing factors that control the synthesis and secretion of anterior pituitary hormones, corticotropin-releasing hormone (3, 4) (CRH), the major activator of the HPA axis, is the first to be expressed in the fetus. The early activation of the pituitary–adrenal axis is likely required because glucocorticoid secretion is necessary for the maturation of many essential fetal organs, including lung (5) and gut (6). This point is underscored by the fact that mice with an inactivating mutation in the gene encoding CRH (Crh−/−) born of Crh−/− dams die at birth with abnormal lung development and respiratory insufficiency, and that this outcome can be prevented by prenatal maternal glucocorticoid treatment (7).
Why, then, is it that children born with the inability to synthesize cortisol because of mutations in the gene encoding POMC (8), as well as those with more variable defects because of mutations in genes encoding DAX-1 (9), steroidogenic enzymes (10), or the ACTH receptor (11), have never been found to have respiratory insufficiency or abnormal lung development? In such infants, the transfer of maternal glucocorticoid across the placenta might promote fetal organ maturation in the absence of fetal glucocorticoid production. Whereas Crh−/− neonatal mice born to Crh−/− mothers die, Crh−/− neonates arising from heterozygous (Crh+/−) mice [which have a normal HPA axis (7, 12)] are born with normal lungs (7). Using this model, we characterized the relative contributions of fetal and maternal adrenal steroid production to the activity of the fetal HPA axis and related these findings to fetal lung development. We found that, in the presence of fetal adrenal insufficiency, normal fetal lung development is maintained by the transfer of maternal glucocorticoid to the fetus specifically during the circadian peak in maternal glucocorticoid secretion.
Materials and Methods
Animal Housing.
CRH-deficient (Crh−/−) and wild-type (Crh+/+) mice of 129×C57BL/6 genetic background were housed on a 12:12 light/dark cycle (lights on at 7:00 a.m.) with ad libitum access to rodent chow and water. The mice were housed and cared for according to National Institutes of Health guidelines, and all animal experiments were approved by the Animal Care and Use Committee of Children's Hospital, Boston.
Timed Pregnancies and Tissue Preparation.
Estrus females were mated with stud males. The presence of a vaginal plug on the morning after introduction of the female into the male cage was set as embryonic day 0.5 (e0.5). Females were subsequently isolated until the time of tissue harvesting to ensure accurate gestational timing. In the experiments where Crh−/− pregnant females were treated with glucocorticoid, corticosterone was added in the drinking water beginning on e12.5, at a final concentration of either 7.5 or 10 μg/ml. Gravid females were killed by decapitation, trunk blood was collected from each, and fetuses from all embryonic time points and genotypes were dissected from the uterus aseptically. Except where otherwise indicated, the Crh−/− fetuses that we studied were carried by Crh−/− mothers.
Trunk blood was collected from each embryo, and the placenta or the tail of each was obtained for DNA extraction for sex and genotype determination. Fetal blood could not be collected before e16.5 because of inadequate blood volumes. For in situ hybridization histochemistry, the decapitated body and head were rapidly frozen on dry ice and subsequently stored at −80°C. Frozen sagittal sections (12 μM) were cut on a cryostat (Leica, Allendale, NJ), mounted on slides (Fisher SuperFrost, Fisher Scientific), and stored at −20°C with desiccant until use. For assessment of the histopathology and architecture of lungs and adrenals, fetal bodies were fixed in Bouins' fixative (90 ml saturated picric acid/10 ml formaldehyde 40%/10 ml glacial acetic acid), paraffin embedded, sliced (sagittal sections, 5 μM) in a rotary microtome (Reichert), and stained with hematoxylin and eosin. Lungs were not inflated before fixation because of their small size.
Identification of the Sex and Genotype of the Embryos by PCR.
The gender and CRH genotype of each embryo were identified by PCR amplification of genomic DNA by using primers specific for the mouse SRY and CRH (13) genes, respectively. PCR reactions were performed in a 100-μl volume containing 1× PCR buffer, deoxynucleotides (Boehringer Mannheim) at a final concentration of 0.2 nM each, 30 pmol of the specific primers (BioSource International, Menlo Park, CA), and Taq DNA polymerase (PGC Scientifics, Gaithersburg, MD). PCR products were analyzed by electrophoresis through 1% agarose gels.
Preparation of cRNA Probes for in Situ Hybridization.
Wild-type adrenals were used for RNA preparation by using TRI reagent (Sigma). Two micrograms of total RNA was reverse transcribed into cDNA as previously described (13). The templates for the mouse-specific cRNA probes encoding steroidogenic acute regulatory protein (StAR), side chain cleavage enzyme (SCC), and the rat 11β-hydroxylase gene were obtained by PCR amplification of the above adrenal cDNA by using specific oligonucleotide primers (BioSource International):
StAR sense: 5′-AGCTCAACTGGAGAGCACTG
StAR antisense: 5′-GTGGAACCTCTGCGCTTGG
SCC sense: 5′-GCACACAACTTGAAGGTACAGGAG
SCC antisense: 5′-CAGCCAAAGCCCAAGTACCGGAAG
11β-hydroxylase sense: 5′-TCACCAAATGTATCAAGAATGTGT
11β-hydroxylase antisense: 5′-CCATCTGCACATCCTCTTTCTCTT
In all cases, the downstream antisense primer also included at its 5′-end 23 bases encoding the antisense sequence of the T3 RNA polymerase recognition site (14). When amplified by PCR, this placed the T3 recognition site at the 5′-end of the antisense strand of the double-stranded DNA template. PCR products were analyzed as described above, and the DNA fragments of interest were excised from agarose gels, purified, and subsequently sequenced to confirm their identities.
In Situ Hybridization Histochemistry.
Before in situ hybridization, mounted tissue sections were fixed with 4% paraformaldehyde in PBS for 15 min, rinsed in 2× SSC (20× SCC: 3 M sodium chloride/0.3 M sodium citrate), and treated as previously described (15). Antisense riboprobes were radiolabeled by using [35S]UTP (NEN). After dilution in hybridization buffer [50% deionized formamide/10% dextran sulfate (Pharmacia LKB)/0.5 M sodium chloride/1× Denhardt's solution (0.1% polyvinylpyrrolidine/0.1% BSA/0.1% Ficoll, type 400)/10 mM Tris, pH 8/1 mM EDTA, pH 8/500 μg/ml yeast tRNA (BRL)/10 mM DTT (Sigma)] to yield 2–3 × 107 cpm/ml, 80–100 μl of a radiolabeled cRNA probe was applied to each slide and hybridized for 16–20 h in a humidified chamber at 55°C for POMC and at 60°C for StAR, SCC, and 11β-hydroxylase mRNAs.
After hybridization, slides were washed twice in 2× SSC at room temperature (RT), subjected to RNase digestion at 37°C for 30 min, and again washed with 0.5× SCC 3 times for 10 min each time at RT and for 30 min at 60°C. Slides were dehydrated in increasing concentrations of ethanol (50%, 70%, 95%, and 100%), air dried, and exposed to Kodak X-Omat AR x-ray film (Kodak) for 12–24 h. The slides were subsequently dipped in Kodak NTB-2 nuclear emulsion (Kodak), diluted 1:1, and exposed for 1–2 weeks.
Image Analysis.
For quantitative densitometric analysis and adrenal size analysis, autoradiographic images from all of the slides were digitized by using the public-domain analysis software, nih image (http://rsb.info.nih.gov/nih-image/). The cross-sectional areas of all adrenal tissue sections of each embryo were measured in nih image by tracing around the perimeter of each section via the computer cursor in an unblinded fashion. That section with the largest measured cross-sectional area was used as an index of adrenal size for each embryo. The expression of steroidogenic enzyme mRNAs was assessed in all sections from each embryo, and the mean level of expression was calculated for each specimen, after subtraction of background. The mean expression of each steroidogenic enzyme in the kidney of each embryo was taken as background.
Corticosterone RIA.
Blood from each pregnant female and fetus was centrifuged at 3,000 rpm (1,900 × g) at 4°C for 10 min, and plasma was collected and stored at −20°C until use. Plasma corticosterone levels were measured by using a commercial RIA kit (ICN).
Statistical Analysis.
Data were analyzed by one-way analysis of variance, followed by post hoc multiple comparison tests. Significance (two-tailed) was accepted at P < 0.05. All data are expressed as means ± SEM. As no difference was observed between sexes of each genotype, data from both sexes of a given genotype were pooled.
Results and Discussion
Fetal Pituitary Expression of POMC mRNA.
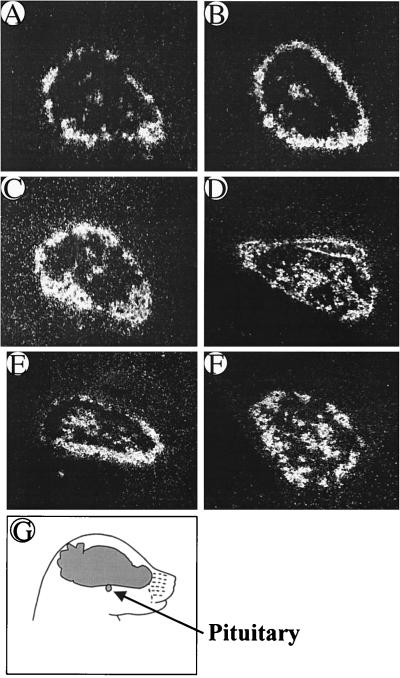
Pituitary POMC mRNA expression first appeared in the intermediate lobe around the perimeter of the gland, with subsequent expression extending into the central portion of the anterior lobe. Similar timing, pattern, and intensity of expression of POMC mRNA were found in fetuses of both the Crh+/+ and Crh−/− genotypes (Fig. 1). This is not surprising, given that the onset of pituitary POMC expression precedes that of hypothalamic CRH expression in the normal mouse fetus (2, 3). Fetal mice with mutations in either the glucocorticoid receptor (16) or 21-hydroxylase (17) genes have elevated pituitary POMC mRNA levels, most likely because of the absence of the suppressive effect of glucocorticoid on POMC synthesis. Given the low levels of blood corticosterone found in Crh−/− fetal mice (see below), it might have been predicted that POMC mRNA levels would also be elevated in them. However, the normal levels of POMC mRNA in Crh−/− fetuses suggest that CRH is required to stimulate POMC synthesis in the setting of fetal glucocorticoid deficiency, as in the adult (18).
Figure 1.
Representative pituitary sections of POMC mRNA expression in Crh+/+ (A, C, and E) and Crh−/− (B, D, and F) fetal mice from e16.5 (A and B), e17.5 (C and D), and e18.5 (E and F). Fresh-frozen midsagittal sections (oriented as depicted in G) were subjected to in situ hybridization by using a mouse POMC riboprobe. No difference was observed in POMC mRNA expression between the two genotypes at any age (n = 5 embryos/group).
Fetal Adrenal Morphology and Steroidogenic Enzyme Gene Expression.
The effect of CRH deficiency on adrenal size and steroidogenic enzyme gene expression was examined between e14.5 and e18.5. Fetal adrenal size tended to increase progressively in Crh+/+ and Crh−/− mice through e17.5, when it was significantly greater in Crh+/+ mice than at any other time in either genotype (Fig. 2A). Consistent with the peak in wild-type adrenal size on e17.5, expression of the mRNA encoding StAR, a key regulator of adrenal glucocorticoid synthesis (19, 20), also peaked in Crh+/+ fetuses on this day of gestation (Fig. 2B). Hypothalamic CRH shows a similar pattern of expression in normal fetal mice, peaking on e16.5–17.5 and declining before birth (3). StAR mRNA levels in Crh−/− mice showed no variation during gestation and at all times were lower than the peak value in Crh+/+ mice (Fig. 2B). In contrast to StAR expression, mRNAs encoding SCC and 11β-hydroxylase, which catalyze the initial and final steps of glucocorticoid biosynthesis, respectively, were invariant within and between genotypes during the period examined (data not shown).
Figure 2.
Adrenal size and StAR mRNA expression in e15.5–18.5 embryos derived from either Crh+/+ × Crh+/+ matings (WT, black bars) and Crh−/− × Crh−/− matings [knockout (KO), white bars]. Fresh-frozen sagittal sections from whole embryos were hybridized with a mouse StAR riboprobe. (A) Adrenal gland size was calculated by using the perimeter of the largest adrenal cross section from each embryo. (B) StAR mRNA adrenal content in Crh+/+ (WT) and Crh−/− (KO) fetal mice. For both A and B: *, P < 0.05 vs. KO fetal mice at same age; $, P < 0.05 vs. WT e15.5 and WT e16.5 (n = 8 animals/group).
The lack of difference in SCC and 11β-hydroxylase mRNAs between normal and Crh−/− mice during gestation is consistent with other rodent studies, which suggest that their expression during fetal life does not depend on hypothalamic–pituitary activation (21) and that StAR expression, rather than these steroidogenic enzymes, is the rate-limiting factor in steroidogenesis (19, 20). The above changes in adrenal size and StAR mRNA content in normal fetuses are likely to be CRH dependent, as they are not observed in Crh−/− mice and correlate temporally with changes in fetal hypothalamic CRH mRNA levels in normal mice (3). Further, given the similar expression of POMC mRNA in Crh+/+ and Crh−/− fetuses, CRH might exert its effects directly on the fetal adrenal (22) rather than via stimulation of pituitary ACTH secretion.
Maternal and Fetal Adrenal Glucocorticoid Secretion.
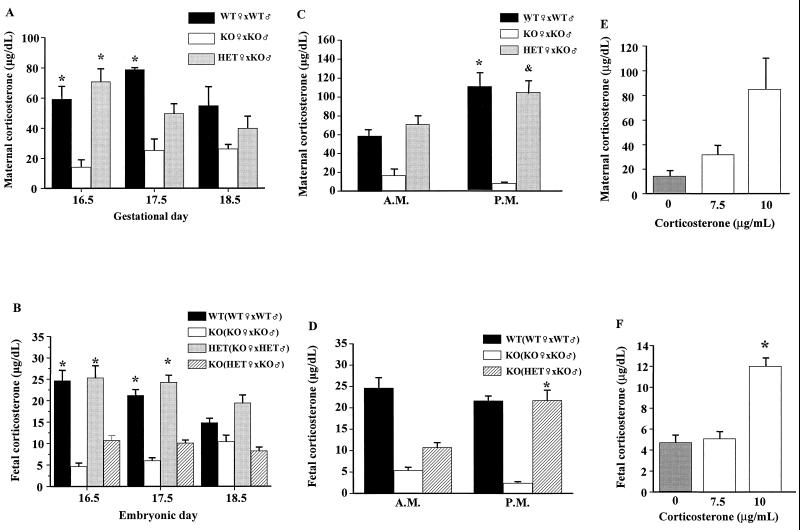
We next examined the impact of CRH deficiency during pregnancy on plasma corticosterone levels in the maternal and fetal circulations. By the morning of e16.5, maternal levels of plasma corticosterone in Crh+/+ and Crh+/− mothers were ≈60 μg/dl (Fig. 3A), 20-fold greater than those in nonpregnant normal females [3.5 ± 0.5 μg/dl (23)] and consistent with previously reported values in pregnant mice (24, 25). Even in Crh−/− mothers, plasma corticosterone was ≈10 μg/dl, 10-fold elevated compared with levels in nonpregnant Crh−/− females [0.9 ± 0.4 μg/dl (23)]. Corticosterone remained elevated during the remainder of gestation, but by the last day of pregnancy (e18.5) there were significant decreases in levels in Crh+/+ and Crh+/− mothers so that they were similar to those measured in Crh−/− dams (Fig. 3A).
Figure 3.
Maternal and fetal corticosterone levels at late gestational ages. (A) Morning corticosterone levels in Crh+/+ (WT, black bars), Crh−/− (KO, white bars) and Crh+/− [heterozygote (HET), gray bars] pregnant females mated with males of the indicated genotype. *, P < 0.05 vs. KO of same age (n = three to five animals/group); (B) fetal morning corticosterone levels in Crh+/+ (WT, black bars), Crh−/− (KO, white and hatched bars), and Crh+/− (HET, gray bars) fetuses derived from parents of the genotypes indicated in the parentheses. *, P < 0.05 vs. KO at same gestational age (n = at least eight fetuses/group); (C) morning and evening maternal corticosterone levels in Crh+/+ (WT, black bars), Crh−/− (KO, white bars), and Crh+/− (HET, gray bars) pregnant females on e16.5 mated with males of the indicated genotype. The morning values in each genotype are the same used in A at e16.5. *, P < 0.05 vs. WT morning and P < 0.05 vs. HET morning (n = at least three pregnant females/group); (D) morning and evening fetal corticosterone levels in Crh+/+ (WT, black bars) and Crh−/− (KO, white and hatched bars) fetuses on e16.5 derived from parents of the genotypes indicated in the parentheses. *, P < 0.05 vs. KO morning values of the same genotype (n = at least eight fetuses of both sexes); (E) morning corticosterone levels in Crh−/− mothers untreated (gray bars) or treated with the indicated concentrations of corticosterone (7.5 or 10 μg/ml, white bars) in the drinking water (n = 3 animals/group). (F) Fetal morning corticosterone levels in Crh−/− fetuses derived from Crh−/− mothers untreated (gray bars) or treated with the indicated concentrations of corticosterone in the drinking water (7.5 and 10 μg/ml, white bars). *, P < 0.001 vs. Crh−/− treated with 7.5 μg/ml corticosterone (n = at least eight fetuses from both sexes).
In the human, peak cortisol levels rise 2- to 3-fold, with the diurnal rhythm maintained (26, 27). Although some of the increase is because of a rise in transcortin, free levels of cortisol do increase as well, to 2-fold above nonpregnant values (26). Whereas the increase in maternal cortisol secretion during human pregnancy may depend on placental CRH secretion into maternal blood (28), our findings indicate that in the mouse, CRH [which is absent from rodent placenta (29)] is not absolutely required, because a modest increase in corticosterone levels is observed in Crh−/− mothers carrying only Crh−/− fetuses (Fig. 3A). Corticosterone is even higher in Crh−/− mothers carrying Crh+/− fetuses (data not shown), suggesting that the increase in maternal glucocorticoid levels is because of both maternal adrenal secretion and the transfer of corticosterone from fetus to mother through the placenta. This is consistent with previous reports of detectable plasma corticosterone levels in adrenalectomized pregnant rats (24).
Fetal plasma corticosterone ranged between 15 and 25 μg/dl in Crh+/+ fetuses and tended to decrease by e18.5 (Fig. 3B, black bars). Similar values were found in Crh+/− fetuses carried by Crh−/− mothers, indicating an independence of fetal corticosterone levels from maternal adrenal activity (Fig. 3B, gray bars), at least after e16.5. Based on the adrenal findings in Crh−/− fetuses carried by Crh−/− mothers (Fig. 2) and the fact that Crh−/− neonates born of Crh−/− mothers die at birth, the low levels of plasma corticosterone that we found in these Crh−/− fetuses were expected (Fig. 3B, white bars). Given that Crh−/− neonates born from Crh+/− mothers can survive after birth without corticosterone treatment (7), we expected them to have corticosterone levels similar to those found in wild-type (WT) fetuses (Fig. 3B, black bars). Instead, corticosterone levels were not significantly different in Crh−/− fetuses carried by either Crh−/− or Crh+/− mothers (Fig. 3B, white and hatched bars).
As the daily rhythm in serum corticosterone is preserved in pregnant rodents (25), we considered that the circadian evening rise in blood corticosterone in Crh+/− mothers might be associated with a concomitant rise in their Crh−/− fetuses. A diurnal increase in evening plasma corticosterone was observed in both Crh+/+ and Crh+/− mothers (Fig. 3C, black and hatched bars), whereas Crh−/− mothers, as expected (23), had no diurnal rhythm (Fig. 3C, white bars). Measurement of corticosterone levels in Crh−/− fetuses carried by Crh+/− mothers showed a significant rise in the evening vs. morning plasma corticosterone concentration, with levels rising to those found in Crh+/+ fetuses carried by Crh+/+ mothers (Fig. 3D, black and striped bars). However, Crh−/− fetuses carried by Crh−/− mothers had evening corticosterone levels similar to their low morning values, resulting in a 10-fold difference in evening values between the fetal knockouts carried by the two different maternal genotypes (Fig. 3D, white vs. striped bars). Thus, a diurnal rise in maternal glucocorticoid secretion correlates with a concomitant rise of the hormone in fetal blood.
These data indicate that a peak in fetal glucocorticoid concentration normally occurs on e16.5–17.5 and may come from either a fetal or maternal source. Lack of this peak is associated with abnormal pulmonary development and neonatal death in Crh−/− mice born to Crh−/− mothers. To directly test the causal relationship between the level of fetal corticosterone and lung development, we determined the effect of exogenous pulses of corticosterone given to pregnant Crh−/− dams carrying Crh−/− offspring. We previously had found that treatment of Crh−/− pregnant females with corticosterone, 30 μg/ml in drinking water, from e12.5 until birth results in normal neonatal lung architecture and normal viability of Crh−/− offspring (7). We next found that 10 μg/ml was the lowest concentration of corticosterone that was compatible with neonatal survival when given during this period: whereas concentrations of 10 and 15 μg/ml were associated with survival of all offspring with normal neonatal lung architecture, a concentration of 7.5 μg/ml resulting in perinatal death of all offspring (n = 3 pregnancies for each dose, data not shown). As mice are nocturnal drinkers, we measured maternal and fetal plasma corticosterone levels on the morning of e16.5, at the end of a 12-h period of corticosterone ingestion (30). The dose of 7.5 μg/ml corticosterone had only a modest effect on maternal (Fig. 3E) and no effect on fetal (Fig. 3F) plasma corticosterone levels. On the other hand, the dose of 10 μg/ml produced morning maternal plasma levels similar to those observed in Crh+/+ dams in the evening (Fig. 3E) and a parallel increase in fetal corticosterone levels (Fig. 3F). Finally, to more closely mimic the endogenous peak in plasma corticosterone of Crh+/+ fetuses on e17.5 (Fig. 3B), we confined the administration of 10 μg/ml of the hormone from e12.5 to e17.5 to Crh−/− mothers carrying Crh−/− fetuses. As shown previously (7), untreated Crh+/+ neonates born of Crh+/+ dams had 100% viability and normal lung architecture (Fig. 4A), as did Crh−/− neonates born of Crh−/− dams treated from e12.5 to birth on e19.5 (Fig. 4B). Crh−/− neonates born of Crh−/− dams with treatment confined from e12.5 to e17.5 were born on e19.5 with near-normal viability (74%, n = 6 pregnancies) and slightly thickened but otherwise normal septae (Fig. 4C). Thus, exposure to corticosterone for this limited period is sufficient to stimulate lung development to the point of postnatal viability.
Figure 4.
Representative lung midsagittal sections of 1-day-old newborn mice. (A) Crh+/+ newborn mouse derived from Crh+/+ × Crh+/+ mating. (B) Crh−/− newborn mouse derived from Crh−/− × Crh−/− mating, with the dam treated with 10 μg/ml corticosterone from e12.5 to spontaneous delivery on e19.5. (C) Crh−/− newborn mouse derived from Crh−/− × Crh−/− mating, with the dam treated with 10 μg/ml corticosterone from e12.5 to e17.5, with spontaneous delivery on e19.5.
Our data indicate that the normal development and function of the fetal adrenal gland require CRH. The absence of fetal CRH causes poor adrenal growth, diminished corticosterone secretion, and impaired pulmonary development (31). However, when homozygous CRH deficient fetuses are carried by heterozygous (Crh+/−) mothers, the effect of fetal adrenal insufficiency is counteracted by the transfer of maternal glucocorticoid to the fetus, so that normal fetal lung development can occur. The transfer of glucocorticoid from mother to fetus is regulated by placental 11-β hydroxysteroid dehydrogenase type 2 (32), as excess glucocorticoid can prematurely hasten differentiation and limit the growth of fetal lung and other tissues (33). This enzyme, when completely absent in mice (34) and humans (35), can result in growth retardation and neonatal death.
Perhaps it is only during the circadian peak in maternal glucocorticoid secretion that levels increase to a concentration that saturates the placental enzyme, allowing for transfer of active steroid, which escapes inactivation, to the fetus (Fig. 5). Rat placental 11-β hydroxysteroid dehydrogenase type 2 has a Km for corticosterone of ≈20 nM (36). As free corticosterone is approximately 5% of the total during the last 5 days of rodent pregnancy (37), diurnal levels of free corticosterone in Crh+/+ mothers (80–160 nM) are high enough to saturate the enzyme and escape inactivation. Moreover, the free levels of corticosterone (120 nM) achieved in Crh−/− mothers treated with corticosterone, 10 μg/ml, are also sufficiently above the Km of the enzyme to escape inactivation via the mechanism proposed in Fig. 5. Thus, it appears that both the maternal and fetal adrenal contribute to the level of fetal plasma corticosterone. Both Crh+/− fetuses carried by Crh−/− mothers (Fig. 3B, gray bars) and Crh−/− fetuses carried by Crh+/− mothers (Fig. 3D, hatched bar) have normal corticosterone levels, with the maximal transfer from mother to fetus appearing to occur during the circadian evening peak in maternal corticosterone.
Figure 5.
Saturation/escape model of transfer of glucocorticoid from mother to fetus through the placenta to support normal fetal lung development. The black vertical arrows represent the amount of corticosterone transferred in the morning (thin arrow) or the evening (thick arrow). Placental 11-β hydroxysteroid dehydrogenase limits the amount of active corticosterone that is transferred to the fetus. High levels of corticosterone are proposed to saturate the enzyme, to escape inactivation, and to be transferred to the fetus. Fetal corticosterone is derived both from that transferred from the mother and that secreted from the fetal adrenal and supports the development of fetal organs, including lung.
This situation is not unique to CRH-deficient mice. Mice with targeted deletions of the CRH type 1 receptor, carried by mothers homozygous for the same gene deletion, die at birth with pulmonary dysplasia, whereas this does not occur if mothers are heterozygous for the inactive gene (38). Mice with targeted inactivation of the gene encoding POMC (the precursor of corticotropin) have undetectable levels of glucocorticoid (39). Heterozygotes demonstrate partial haplo-insufficiency, with blood corticosterone values ≈20% lower than those of normal mice (39), and homozygous POMC-deficient fetuses carried by heterozygous mothers survive at only one-quarter of the expected frequency. Although not yet determined, if these pregnant dams lack the normal gestational increase and/or circadian rise in plasma corticosterone, this would further establish the significance of maternal–fetal transfer of glucocorticoid for normal fetal development.
In humans, fetuses lacking adrenal gland development (8) likely depend on the transplacental transfer of glucocorticoid from their normal mothers to allow for normal fetal lung development. This possibility raises the larger question of whether transfer of glucocorticoid from mother to fetus at earlier times during gestation, before fetal adrenal activation, is required for proper fetal organ maturation even when fetal glucocorticoid production is normal. Although it has been long accepted that human fetuses lacking the ability to synthesize thyroxine depend on maternal transfer of hormone for normal brain development (40, 41), it has been recognized only recently that maternal–fetal thyroid hormone transfer is necessary for the brain development of euthyroid fetuses (42). Thus, the increase during gestation in maternal free cortisol (26, 27) may function to promote organ maturation in normal fetuses, as well as in fetuses with defects in adrenal function.
Acknowledgments
We thank Mr. Chris Lage and Drs. Lauren Jacobson, Fred Grant, and Katia Karalis for their help and suggestions. This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute Grant 5P50HL56398 (J.M.).
Abbreviations
- HPA
hypothalamic-pituitary-adrenal
- POMC
proopiomelanocortin
- ACTH
adrenocorticotropin
- CRH
corticotropin-releasing hormone
- en
embryonic day n
- StAR
steroidogenic acute regulatory protein
- SCC
side chain cleavage enzyme
- WT
wild type
- KO
knockout
References
- 1.Asa S L, Kovacs K, Melmed S. In: The Hypothalamic-Pituitary Axis. Melmed S, editor. Cambridge: Blackwell Science; 1995. pp. 3–44. [Google Scholar]
- 2.Japon M A, Rubinstein M, Low M J. J Histochem Cytochem. 1994;42:1117–1125. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- 3.Keegan C E, Herman J P, Karolyi I J, O'Shea K S, Camper S A, Seasholtz A F. Endocrinology. 1994;134:2547–2555. doi: 10.1210/endo.134.6.8194481. [DOI] [PubMed] [Google Scholar]
- 4.Rundle S E, Funder J W. Neuroendocrinology. 1988;47:374–378. doi: 10.1159/000124941. [DOI] [PubMed] [Google Scholar]
- 5.Ballard P L. Baillieres Clin Endocrinol Metab. 1989;3:723–753. doi: 10.1016/s0950-351x(89)80051-5. [DOI] [PubMed] [Google Scholar]
- 6.Majumdar A P, Nielsen H. Scand J Gastroenterol. 1985;20:65–71. doi: 10.3109/00365528509089634. [DOI] [PubMed] [Google Scholar]
- 7.Muglia L, Jacobson L, Dikkes P, Majzoub J A. Nature (London) 1995;373:427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- 8.Krude I, Gruters I. Trends Endocrinol Metab. 2000;11:15–22. doi: 10.1016/s1043-2760(99)00213-1. [DOI] [PubMed] [Google Scholar]
- 9.Zanaria E, Muscatelli F, Bardoni B, Strom T M, Guioli S, Guo W, Lalli E, Moser C, Walker A P, McCabe E R. Nature (London) 1994;372:635–641. doi: 10.1038/372635a0. [DOI] [PubMed] [Google Scholar]
- 10.Newfield R S, New M I. Ann NY Acad Sci. 1997;816:219–229. doi: 10.1111/j.1749-6632.1997.tb52145.x. [DOI] [PubMed] [Google Scholar]
- 11.Weber A, Clark A J. Hum Mol Genet. 1994;3:585–588. doi: 10.1093/hmg/3.4.585. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson L. Endocrinology. 1999;140:310–317. doi: 10.1210/endo.140.1.6416. [DOI] [PubMed] [Google Scholar]
- 13.Muglia L J, Jenkins N A, Gilbert D J, Copeland N G, Majzoub J A. J Clin Invest. 1994;93:2066–2072. doi: 10.1172/JCI117201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant F D, Reventos J, Gordon J W, Kawabata S, Miller M, Majzoub J A. Mol Endocrinol. 1993;7:659–667. doi: 10.1210/mend.7.5.8100353. [DOI] [PubMed] [Google Scholar]
- 15.Weninger S C, Dunn A J, Muglia L J, Dikkes P, Miczek K A, Swiergiel A H, Berridge C W, Majzoub J A. Proc Natl Acad Sci USA. 1999;96:8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichardt H M, Schutz G. Mol Med. 1996;2:735–744. [PMC free article] [PubMed] [Google Scholar]
- 17.Tajima T, Ma X M, Bornstein S R, Aguilera G. Endocrinology. 1999;140:3354–3362. doi: 10.1210/endo.140.7.6755. [DOI] [PubMed] [Google Scholar]
- 18.Muglia L J, Jacobson L, Luedke C E, Vogt S K, Schaefer M L, Dikkes P, Fukuda S, Saiki Y, Suda T, Majzoub J A. J Clin Invest. 2000;105:1269–1277. doi: 10.1172/JCI5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stocco D M, Clark B J. Steroids. 1997;62:29–36. doi: 10.1016/s0039-128x(96)00155-9. [DOI] [PubMed] [Google Scholar]
- 20.Miller W L, Strauss J F. J Steroid Biochem Mol Biol. 1999;69:131–141. doi: 10.1016/s0960-0760(98)00153-8. [DOI] [PubMed] [Google Scholar]
- 21.Wotus C, Levay-Young B K, Rogers L M, Gomez-Sanchez C E, Engeland W C. Endocrinology. 1998;139:4397–4403. doi: 10.1210/endo.139.10.6230. [DOI] [PubMed] [Google Scholar]
- 22.Smith R, Mesiano S, Chan E C, Brown S, Jaffe R B. J Clin Endocrinol Metab. 1998;83:2916–2920. doi: 10.1210/jcem.83.8.5020. [DOI] [PubMed] [Google Scholar]
- 23.Muglia L J, Jacobson L, Weninger S C, Luedke C E, Bae D S, Jeong K H, Majzoub J A. J Clin Invest. 1997;99:2923–2929. doi: 10.1172/JCI119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen A, Guillon Y. Biol Neonate. 1985;47:163–169. doi: 10.1159/000242108. [DOI] [PubMed] [Google Scholar]
- 25.Montano M M, Wang M H, Even M D, vom Saal F S. Physiol Behav. 1991;50:323–329. doi: 10.1016/0031-9384(91)90073-w. [DOI] [PubMed] [Google Scholar]
- 26.Goland R S, Jozak S, Conwell I. Am J Obstet Gynecol. 1994;171:1287–1291. doi: 10.1016/0002-9378(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 27.Magiakou M A, Mastorakos G, Rabin D, Margioris A N, Dubbert B, Calogero A E, Tsigos C, Munson P J, Chrousos G P. Clin Endocrinol (Oxford) 1996;44:419–428. doi: 10.1046/j.1365-2265.1996.683505.x. [DOI] [PubMed] [Google Scholar]
- 28.Majzoub J A, Karalis K P. Am J Obstet Gynecol. 1999;180:S242–S246. doi: 10.1016/s0002-9378(99)70708-8. [DOI] [PubMed] [Google Scholar]
- 29.Robinson B G, Arbiser J L, Emanuel R L, Majzoub J A. Mol Cell Endocrinol. 1989;62:337–341. doi: 10.1016/0303-7207(89)90022-1. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson L, Akana S F, Cascio C S, Scribner K, Shinsako J, Dallman M F. Endocrinology. 1989;124:2144–2152. doi: 10.1210/endo-124-5-2144. [DOI] [PubMed] [Google Scholar]
- 31.Muglia L J, Bae D S, Brown T T, Vogt S K, Alvarez J G, Sunday M E, Majzoub J A. Am J Respir Cell Mol Biol. 1999;20:181–188. doi: 10.1165/ajrcmb.20.2.3381. [DOI] [PubMed] [Google Scholar]
- 32.Edwards C R, Benediktsson R, Lindsay R S, Seckl J R. Steroids. 1996;61:263–269. doi: 10.1016/0039-128x(96)00033-5. [DOI] [PubMed] [Google Scholar]
- 33.Carson S H, Taeusch H W J, Avery M E. J Appl Physiol. 1973;34:660–663. doi: 10.1152/jappl.1973.34.5.660. [DOI] [PubMed] [Google Scholar]
- 34.Kotelevtsev Y, Brown R W, Fleming S, Kenyon C, Edwards C R, Seckl J R, Mullins J J. J Clin Invest. 1999;103:683–689. doi: 10.1172/JCI4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krozowski Z, Li K X, Koyama K, Smith R E, Obeyesekere V R, Stein-Oakley A, Sasano H, Coulter C, Cole T, Sheppard K E. J Steroid Biochem Mol Biol. 1999;69:391–401. doi: 10.1016/s0960-0760(99)00074-6. [DOI] [PubMed] [Google Scholar]
- 36.Waddell B J, Benediktsson R, Brown R W, Seckl J R. Endocrinology. 1998;139:1517–1523. doi: 10.1210/endo.139.4.5900. [DOI] [PubMed] [Google Scholar]
- 37.Gewolb I H, Warshaw J B. Pediatr Res. 1986;20:155–160. doi: 10.1203/00006450-198602000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Smith G W, Aubry J M, Dellu F, Contarino A, Bilezikjian L M, Gold L H, Chen R, Marchuk Y, Hauser C, Bentley C A, et al. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 39.Yaswen L, Diehl N, Brennan M B, Hochgeschwender U. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 40.Cheron R G, Kaplan M M, Larsen P R, Selenkow H A, Crigler J F J. N Engl J Med. 1981;304:525–528. doi: 10.1056/NEJM198102263040907. [DOI] [PubMed] [Google Scholar]
- 41.Vulsma T, Gons M H, de Vijlder J J. N Engl J Med. 1989;321:13–16. doi: 10.1056/NEJM198907063210103. [DOI] [PubMed] [Google Scholar]
- 42.Sherman S I, Gopal J, Haugen B R, Chiu A C, Whaley K, Nowlakha P, Duvic M. N Engl J Med. 1999;340:1075–1079. doi: 10.1056/NEJM199904083401404. [DOI] [PubMed] [Google Scholar]