Abstract
The catalytic domain of acetylcholinesterase AChET subunits is followed by a C-terminal T peptide which mediates their association with the proline-rich attachment domain (PRAD) of anchoring proteins. Addition of the T peptide induced intracellular degradation and concomitantly reduced to variable degrees the secretion of AChE species differing in their oligomerization capacity and of human alkaline phosphatase. The T peptide forms an amphiphilic α-helix, containing a series of conserved aromatic residues. Replacement of two, four or five aromatic residues gradually suppressed degradation and increased secretion. Co-expression with a PRAD- containing protein induced the assembly of PRAD-linked tetramers in the endoplasmic reticulum (ER) and allowed partial secretion of a dimerization- defective mutant; by masking the aromatic side chains, hetero-oligomerization rescued this enzyme from degradation. Degradation was due to ERAD, since it was not blocked by brefeldin A but was sensitive to proteasome inhibitors. Kifunensine reduced degradation, suggesting a cooperativity between the glycosylated catalytic domain and the non-glycosylated T peptide. This system appears particularly well suited to analyze the mechanisms which determine the degradation of correctly folded multidomain proteins in the ER.
Keywords: acetylcholinesterase/amphiphilic helix/ERAD/oligomerization//T peptide/WAT domain
Introduction
In the major variant of vertebrate acetylcholinesterase (AChET), the catalytic domain is associated with a C-terminal peptide of 40 residues (Massoulié et al., 1993; Massoulié, 2002). This T peptide allows AChET subunits to assemble as tetramers, associated with the ColQ or PRiMA anchoring proteins. The association of AChET subunits with ColQ produces the collagen-tailed forms which play an essential role in the neuromuscular junction (Feng et al., 1999), while their association with PRiMA produces the membrane-bound tetramers which represent the predominant AChE species in the mammalian nervous system (Fernandez et al., 1996; Perrier et al., 2002). The assembly of these hetero-oligomers is based on an association of four T peptides with short proline-rich attachment domains (PRADs), located in the N-terminal regions of ColQ or PRiMA. The T peptide in fact represents an autonomous interaction domain, the ‘tryptophan (W) amphiphilic tetramerization’ domain or WAT, which can associate with a PRAD in the absence of the catalytic domain, or in association with a foreign protein (Bon et al., 1997; Simon et al., 1998).
When AChET subunits are expressed in transfected cells without an anchoring protein containing a PRAD, they can form homo-oligomers, mostly dimers and tetramers. Truncated subunits, lacking a C-terminal T peptide, remain monomeric (Duval et al., 1992).
The T peptide contains a series of seven conserved aromatic residues, including three evenly spaced tryptophans, and can organize as an amphiphilic α-helix in which the aromatic residues form a hydrophobic cluster (Massoulié et al., 1993; Giles, 1997). The presence of this peptide confers an amphiphilic character to AChET monomers and dimers (Duval et al., 1992); in contrast, PRAD-linked tetramers are not amphiphilic, indicating that the aromatic side chains are not accessible, in agreement with a crystallographic study of the complex formed by four T peptides with a PRAD (M.Harel, S.Bon, W.Q.Liu, H.Dvir, S.Belbeoc’h, M.Vidal, C.Garbay, I.Silman, J.Massoulié and J.Sussman, in preparation). The T peptide also possesses a cysteine near its C-terminus, forming intercatenary disulfide bonds required for dimerization, but not for the formation of tetramers or for association with a PRAD (Velan et al., 1991; Bon et al., 1997).
We recently showed that the presence of the T peptide induces a partial intracellular degradation of AChET subunits, an effect which was considerably increased in the case of subunits in which oligomerization was compromised by a point mutation in the dimer contact zone (F527A) (Morel et al., 2001). When reduced to the catalytic domain, the dimerization-defective mutant (rAChE*stop) was secreted as monomers, but secretion of AChE was practically abolished when the C-terminal T peptide was present (rAChE*T); co-expression with an N-terminal fragment of ColQ (QN) rescued a fraction of this enzyme from degradation and allowed the secretion of PRAD-linked tetramers.
In the absence of a PRAD-containing anchoring protein, the T peptide thus appeared to target active AChET subunits towards degradation rather than secretion. In the present study, we analyze the nature of this intracellular degradation, and we attempt to assess a possible relationship between oligomerization and degradation and to identify its determinants in the T peptide. For this purpose, we analyzed the cellular fate of wild-type rat AChET subunits and of the dimerization-defective mutant, rAChE*T; we also added a T peptide to the homologous AChEB enzyme from the parasitic nematode Nippostrongylus brasiliensis which normally is secreted as a monomer (Hussein et al., 1999) as well as to human alkaline phosphatase (hAP), a non-homologous protein. We show that the T peptide induces variable levels of degradation, which depends on the aromatic residues of this peptide but not on the cysteine, and that it is due to an ERAD mechanism. This study provides new insights into the determinants that target correctly folded, active proteins towards degradation in the secretory pathway.
Results
Constructs used in this study
Figure 1A illustrates the major types of oligomers obtained by expression of AChE subunits alone, or with Torpedo QN, which contains a PRAD. While truncated AChE subunits (AChEstop) only produced monomers, AChET subunits containing the wild-type rat T peptide produced monomers, dimers and tetramers when expressed alone, as well as PRAD-linked tetramers when expressed with QN, as previously described (Bon and Massoulié, 1997). The formation of dimers depends on the presence of cysteine 37, and the association with a PRAD depends on the aromatic residues, as shown by mutation of individual residues (S.Belbeoc’h, J.Leroy, A.Ayon, J.Massoulié and S.Bon, in preparation), or groups of residues (see below).
Fig. 1. (A) Schematic representation of different molecular forms of AChE. An asterisk indicates the presence of the mutation F527A located in the dimerization surface. The catalytic subunits (rAChE and rAChE*) are shown as spheres, and the WAT domains as zigzags. The PRAD is represented as a shaded square (light gray). The intercaternary disulfide bonds join two T peptides (WAT domains) to each other in rAChET dimers. In PRAD-linked tetramers, one PRAD interacts with four WAT domains. (B) Schematic representation of protein constructs used in this study. These constructs were inserted in the pEF-BOS vector or in the pcDNA3 vector for expression in COS cells. The WAT domains were added at the C-terminus of N.brasiliensis AChE and alkaline phosphatase. The letters r, n and h stand respectively for rat, N.brasiliensis and human. (C) Sequences of the WAT domains that were used in this study. In mutants ‘2b’, ‘2c’, ‘4’ and ‘5’, aromatic residues were replaced by glycines, as illustrated, and also by serines. The complete sequence of the mature QN protein, which corresponds to the N-terminal region of rat ColQ and contains the PRAD domain (box), is also shown. (D) Effects of mutations in the rat WAT domain on secreted activities of AChE and alkaline phosphatase, expressed as a percentage of the activity obtained for the truncated subunits characterized by the absence of the WAT domain (rAChEstop, rAChE*stop, nAChEstop and hAPstop). Boxes correspond to secreted activity recovered in the medium, 3 days after transfection. The results are expressed as the mean ± SD of 15 independent experiments. The T peptide decreases secretion to different extents, depending on the protein to which it is attached. Mutation of tryptophans and phenylalanines increased the secreted activity, reaching a significant fraction of that observed for the truncated subunits.
In this study, we analyze the influence of the C-terminal T peptide on the cellular fate (intracellular degradation or secretion) of three AChE species: the wild-type rat catalytic domain (rAChE), the dimerization-defective mutant F527A (rAChE*) and the normally monomeric Nippostrongylus enzyme (nAChE); we also added the rat T peptide at the C-terminus of hAP, in place of the normal glycosylphosphatidylinositol (GPI) addition signal. These constructs are illustrated in Figure 1B. They contained either a wild-type rat T peptide, or a modified T peptide in which we replaced aromatic residues by glycines or serines, either individually, in pairs or in groups of four or five, as shown in Figure 1C; we also replaced C37 by a serine; in addition, the double mutation C37K/S38D introduced a C-terminal KDEL motif. The different constructs were expressed in COS7 cells, with or without QN.
Role of aromatic residues in degradation and oligomerization
Figure 1D shows that addition of a T peptide decreased the secretion of each of the four proteins, to different fractions of that observed in its absence (AChEstop or hAPstop), in the order rAChE*T < hAPT < rAChET < nAChET. Mutation of aromatic residues restored secretion, in a progressive manner: a significant effect was observed by mutation of a pair of residues in the case of the more degraded proteins, rAChE*T and hAPT, and by mutation of four or five residues in all cases; it must be noted that mutation of the proximal pair (W10 and F14) had less effect than that of the distal pair (W24 and F28). When the five residues (W10, F14, W17, W24 and F28) were replaced by glycines or serines, the rate of secretion approached that observed without a T peptide. Replacement of aromatic residues by serines increased secretion more than their replacement by glycines, suggesting that hydrophilicity was an important factor. In any case, degradation induced by the T peptide clearly required the presence of aromatic residues and could be essentially suppressed by their mutation.
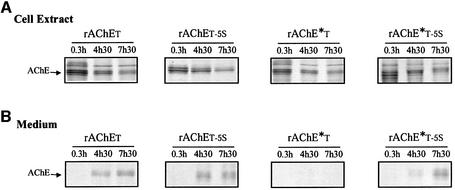
These results were confirmed and expanded by metabolic labeling of rAChET and rAChE*T proteins containing a wild-type or a mutated T peptide (Figure 2). The newly synthesized proteins decreased in the cells at similar rates in all cases during the chase period (Figure 2A), whether they were secreted or not (Figure 2B). This indicates that the non-secreted fraction is degraded intracellularly, rather than accumulated in the cells. Figure 2B shows that the mutation of five aromatic residues allowed some secretion of the dimerization-defective mutant: rAChE*T-5S was partially secreted, while rAChE*T was not, in agreement with our analysis of AChE activity.
Fig. 2. The polypeptides produced by wild-type rAChET, rAChET-5S, rAChE*T or rAChE*T-5S mutants were labeled for 30 min with [35S]methionione and analyzed by reducing polyacrylamide gel electrophoresis, in cell extracts (A) and in the culture medium (B) after the indicated chase periods. Monomers formed a triplet because of heterogeneity in N-glycosylation, since treatment of the cellular enzyme by N-glycanase produced a single, faster migrating band (not shown). rAChE*T did not accumulate in the cells and did not appear in the medium. In contrast, the wild-type rAChET enzyme and the ‘5S’ mutants were secreted efficiently.
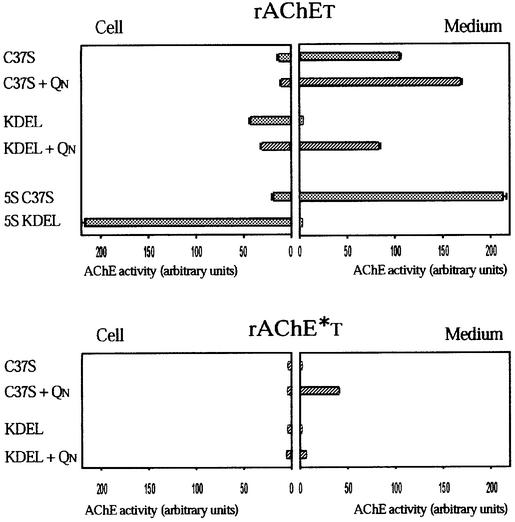
The oligomeric state of the secreted enzymes was determined by non-denaturing electrophoresis and by sedimentation in sucrose gradients, as illustrated in Figure 3B and C. When rAChET is expressed alone, this enzyme is secreted as monomers (G1), dimers (G2) and tetramers (G4), reaching ∼40% of the secretion level observed with the truncated monomeric species, rAChEstop; it should be noted that the proportion of oligomers is higher in the medium than in the cell extracts (Bon and Massoulié, 1997). However, monomers represent a high proportion of AChE activity in the medium, indicating that they are also secreted efficiently. Co-expression with QN recruited a major proportion of rAChET subunits into G4–QN hetero-oligomers, and increased secretion by 30%.
Fig. 3. Effect of QN on secretion and oligomerization of rAChET, rAChE*T, nAChET and hAPT subunits. The different subunits were co-expressed with a defective QN from which the PRAD domain was deleted (–) or with a complete QN (+QN). (A) Analysis of the presence of QN on secreted activity, expressed as a percentage of the truncated subunits (rAChEstop, rAChE*stop, nAChEstop and hAPstop). Co-expression with QN significantly increased the secretion of rAChE*T and hAPT and had little effect on rAChET and nAChET. (B) Sedimentation profiles obtained from the culture medium of COS cells expressing the different subunits. When co-expressed with QN, rAChET, rAChE*T and hAPT were mostly secreted as QN-linked tetramers, while nAChET only produced a small proportion of these oligomers. (C) Electrophoretic analysis of secreted AChE in non-denaturing conditions. Co-expression of the wild-type rAChET with QN induced the secretion of soluble QN-linked tetramers (G4–QN). Mutation of two or more aromatic residues in ‘2aS’, ‘2bS’, ‘4S’ and ‘5S’ mutants totally abolished the association with QN.
When expressed without QN, the Nippostrongylus nAChET enzyme remained mostly monomeric, with a very low proportion of dimers. Co-expression with QN produced a small proportion of QN-linked tetramers, both in the cell extracts and in the medium. Thus, nAChET subunits had very little tendency to form homo- or hetero-oligomers. In addition, hetero-oligomerization had no significant effect on the level of secretion.
The dimerization-defective rAChE*T subunits were practically not secreted when expressed without QN, but co-expression with QN allowed the secretion of G4–QN oligomers, representing ∼40% of the secretion of the corresponding truncated enzyme, rAChE*stop.
hAP possessing a T peptide produced monomers and dimers, but no tetramers without QN, and secretion was only ∼5% of that obtained with hAPstop. Co-expression with QN produced QN-linked tetramers and increased secretion to ∼25% of hAPstop (Figure 3). This demonstrates that the T peptide is sufficient to allow the formation of a hetero-oligomeric complex, in which the degradation determinants are masked.
We found that QN did not interact with AChE or hAP proteins carrying modified T peptides in which two or more aromatic residues were mutated, and did not affect their secretion. This is shown for rAChET-5S subunits in Figure 3. Such mutants of rat AChE produced monomers and dimers, but no tetramers, illustrating the importance of aromatic residues for tetramerization, with or without a PRAD.
Role of the C-terminal cysteine
To evaluate whether a free cysteine (C37) contributed to the degradation induced by the T peptide, we replaced it by a serine in rAChET and nAChET, with or without mutation of five aromatic residues. The rAChET and nAChET constructs containing a wild-type or ‘5S’ T peptide were secreted mostly as monomers and dimers: in the case of rAChET, the proportions of these two forms were comparable, while >90% of nAChET remained monomeric (Figure 3B), in agreement with its low capacity for oligomerization. As expected, the C37S mutation totally suppressed dimerization (not shown).
The replacement of the cysteine by a serine reduced both cellular and secreted activity of the wild-type rAChET by ∼40% (Figure 4). The reduction of secretion reflects an increased intracellular degradation and, in fact, the C37S mutation had much less effect on the rAChET-5S mutant, which is not degraded.
Fig. 4. Effect of mutation of the C-terminal cysteine (C37S) in the T peptide on cellular and secreted AChE activity, expressed as a percentage of the wild-type (WT) subunits. Boxes on the left correspond to cell-associated activity and boxes on the right correspond to secreted activity recovered in the medium, 3 days after transfection. The results are expressed as the mean ± SD of 12 independent experiments. Mutation of the cysteine reduced both cellular and secreted activity of rAChET and reduced the cellular activity of nAChET. In contrast, there was no effect on the ‘5S’ or ‘5G’ mutants.
To test the hypothesis of a retention effect due to the free C37 cysteine without complications due to degradation and dimerization, we compared mutants nAChET-5S and nAChET-5S/CS: these mutants are not degraded because of mutation of the aromatic residues, and, even when C37 was present, nAChET-5S remained predominantly monomeric. The cellular and secreted activities of the two mutants were essentially identical, showing that, in this case, the C-terminal cysteine did not act as a significant retention signal.5
Fig. 5. Effect of a KDEL motif in the rat T peptide on cellular and secreted AChE activity. AChE subunits were co-expressed with a PRAD-deleted or complete QN (+QN), and the results of 12 independent experiments are represented as in Figure 4. The presence of the KDEL motif blocked secretion and increased the cellular activity of rAChET-KDEL and of rAChET-5S-KDEL, but not of the dimerization-defective mutant, rAChE*T-KDEL. Co-expression with QN partially restored the secretion of rAChET-KDEL and to a lesser degree of rAChE*T-KDEL.
An endoplasmic reticulum retention motif (KDEL) did not prevent degradation of AChET
In order to study the mechanism of degradation induced by the T peptide, we analyzed AChE mutants containing a KDEL motif: the tetrapeptide sequence CSDL at the C-terminus of the T peptide was replaced by the KDEL motif in the rat AChE subunits rAChET, rAChET-5S and rAChE*T (Figure 1C). We found that KDEL mutants produced molecular forms very similar to the corresponding C37S mutants which also lacked the C-terminal cysteine of the T peptide (not shown).
The presence of the KDEL motif reduced secretion to a very low level, even 3 days after transfection, and increased the cellular level more than three times compared with rAChET-CS, indicating an efficient retention. In the case of the rAChET-5S/KDEL mutant, secretion was blocked and the level of cellular activity was increased ∼10 times compared with the corresponding mutant in which only C37 was mutated (rAChET-5S/CS). Because the rate of biosynthesis was the same for all constructs and because the different KDEL mutants were not secreted, the levels of cellular activity were inversely related to their rates of degradation. Therefore, the fact that the level of cellular activity was lower (∼20%) for rAChET-KDEL than for the corresponding ‘5S’ mutant (rAChET-5S/KDEL) indicates that the former is actively degraded intracellularly. Degradation is particularly obvious in the case of the dimerization-defective mutant rAChE*T-KDEL, for which both secreted and cellular activities remained as low as when only the cysteine was mutated (rAChE*T-CS). The fact that degradation is not prevented by endoplasmic reticulum (ER) retention through the KDEL motif suggests that it occurs through the ERAD pathway.
The presence of an ER retention signal did not prevent assembly with QN; in fact, QN partially restored the secretion of the wild-type and dimerization-defective mutant containing a KDEL motif (rACHET-KDEL and rAChE*T-KDEL), in the form of PRAD-associated tetramers (G4–QN). This suggests that association between WAT and PRAD domains may occur in the ER, and that the KDEL motif is hardly accessible to its receptor in the G4–QN quaternary assembly.
Brefeldin A does not prevent AChET degradation
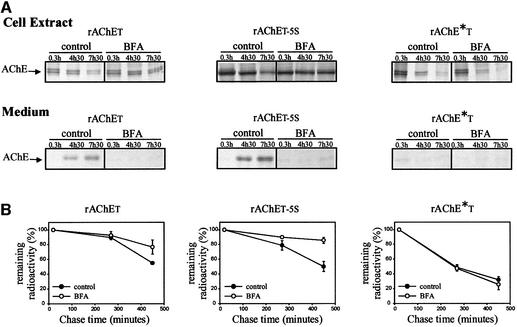
To test the hypothesis that AChE subunits possessing a WAT domain are degraded by the ERAD process, we used brefeldin A (BFA) which completely stops the vesicular export of secretory proteins from the ER (Misumi et al., 1986; Fujiwara et al., 1988). The rate of protein degradation was determined after metabolic labeling, by SDS–PAGE analysis under reducing conditions, for the wild-type rAChET, the dimerization-defective mutant rAChE*T and the rAChET-5S mutant (Figure 6). During the chase period after metabolic labeling, at least up to 7.5 h, we observed that the remaining intracellular AChE was still sensitive to digestion with endoglycosidase H (data not shown). When the cells were treated with BFA, no labeled AChE appeared in the medium even after a 7.5 h chase period, for any of the studied proteins. The wild-type and the dimerization-defective mutant subunits were not accumulated in cells, confirming that their retention in an ER–Golgi compartment did not block their degradation. In contrast, we found that the rAChET-5S mutant persisted during the chase period in cells treated with BFA, in agreement with an inefficient degradation of this mutant.
Fig. 6. Effect of brefeldin A on metabolically labeled wild-type rAChET, rAChET-5S and rAChE*T mutants. (A) The AChE polypeptides were labeled for 30 min with [35S]methionine and analyzed by reducing polyacrylamide gel electrophoresis after the indicated chase periods, in cell extracts and in the culture medium. Cells were incubated for 0.3, 4.5 or 7.5 h with or without 4.2 µg/ml BFA as indicated. (B) The radioactivity remaining in cell extracts was measured by PhosphorImager analysis and expressed as a percentage of the initial label (means ± SD of five independent experiments). In BFA-treated cells, no labeled AChE appeared in the medium. The dimerization-defective mutant was not accumulated in cells, in contrast to the rAChET-5S mutant. The level of wild-type rAChET was moderately increased in BFA-treated cells.
We also found that the rate of AChET degradation was not affected by addition of chloroquine in the culture medium during the chase (data not shown), showing that acidic compartments of the endocytic pathway are not involved in the degradation of this protein.
Proteasome inhibitors increase the cellular level of rAChET
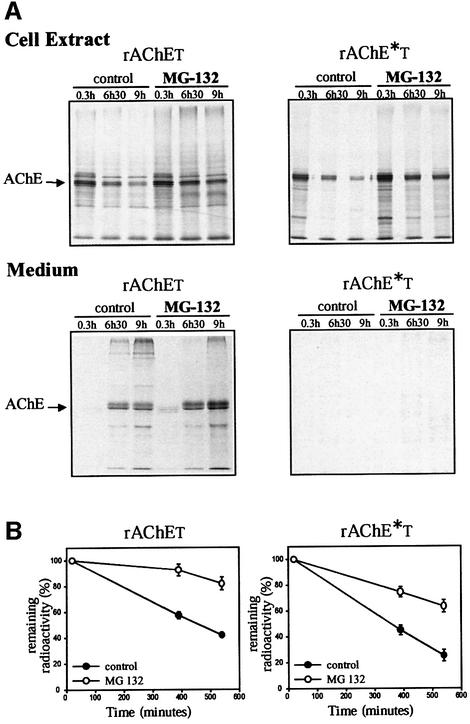
To see whether the proteasome pathway is involved in the degradation of rAChET, we studied the effect of different proteasome inhibitors. We followed the fate of metabolically labeled AChE protein by incubating transfected COS cells in the presence or absence of MG-132 (50 μM) for up to 9 h (Figure 7). This inhibitor reduced the decrease of the wild-type rAChET protein in the cells and increased its release into the medium. The amount of the dimerization-defective mutant decreased less rapidly in the cells, but this protein was hardly detected in the medium, even in the presence of the inhibitor.
Fig. 7. Effect of the proteasome inhibitor MG-132 on metabolically labeled wild-type rAChET and the dimerization-defective mutant rAChE*T. Cells were incubated for 0.3, 6.5 or 9 h with or without 50 µM MG-132 as indicated. (A) The labeled polypeptides were analyzed as in Figure 6 (means ± SD of five experiments). (B) The radioactivity remaining in cell extracts was measured by PhosphorImager analysis and expressed as a percentage of the initial label. The proteasome inhibitor MG-132 reduced the decrease of rAChET in the cells and increased its release in the medium.The amount of rAChE*T was also increased in the cells, but this mutant was hardly detected in the medium.
We obtained a more precise determination of the fate of AChE by following its activity in the presence or absence of inhibitors. We monitored AChE activity in COS cells expressing rAChE*T and rAChET-5S during treatment with MG-132 or clasto-lactacystin-β-lactone. In both cases, we observed an increase of cellular activity. In the presence of 50 µM β-lactone, the cellular activity was already significantly increased after 1 h, and it was 2.5-fold higher than the control after 6 h; in addition, the secreted activity was increased 2-fold (Figure 8). We observed no change in activity for cells expressing the ‘5S’ mutant, confirming the fact that it was not significantly degraded.
Fig. 8. Effect of the proteasome inhibitor clasto-lactacystin-β-lactone on cellular and secreted AChE activity, expressed as a percentage of AChE activity in untreated cells. Three days after transfection, the culture medium was changed and cells were incubated for 1, 2 or 6 h with or without 50 µM β-lactone, then AChE activities were measured. The results represent the means ± SD of three independent experiments. The inhibitor induced an increase of cellular activity for rAChE*T; although the secreted activity remained very low, it was also reproducibly increased. There was no effect on rAChET-5S, indicating that this mutant is not degraded by the proteasome pathway.
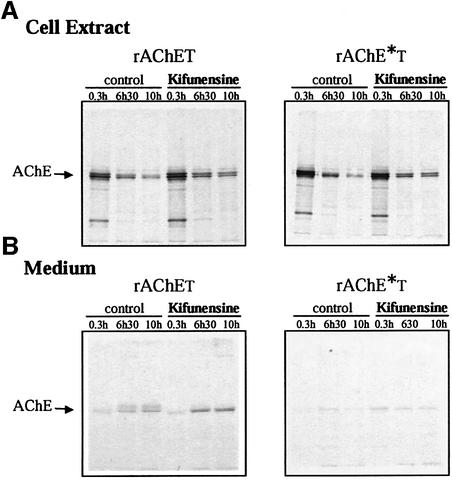
We also studied the effect of kifunensine, a specific inhibitor of mannosidase I activity in the ER (Tokunaga et al., 2000; Fagioli and Sitia, 2001). Kifunensine reduced the degradation of rAChET and rAChET*, in a manner similar to the proteasome inhibitors (Figure 9), suggesting that degradation involves binding of their carbohydrate moieties to the ER lectin chaperones.
Fig. 9. Effect of kifunensine on metabolically labeled wild-type rAChET and the dimerization-defective mutant, rAChE*T. Cells were incubated for 0.3, 6.5 or 10 h with or without 4 µg/ml kifunensine as indicated. The loss of cellular polypeptides (A) and their release in the culture medium (B) were analyzed as in Figures 6 and 7. The decrease of rAChET was reduced in the treated cells, and the release in the medium was increased. The rAChE*T mutant was not significantly secreted but its degradation was reduced in the treated cells.
Discussion
We show that the C-terminal T peptide of AChE, or WAT domain, induced variable degrees of degradation when associated with three types of AChE subunits which differ in their capacity to oligomerize, and with a heterologous protein, hAP. We present several lines of evidence indicating that this degradation corresponds to an ERAD mechanism (reviewed in Brodsky and McCracken, 1999; Ellgard and Helenius, 2003). First, ER retention by addition of a KDEL motif did not prevent the degradation of AChET, as in the case of TCR subunits (Yamamoto et al., 2001). Secondly, degradation of AChET subunits was not blocked by inhibitors of lysosomal proteolysis. Thirdly, it was not blocked by BFA, which prevents the vesicular export of secretory proteins from the ER (Misumi et al., 1986; Fujiwara et al., 1988) and does not prevent the degradation of ERAD substrates (Klausner and Sitia, 1990; Zanusso et al., 1999). Finally, proteasome inhibitors, MG-132 and clasto-lactacystin-β-lactone, induced an increase in the amount of rAChET in the cells as well in the medium. In contrast, only small changes were observed for the non-degraded rAChET-5S mutant, in which five aromatic residues were mutated to serines.
While the wild-type rat enzyme readily forms homodimers and homotetramers, a single mutation in the dimerization contact zone, F527A, strongly reduces this capacity in rAChE*T. We also used AChE from the parasitic nematode N.brasiliensis, which normally is secreted as a monomer, and in fact contains an alanine residue at the position homologous to rat F527, as in the dimerization-defective mutant. This enzyme proved to remain mostly monomeric, even when associated with the rat T peptide. The comparison of these enzymes did not show any direct relationship between homo-oligomerization and the level of degradation induced by the T peptide.
The T peptide constitutes an autonomous interaction domain, the WAT domain, which is normally associated with AChE, but can also confer to foreign proteins the capacity to associate with QN, the N-terminal fragment of collagen ColQ, through its PRAD. Co-expression with QN recruited variable fractions of rAChET, rAChE*T, nAChET and hAPT subunits into PRAD-linked tetramers; this confirmed the capacity of the T peptide to act as an autonomous interaction domain (WAT), but also indicated that the catalytic domain influenced the capacity of the different constructs to oligomerize. The formation of QN-associated tetramers allowed a significant secretion of the dimerization-defective mutant rAChE*T and of hAPT, which are almost totally degraded intracellularly when expressed alone, suggesting that the degradation signal is masked in the PRAD-associated hetero-oligomers. Interestingly, hetero-oligomers could be assembled and secreted with the mutant carrying a KDEL motif, indicating that the association of the T peptides and PRAD can occur in the ER and that the ER retention signal is masked in the G4–QN complex.
In a previous study, we showed that the presence of a T peptide did not modify the initial rate of recovery of AChE activity, after irreversible inhibition of the pre-existing enzyme, for rAChET or rAChE*T, compared with rAChEstop or rAChE*stop, respectively (Morel et al., 2001). Therefore, the folding of the catalytic domain was not affected by the C-terminal T peptide. In addition, the T peptide did function as an autonomous interaction domain, conferring the capacity to form PRAD-associated tetramers to these foreign proteins, although the efficiency of hetero-oligomerization depended on the nature of the catalytic domain. The T peptide induced degradation of correctly folded proteins, and this degradation varied widely with the nature of the catalytic domain, in a manner which was not related to its capacity for oligomerization. This shows that the ER quality control is sensitive to the combination of the two domains, rather than to an individual ‘signal’ carried by the T peptide alone. This notion is reinforced by the fact that kifunensine blocked degradation, indicating an involvement of the glycosylated catalytic domain, since the T peptide itself is not glycosylated. Again, we emphasize the fact that the glycan moieties were not associated with abnormally exposed peptides due to misfolding.
By introducing various mutations in the T peptide, we attempted to identify determinants inducing degradation. Replacement of the C-terminal cysteine by a serine reduced the secretion of rat AChE, but had no effect on that of the Nippostrongylus enzyme. Therefore, in our system, a free cysteine in the T peptide does not act as a retention signal, in contrast to the results obtained in the case of other proteins, such as immunoglobulins (Sitia et al., 1990). The T peptide also contains seven aromatic residues and can form an amphiphilic α-helix (Massoulié et al., 1993); these aromatic residues play a critical role in the heteromeric association with a PRAD (Blong et al., 1997; S.Belbeoc’h, J.Leroy, A.Ayon, J.Massoulié and S.Bon, in preparation). The present study shows that they are also involved in the degradation process: first, the more degraded proteins were stabilized by mutation of W24 and F28 to glycines or serines, and degradation was essentially abolished by mutation of five residues (W10, F14, W17, W24 and F28), since the corresponding mutants were either secreted, or accumulated in the cells when they contained a C-terminal KDEL motif; secondly, the dimerization-defective mutant rAChE*T and hAPT were almost entirely degraded when expressed without QN, but could be secreted as PRAD-linked tetramers in which all aromatic residues are oriented towards the inside of a compact barrel of helices, as recently shown by crystallographic determination of the three-dimensional structure of a PRAD–WAT complex (M.Harel, S.Bon, W.Q.Liu, H.Dvir, S.Belbeoc’h, M.Vidal, C.Garbay, I.Silman, J.Massoulié and J.Sussman, in preparation).
When the wild-type rat enzyme was co-expressed with QN, PRAD-linked tetramers represented the major secreted component, but the level of secretion was not markedly increased. This reflects a complex balance between degradation, heteromeric association with QN, which both occur in the ER, and secretion of AChET monomers and dimers. It is important to note that in vertebrate tissues, AChET subunits are mostly present as hetero-oligomers with the PRAD-containing anchoring proteins, ColQ and PRiMA, so that unassembled subunits are probably degraded by the ERAD mechanism analyzed in this study.
AChE and its C-terminal T peptide, or WAT domain, constitute an extremely favorable experimental model for the study of protein traffic in the secretory pathway, for several reasons. First, it is convenient to follow the fate of the enzyme and its oligomerization state, with a sensitive and specific assay. Secondly, we can compare a range of homologous enzymes which differ in their oligomerization capacities. In the present study, we have shown that the 40 residue T peptide behaves as an ERAD enhancer whose efficiency depends on the nature of the catalytic domain with which it is associated, and that its aromatic residues participate in the targeting of the proteins towards degradation. The features of the T peptide and of its combination with the glycosylated catalytic domain which are recognized by the quality control mechanism of the ER remain to be determined. In the present experiments, engagement of this mechanism did not require misfolding and did not depend on small peptide determinants, but rather on combined elements characterizing the whole multidomain AChET protein.
Materials and methods
Chemical and reagents
Clasto-lactacystin-β-lactone, chloroquine and BFA were purchased from Sigma and used at a final concentration of 50 μM, 100 μM and 4.2 μg/ml, respectively. The proteasome inhibitor MG-132 was purchased from MolBiol (Tebu) and used at a final concentration of 50 μM. The ER mannosidase I inhibitor kifunensine was purchased from Toronto Research Chemicals and used at a final concentration of 4 μg/ml. Stock solutions were prepared in dimethylsulfoxide (DMSO) (clasto- lactacystin-β-lactone, MG-132), methanol (BFA) or hot water (chloroquine, kifunensine).
Site-directed mutagenesis and transfection in COS cells
All constructs were expressed in the pEF-BOS vector (Legay et al., 1993) or the pCDNA3 vector (InVitrogen). Mutagenesis was performed by the method of Kunkel et al. (1987). For construction of nAChET, the C-terminal T peptide of rat AChE was added by PCR at the C-terminus of N.brasiliensis AChEB (Hussein et al., 1999) before the stop codon. For construction of hAPT, the T peptide of rat AChE was added by PCR at the C-terminus of hAP in place of the hydrophobic sequence responsible for the GPI anchoring of the wild-type enzyme (Hawrylak and Stinson, 1988). COS cells were transfected by the DEAE–dextran method, as previously described (Bon et al., 1997), using 3 μg of DNA encoding AChE or hAP per 100 mm dish. Co-expression with QN/stop551 (Bon et al., 1997) was obtained by adding 5 μg of DNA encoding this protein; to obtain an equivalent level of protein expression in the corresponding controls, the different constructs were transfected with the same amount of DNA encoding a defective QN/stop551, from which the PRAD motif was deleted. In the latter case, there was no evidence for the production of hetero-oligomers, indicating that COS cells do not express any detectable endogenous PRAD-containing protein. After transfection, COS cells were incubated for 2–3 days at 37°C, in a medium containing 10% Nuserum (Inotech, Dottikon, Switzerland), which had been pre-treated with 10–5 M soman to inactivate serum cholinesterases.
Cell extracts
The cells were extracted at 20°C with TMg buffer (1% Triton X-100, 50 mM Tris–HCl pH 7.5, 10 mM MgCl2) or with BMg buffer, containing 1% Brij-97 instead of Triton X-100, and then centrifuged at 13 000 r.p.m. for 30 min. Media were also centrifuged at 13 000 r.p.m. for 30 min before analysis.
AChE and AP activity assays
AChE activity was determined by the colorimetric method of Ellman et al. (1961) at room temperature as described previously. AP activity was determined by using a colorimetric method: 200 μl of assay medium (1 mM p-nitrophenyl phosphate in 100 mM Tris–HCl pH 8) were added to 10 μl of enzymes samples. The reaction was monitored at 414 nm with a Labsystems Multiskan RC automatic plate reader (Helsinki, Finland); the optical density was recorded at 20 s intervals over a period of 5 min.
Metabolic labeling
Two days after transfection, COS cells were pre-incubated for 45 min in Dulbecco’s modified Eagle medium (Gibco-BRL) without methionine and labeled for 30 min with 50–100 mCi/ml [35S]methionine (Amersham Pharmacia Biotech). After labeling, the cells were rinsed with phosphate-buffered saline (PBS) and chased in medium containing 10% Nuserum; at various times, the medium was collected and the cells were extracted. The cells extracts (150 µl) and the media (1 ml) were incubated for 1 h with 60 µl of a suspension of protein G–Sepharose 4B beads (Sigma) to eliminate non-specifically bound radioactive material. After centrifugation at 13 000 r.p.m. for 5 min, the supernatants were incubated overnight at 4°C with a 1:500 dilution of the rabbit polyclonal anti-rat AChE antiserum (A63) (Marsh et al., 1984) under rotary agitation, and then mixed with 100 µl of a suspension of protein G–Sepharose 4B beads (Sigma) and incubated for 1 h at 4°C. The beads were rinsed three times with extraction buffer. The immunoprecipitated AChE sample was analyzed by electrophoresis under reducing and denaturating conditions (SDS–PAGE), and the distribution of radioactive protein was determined with a Fuji image analyzer. All inhibitors were added 1 h before metabolic labeling and cells were maintained in the presence of the compound throughout the experiment.
Sedimentation and electrophoresis analyses
Centrifugation was performed in 5–20% sucrose gradients (50 mM Tris–HCl pH 7.5, 50 mM MgCl2, in the presence of either 1% Brij-96 or 0.2% Triton X-100) in a Beckman SW41 rotor, at 36 000 r.p.m., for 16 h at 6°C. The gradients contained Escherichia coli β-galactosidase (16S) and alkaline phosphatase (6.1S) as internal sedimentation standards. Electrophoresis in non-denaturating polyacrylamide gels was performed as described previously (Duval et al., 1992), and AChE activity was revealed by the histochemical method of Karnovsky and Roots (1964).
Acknowledgments
Acknowledgements
This work was supported by grants form the Centre National de la Recherche Scientifique, the Association Française contre les Myopathies, the Direction des Forces et de la Prospective, and the European Community; S.B. was the recipient of a PhD grant from the French Ministry of Research.
References
- Blong R.M., Bedows,E. and Lockridge,O. (1997) Tetramerization domain of human butyrylcholinesterase is at the C-terminus. Biochem. J., 327, 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon S. and Massoulié,J. (1997) Quaternary associations of acetyl cholinesterase. I. Oligomeric associations of T subunits with and without the amino-terminal domain of the collagen tail. J. Biol. Chem., 272, 3007–3015. [DOI] [PubMed] [Google Scholar]
- Bon S., Coussen,F. and Massoulié,J. (1997) Quaternary associations of acetylcholinesterase. II. The polyproline attachment domain of the collagen tail. J. Biol. Chem., 272, 3016–3021. [DOI] [PubMed] [Google Scholar]
- Brodsky J.L. and McCracken,A.A. (1999) ER protein quality control and proteasome-mediated protein degradation. Semin. Cell Dev. Biol., 5, 507–513. [DOI] [PubMed] [Google Scholar]
- Duval N., Massoulié,J. and Bon,S. (1992) H and T subunits of acetylcholinesterase from Torpedo, expressed in COS cells, generate all types of globular forms. J. Cell Biol., 118, 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgard L. and Helenius,A. (2003) Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol., 3, 181–191. [DOI] [PubMed] [Google Scholar]
- Ellman G.L., Courtney,K.D., Andres,V. and Featherstone,R.M. (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol., 7, 88–95. [DOI] [PubMed] [Google Scholar]
- Fagioli C. and Sitia,R. (2001) Glycoprotein quality control in the endoplasmic reticulum. Mannose trimming by endoplasmic reticulum mannosidase I times the proteasomal degradation of unassembled immunoglobulin subunits. J. Biol. Chem., 16, 12885–12892. [DOI] [PubMed] [Google Scholar]
- Feng G., Krejci,E., Molgo,J., Cunningham,J.M., Massoulié,J. and Sanes,J.R. (1999) Genetic analysis of collagen Q: roles in acetylcholinesterase and butyrylcholinesterase assembly and in synaptic structure and function. J. Cell Biol., 144, 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H.L., Moreno,R.D. and Inestrosa,N.C. (1996) Tetrameric (G4) acetylcholinesterase: structure, localization and physiological regulation. J. Neurochem., 66, 1335–1346. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Oda,K., Yokota,S., Takatsuki,A. and Ikehara,Y. (1988) Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem., 263, 18545–18552. [PubMed] [Google Scholar]
- Giles K. (1997) Interactions underlying subunit association in cholinesterases. Protein Eng., 10, 677–685. [DOI] [PubMed] [Google Scholar]
- Hawrylak K. and Stinson,R.A. (1988) The solubilization of tetrameric alkaline phosphatase from human liver and its conversion into various forms by phosphatidylinositol phospholipase C or proteolysis. J. Biol. Chem., 263, 14368–14373. [PubMed] [Google Scholar]
- Hussein A.S., Chacon,M.R., Smith,A.M., Tosado-Acevedo,R. and Selkirk,M.E. (1999) Cloning, expression and properties of a nonneuronal secreted acetylcholinesterase from the parasitic nematode Nippostrongylus brasiliensis. J. Biol. Chem., 274, 9312–9319. [DOI] [PubMed] [Google Scholar]
- Karnovsky M.J. and Roots,L. (1964) A direct-coloring thiocholine method for cholinesterases. J. Histochem. Cytochem., 12, 219–222. [DOI] [PubMed] [Google Scholar]
- Klausner R.D. and Sitia,R. (1990) Protein degradation in the endoplasmic reticulum. Cell, 62, 611–614. [DOI] [PubMed] [Google Scholar]
- Kunkel T.A., Roberts,J.D. and Zakour,R.A. (1987) Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol., 154, 367–382. [DOI] [PubMed] [Google Scholar]
- Legay C., Bon,S., Vernier,P., Coussen,F. and Massoulié,J. (1993) Cloning and expression of a rat acetylcholinesterase subunit: generation of multiple molecular forms, complementarity with a Torpedo collagenic subunit. J. Neurochem., 60, 337–346. [DOI] [PubMed] [Google Scholar]
- Marsh D., Grassi,J., Vigny,M. and Massoulié,J. (1984) An immunological study of rat acetylcholinesterase: comparison with acetylcholinesterases from other vertebrates. J. Neurochem., 43, 204–213. [DOI] [PubMed] [Google Scholar]
- Massoulié J. (2002) The origin of the molecular diversity and functional anchoring of cholinesterases. NeuroSignals, 11, 130–143. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Pezzementi,L., Bon,S., Krejci,E. and Vallette,F.M. (1993) Molecular and cellular biology of cholinesterases. Prog. Neurobiol., 41, 31–91. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Misumi,Y., Miki,K., Takatsuki,A., Tamura,G. and Ikehara,Y. (1986) Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J. Biol. Chem., 261, 11398–11403. [PubMed] [Google Scholar]
- Morel N., Leroy,J., Ayon,A., Massoulié,J. and Bon,S. (2001) Acetylcholinesterase H and T dimers are associated through the same contact; mutations at this interface interfere with the C-terminal T peptide, inducing degradation rather than secretion. J. Biol. Chem., 276, 37379–37389. [DOI] [PubMed] [Google Scholar]
- Perrier A.L., Massoulié,J. and Krejci,E. (2002) PRiMA, the membrane anchor of acetylcholinesterase in brain. Neuron, 33, 275–285. [DOI] [PubMed] [Google Scholar]
- Simon S., Krejci,E. and Massoulié,J. (1998) A four-to-one association between peptide motifs: four C-terminal domains from cholinesterase assemble with one proline-rich attachment domain (PRAD) in the secretory pathway. EMBO J., 17, 6178–6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R., Neuberger,M., Alberini,C., Bet,P., Fra,A., Valetti,C., Williams,G. and Milstein,C. (1990) Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell, 60, 781–790. [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Brostrom,C., Koide,T. and Arvan,P. (2000) Endoplasmic reticulum (ER)-associated degradation of misfolded N-linked glycoproteins is suppressed upon inhibition of ER mannosidase I. J. Biol. Chem., 52, 40757–40764. [DOI] [PubMed] [Google Scholar]
- Velan B. et al. (1991) The effect of elimination of intersubunit disulfide bonds on the activity, assembly and secretion of recombinant human acetylcholinesterase. Expression of acetylcholinesterase Cys-580→Ala mutant. J. Biol. Chem., 266, 23977–23984. [PubMed] [Google Scholar]
- Yamamoto K., Fujii,R., Toyofuku,Y., Saito,T., Koseki,H., Hsu,V.W. and Aoe,T. (2001) The KDEL receptor mediates a retrieval mechanism that contributes to quality control at the endoplasmic reticulum. EMBO J., 20, 3082–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanusso G., Petersen,R.B., Jin,T., Jing,Y., Kanoush,R., Ferrari,S., Gambetti,P. and Singh,N. (1999) Proteasomal degradation and N-terminal protease resistance of the codon 145 mutant prion protein. J. Biol. Chem., 274, 23396–23404. [DOI] [PubMed] [Google Scholar]