Abstract
Proteins of the annexin family are believed to be involved in membrane-related processes, but their precise functions remain unclear. Here, we have made use of several experimental approaches, including pathological conditions, RNA interference and in vitro transport assays, to study the function of annexin II in the endocytic pathway. We find that annexin II is required for the biogenesis of multivesicular transport intermediates destined for late endosomes, by regulating budding from early endosomes—but not the membrane invagination process. Hence, the protein appears to be a necessary component of the machinery controlling endosomal membrane dynamics and multivesicular endosome biogenesis. We also find that annexin II interacts with cholesterol and that its subcellular distribution is modulated by the subcellular distribution of cholesterol, including in cells from patients with the cholesterol-storage disorder Niemann-Pick C. We conclude that annexin II forms cholesterol-containing platforms on early endosomal membranes, and that these platforms regulate the onset of the degradation pathway in animal cells.
Keywords: cholesterol/endosomal carrier vesicle (ECV)/membrane domain/multivesicular body (MVB)/Niemann-Pick type C/RNA interference (RNAi)
Introduction
Endocytosed molecules, including solutes and cell surface components, are delivered to early endosomes, which serve as major sorting stations in the pathway (Gruenberg, 2001). While some receptors are rapidly recycled back to the cell surface, downregulated receptors for growth factors and hormones are transported to late endosomes and lysosomes for degradation. Transport along the degradation pathway occurs via large transport intermediates (0.4–0.5 µm diameter) that accumulate internal membranes and thus exhibit a characteristic multivesicular appearance (here referred to as endosomal carrier vesicle/multivesicular body or ECV/MVB). An endosomal COP complex and ARF1 were proposed to play a role in early-to-late endosome transport, ECV/MVB formation and protein sorting (Whitney et al., 1995; Aniento et al., 1996; Daro et al., 1997; Gu et al., 1997; Piguet et al., 1999; Gu and Gruenberg, 2000). Recent studies also highlight the role of the ESCRT complexes in yeast (Katzmann et al., 2002) and Hrs together with other class E proteins, and presumably a bilayered clathrin coat in mammalian cells (Raiborg et al., 2001; Bishop et al., 2002; Raiborg et al., 2002; Sachse et al., 2002) in the sorting of ubiquitinated proteins into the multivesicular body. However, the mechanisms that regulate the biogenesis of ECV/MVBs have remained elusive.
Annexins form a family of 13 mammalian proteins, some being tissue specific. They are found both in the cytoplasm and bound to membranes, suggesting that they play a role in membrane-related processes, including trafficking (Gerke and Moss, 2002). Annexin XIIIs play a role in polarized transport in epithelial cells (Fiedler et al., 1995; Lecat et al., 2000), annexin I is phosphorylated by the EGF-receptor (Futter et al., 1993) and annexin II may be involved in secretion and endocytosis (Sarafian et al., 1991; Emans et al., 1993; Harder and Gerke, 1993). All contain the endonexin fold, a Ca2+-binding motif, repeated four or eight times, and share the in vitro property to interact with negatively charged phospholipids in a Ca2+-dependent manner. However, annexin II association to early endosomes is Ca2+-independent, but highly sensitive to cholesterol-sequestering agents, indicating that the protein is associated to endosomes, but perhaps not to the plasma membrane (Babiychuk and Draeger, 2000), via an unconventional mechanism (Emans et al., 1993; Harder et al., 1997). Annexin II is also found in most cells bound to the p11 light chain, a member of the S100 protein family, as a heterotetrameric (annexin II)2(p11)2 complex (Johnsson et al., 1988); but p11 is not required for endosome association (Jost et al., 1997; Konig and Gerke, 2000). Similarly, annexin IV, VI and XIIIa and b bind membranes in a Ca2+-independent manner (Lafont et al., 1998; Turpin et al., 1998; Sable and Riches, 1999; Lecat et al., 2000). Annexin II is presumably involved in endosomal membrane dynamics (Emans et al., 1993; Harder and Gerke, 1993), but the role of the protein is not known, much like for other family members.
In this paper, we studied the biochemical properties of annexin II in vitro, and the role of the protein in vivo. We find that annexin II interacts with cholesterol and that its subcellular distribution is modulated by the cholesterol distribution. Our in vivo and in vitro data also show that annexin II regulates the biogenesis of transport intermediates destined for late endosomes. We conclude that annexin II forms cholesterol-containing platforms on early endosomes, and that these platforms play a pivotal role in regulating the onset of the degradation pathway in animal cells.
Results
Binding of annexin II to cholesterol-containing membranes
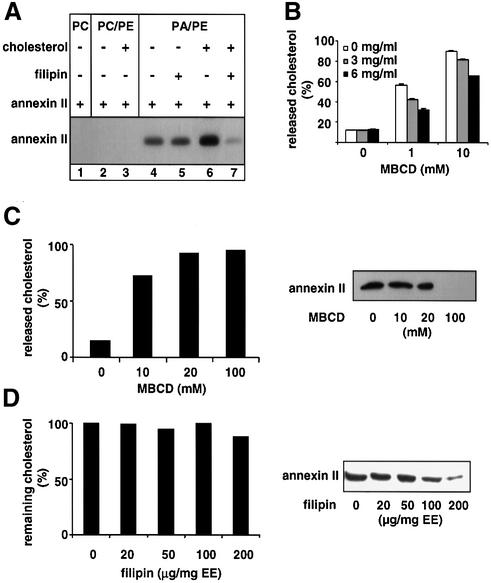
We had found that annexin II is very tightly membrane associated and that this association is Ca2+-independent, but highly sensitive to the cholesterol-clustering agent filipin (Emans et al., 1993; Harder et al., 1997). We thus investigated the mechanism of annexin II membrane association using protein-free liposomes. The properties of monomeric and heterotetrameric annexin II were similar in the in vitro experiments described below, but, unless indicated, data with the natural heterotetrameric form are shown. Also, for the sake of clarity, annexin II in the text refers to the heterotetramer, unless stated otherwise. We found that annexin II becomes associated to liposomes containing the negatively charged phospholipid phosphatidic acid (PA), even in the presence of 5 mM EGTA (Figure 1A, lane 4; free Ca2+ concentration <10 nM), in agreement with previous studies (Powell and Glenney, 1987). In contrast, the protein did not bind to liposomes containing only phosphatidylcholine (PC; Figure 1A, lane 1) or PC and phosphatidyl ethanolamine (PE; Figure 1A, lane 2), suggesting that annexin II can interact with the polar headgroups of PA in vitro, in agreement with previous studies (Blackwood and Ernst, 1990). However, such interactions cannot account for annexin II subcellular distribution in vivo, since the protein is restricted to a limited subset of intracellular membranes.
Fig. 1. Binding to liposomes and early endosomes. (A) Liposomes of the following composition were prepared: PC only, PC/PE (1:1), PC/PE/cholesterol (2:2:1), PA/PE (1:1), PA/PE/cholesterol (2:2:1), and incubated as indicated with 1 µg of purified heterotetrameric annexin II and 5 mM EGTA for 30 min at room temperature. Lane 7 shows liposomes that were subsequently re-incubated with filipin for 30 min at 4°C. Liposomes were then collected by centrifugation and analyzed by SDS–PAGE (6 µmoles lipid/lane) and western blotting using the HH7 antibody against annexin II. (B) PA/PE/[1α-,2α(n)-3H] cholesterol (2:2:1) liposomes were incubated with the indicated annexin II concentrations (as in A), collected by floatation on gradients and reincubated at 4°C for 30 min with MBCD. Liposomes were collected as above and analyzed by liquid scintillation counting. Data are expressed as a percentage of the total cholesterol in each experiment (liposome and released). (C and D) Early endosomes were prepared from BHK cells labeled to equilibrium with [3H]cholesterol, incubated as in (A) with MBCD (C) or filipin [D; µg/mg early endosomal (EE) protein], and collected by centrifugation. Cholesterol release was quantified as in (B), and annexin analyzed as in (A); each lane contained 30 µg protein. Each experiment was repeated more than three times and representative experiments are shown.
Annexin II binding was significantly increased when liposomes containing PA (Figure 1A, lane 6), but not PE (Figure 1A, lane 3), were supplemented with cholesterol in the absence of Ca2+. These interactions were specific, since non-relevant proteins, which can interact with long aliphatic chains, the guanine nucleotide dissociation inhibitor GDI and bovine serum albumin, did not bind PA-liposomes containing cholesterol (data not shown). In addition, annexin bound to cholesterol-containing liposomes (Figure 1A, lane 7), or to endosomes (Figure 1D; Harder et al., 1997), was released by the cholesterol-clustering agent filipin. In contrast, filipin had no effect on the cholesterol-independent binding of annexin II to PA-liposomes (Figure 1A, lane 5), demonstrating that the effects of the drug on annexin II binding were specific.
To our surprise, however, annexin II binding to cholesterol-containing liposomes (data not shown) or endosomes (Figure 1C) was only marginally affected by cholesterol depletion with the cholesterol-solubilizing agent methyl-β-cyclodextrin (MBCD), except at concentrations so high (100 mM) that effects were no longer specific and simply due to membrane solubilization (data not shown). In contrast, filipin did not release cholesterol (Figure 1D), as expected, but efficiently released annexin II from liposomes (Figure 1A) and endosomes (Figure 1D). Cholesterol molecules involved in annexin II binding thus appear to be part of a pool resistant to MBCD, but not filipin. Annexin II on the surface of the bilayer might interact physically with a limited number of cholesterol molecules, forming a protective shield against the action of MBCD in solution, but not against the clustering effect of hydrophobic filipin within the bilayer. If so, annexin II itself should partially protect cholesterol from extraction by MBCD. Indeed, increasing amounts of annexin II inhibited cholesterol extraction from liposomes (Figure 1B). Altogether, our data show that the mechanism of annexin II membrane association can be reconstituted using artificial bilayers. They also show that cholesterol is both necessary and sufficient for this process, provided that PA is also present, and strongly suggest that annexin II interacts physically with cholesterol.
Annexin II localization depends on the subcellular distribution of cholesterol
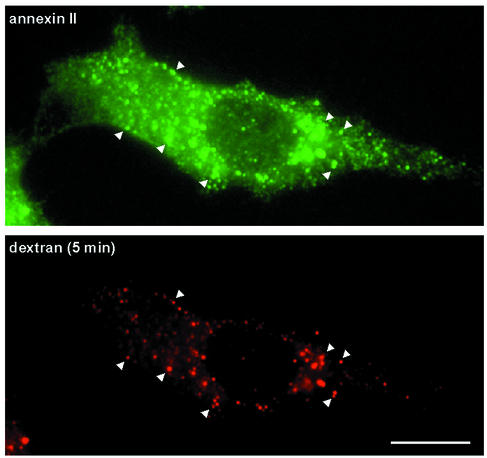
An analysis by immunofluorescence showed that annexin II was present in the cytoplasm, at the plasma membrane and on early endosomes labeled with fluorescent dextran endocytosed for 5 min at 37°C (Figure 2). This distribution agrees well with previous observations (Harder and Gerke, 1993; Jost et al., 1997), including our fractionation and electron microscopy data showing that the protein is associated to early endosomes (Emans et al., 1993), but absent from recycling endosomes purified from MDCK cells (Gagescu et al., 2000). Since the protein binds cholesterol-containing bilayers (Figure 1), we wondered whether its subcellular distribution was influenced by membrane cholesterol.
Fig. 2. Annexin II in early endosomes. BHK cells were incubated for 5 min at 37°C with rhodamine-dextran to label early endosomes, permeabilized and fixed as described in Materials and methods, and then labeled with the monoclonal HH7 antibody against annexin II. Bar: 5 µm.
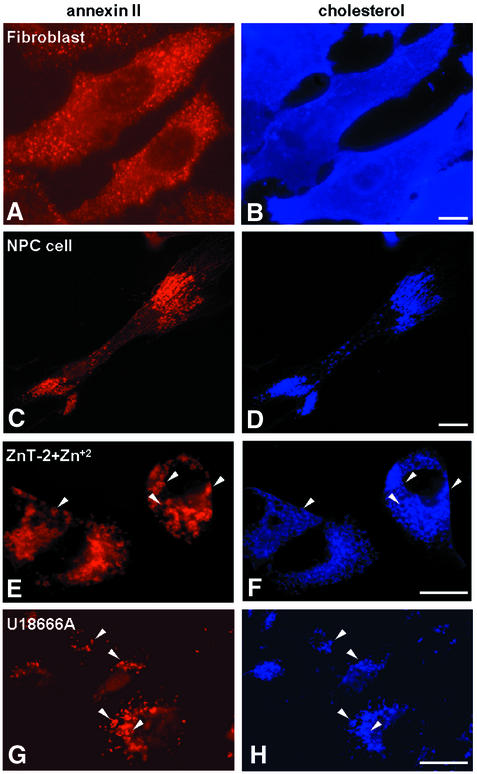
Strikingly, we observed that annexin II distribution was dramatically altered in Niemann-Pick C fibroblasts (Figure 3C), a cholesterol-storage disease accompanied by dramatic cholesterol accumulation in late endosomes and lysosomes (Liscum and Klansek, 1998; Kobayashi et al., 1999). In contrast to healthy fibroblasts (Figure 3A), the protein was largely relocalized to cholesterol-containing late endosomes in NPC fibroblasts (Figure 3C–D). Similarly, an NPC-like cholesterol accumulation in late endosomes can be induced in a non-pathological genetic background by the hydrophobic amine U18666A (Liscum and Faust, 1989; Kobayashi et al., 1999) or by the addition of Zn+2 to cells expressing the Zn transporter ZnT-2 (Kobayashi et al., 1999). Both treatments caused annexin II redistribution to cholesterol-laden late endosomes (Figure 3E–H), but did not affect the subcellular distribution of early endosomal markers, including the rab5 effector EEA1 (Stenmark et al., 1996), the transferrin receptor or rhodamine-dextran endocytosed for 5 min at 37°C (data not shown; see below).
Fig. 3. Redistribution to late endosomes loaded with cholesterol. (A and B) Human skin fibroblasts from healthy individuals were labeled with filipin or anti-annexin II antibodies Annexin II was labeled with rhodamine-labeled secondary antibodies and filipin was directly visualized in the UV light range. (C and D) Human NPC skin fibroblasts double-labeled with filipin and anti-annexin II antibodies were analyzed as in (A and B). (E and F) Cholesterol accumulation was induced in BHK cells expressing the Zn2+ transporter ZnT-2 by incubation for 72 h with 0.1 mM ZnCl2 (Palmiter et al., 1996; Kobayashi et al., 1999). Cells were then processed as in (A–D). (G and H) BHK cells were incubated for 16 h with 3 µg/ml U18666A, and processed as above. Bars: 5 µm.
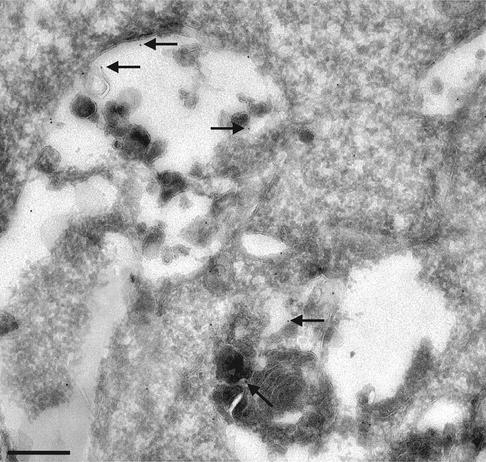
An electron microscopy analysis after U18666A treatment showed that annexin II was no longer present on early endosomes, but associated to large and swollen multivesicular elements (Figure 4) with the very characteristic, altered ultrastructure of cholesterol-laden late endosomes (Kobayashi et al., 1999, 2000; Galve-de Rochemonteix et al., 2000). Annexin II was also detected within these swollen multivesicular endosomes, presumably because the protein had been engulfed within membrane invaginations containing cholesterol. Our data thus demonstrate that genetically or experimentally induced redistribution of cholesterol to late endosomes causes a dramatic redistribution of annexin II to the same compartment, suggesting that annexin II subcellular localization depends on the distribution of cholesterol.
Fig. 4. Ultrastructural analysis of annexin II distribution in late endosomes loaded with cholesterol. BHK cells were incubated with U18666A as above, and processed for cryo-sectioning. Frozen sections were labeled with the HH7 anti-annexin II antibody followed by 10 nm goat anti-mouse gold particles (arrows). Bar: 0.1 µm.
Transport from early to late endosomes after cholesterol accumulation depends on annexin II
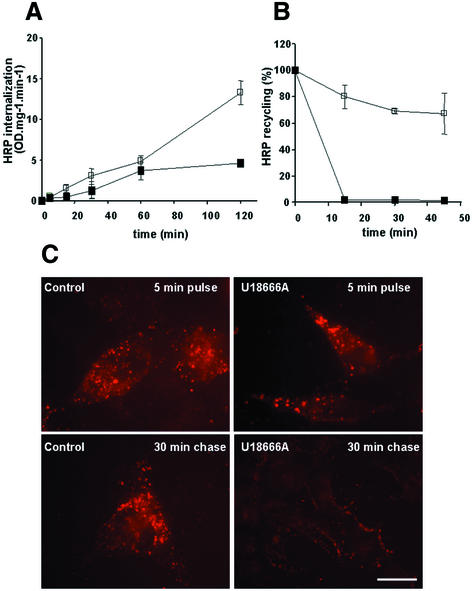
We then investigated whether the redistribution of cholesterol and annexin II to late endosomes interfered with endocytic membrane transport. Endocytosis of the fluid-phase marker horseradish peroxidase (HRP) over short time periods was only slightly reduced by U18666A, when compared with untreated controls (Figure 5A). However, HRP then reached a plateau in U1866A-treated cells, but not in control cells, and failed to accumulate intracellularly (Figure 5A), suggesting that the tracer was recycled to the medium, rather than being transported to late endosomes and lysosomes. Indeed, amounts of pre-internalized HRP regurgitated into the medium were significantly increased in drug-treated cells, when compared with controls (Figure 5B). In agreement with these observations, the distribution of rhodamine-dextran internalized into early endosomes after 5 min at 37°C was similar in control and drug-treated cells. In control cells, however, the marker was then chased to perinuclear vesicles, presumably late endosomes, after subsequent incubation in marker-free medium. In contrast, rhodamine-dextran was no longer detected intracellularly in drug-treated cells (Figure 5C). Hence, transport from early to late endosomes was inhibited after accumulation of cholesterol and annexin II in late endosomes, while internalization and recycling did not appear to be significantly affected.
Fig. 5. Internalization, recycling and intracellular accumulation. (A) BHK cells pretreated (closed symbol) or not (open symbol) with U18666A, as in Figure 3G and H, were incubated with 3 mg/ml HRP at 37°C, for the indicated time periods. Amounts of HRP accumulated in cells were quantified (OD U/min/mg cellular protein) at each time point. (B) BHK cells treated as in (A) were incubated for 5 min at 37°C with 0.5 mg/ml HRP, washed and then reincubated for the indicated time in marker-free medium. At each time point, HRP was quantified in cells and in the medium. The figure shows HRP that remained cell associated, expressed as a percentage of the total (cell associated and regurgitated) HRP. Each panel shows the mean of three series of experiments. (C) BHK cells treated as in (A) were incubated for 5 min at 37°C with 3 mg/ml rhodamine-dextran (pulse), to label early endosomes, or subsequently reincubated for 30 min in the absence of the marker (chase). Cells were then processed for fluorescence microscopy. Bar: 5 µm.
Complete relocalization of annexin II to late endosomes required long (>16 h) incubations with U18666A. Such treatments did not cause irreversible changes, as cells fully recovered after drug wash-out (data not shown). After shorter incubations with the drug (6–12 h), NPC-like cholesterol accumulation already occurred in late endosomes (Kobayashi et al., 1999), but annexin II was only partially redistributed (data not shown), and transport was not inhibited (Kobayashi et al., 2000; Lebrand et al., 2002). These data thus show that the drug did not directly interfere with components of the transport machinery. They might also suggest that transport is inhibited when amounts of annexin II become limiting. We therefore overexpressed a GFP-tagged version of annexin II in U18666A-treated cells, which accumulated in the cytosol but was also properly targeted to early endosomes containing the Rab5 effector EEA1 (see Supplementary data, available at The EMBO Journal Online).
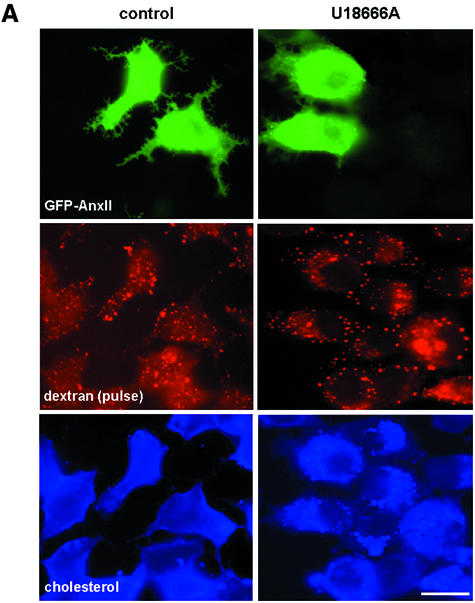
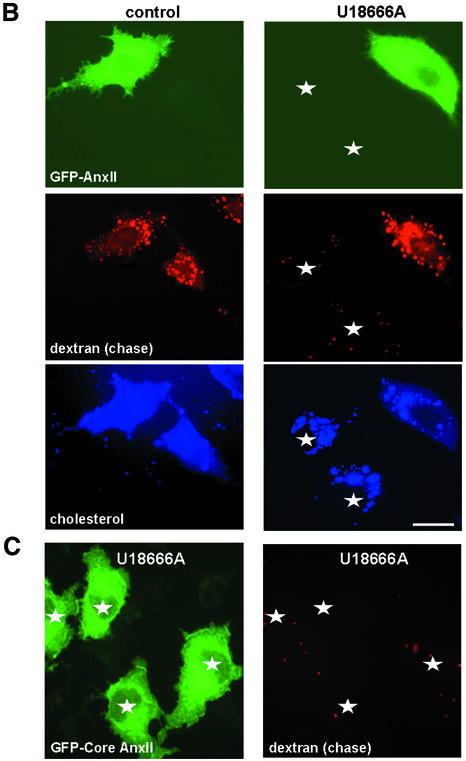
GFP-annexin II overexpression did not prevent cholesterol accumulation in drug-treated cells (Figure 6), indicating that the protein is not directly involved in cholesterol transport. A pulse of rhodamine-dextran internalized for 5 min at 37°C into early endosomes showed the same distribution in U18666A-treated and control cells, containing or not overexpressed GFP–annexin II (Figure 6A). Strikingly, however, rhodamine-dextran was transported to cholesterol-laden late endosomes after a 45 min chase, much like in controls, but only in GFP–annexin II-expressing cells and not in untransfected cells (Figure 6B). In marked contrast, a GFP-tagged annexin mutant lacking the 23 first N-terminal amino acids, which are required for endosomal targeting (Jost et al., 1997), failed to rescue transport to late endosomes (Figure 6C). Neither did the overexpression of the annexin II light-chain p11 (see Supplementary data), consistently with the findings that p11 is not required for annexin binding to endosome in vitro (data not shown) or in vivo (Jost et al., 1997; Konig and Gerke, 2000). These data show that annexin II overexpression could restore transport from early to late endosomes.
Fig. 6. Transport to late endosomes after cholesterol accumulation. (A and B) BHK cells transfected with a plasmid encoding for GFP–annexin II (GFP–AnxII) were pretreated or not with U18666A, and incubated for 5 min at 37°C with 3 mg/ml rhodamine-dextran (pulse), to label early endosomes (A), or reincubated for 40 min in the absence of the marker to label late endosomes (chase) (B). Cells were then processed as in Figure 3 and analyzed by triple-channel fluorescence. Stars show non-transfected cells. (C) Cells were treated as in (B), except that they were transfected with the GFP-tagged core domain of annexin II; the distribution of dextran and the GFP–core is shown. Bar: 5 µm.
Down-modulation of annexin II expression inhibits transport
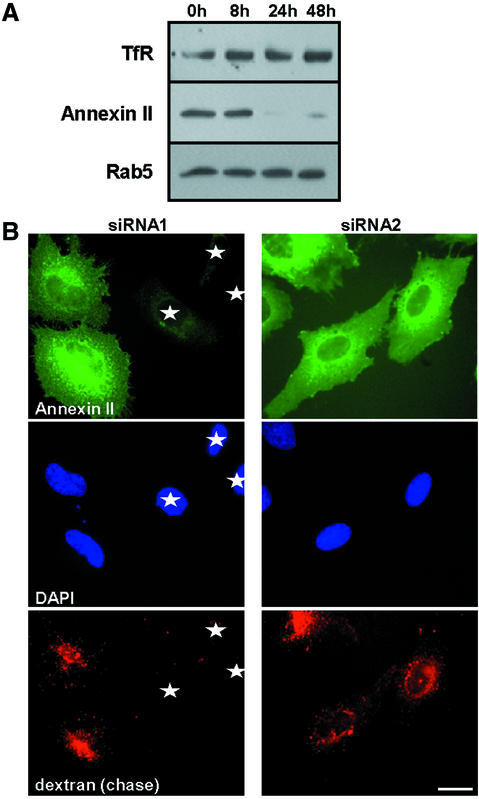
We then investigated whether annexin II had a direct role to play in early to late endosome transport in the absence of cholesterol accumulation. To this end, two siRNAs oligonucleotides complementary to two regions of human annexin II N-terminus were transfected into HeLa cells. Amounts of annexin II were already significantly reduced (>90%) by siRNA1 after 24 h (Figure 7A), but not by siRNA2 or control siRNAs complementary to other sequences (data not shown). Down-modulation of annexin II expression with siRNA1 was specific, since annexin II expression in the hamster BHK cell line was not affected (data not shown). Moreover, amounts of the other early endosomal proteins Rab5 and the transferrin receptor remained unchanged (Figure 7A). Consistently, annexin II could no longer be detected by immunofluorescence in cells transfected with siRNA1, while the protein was not affected by siRNA2 (Figure 7B). Moreover, the distribution of early and late endosomal markers remained unchanged in siRNA1-transfected cells (data not shown). These data thus show that annexin II expression was selectively down modulated by siRNA1 transfection.
Fig. 7. Annexin II down-modulation inhibits solute transport to late endosomes. (A) HeLa cells were transfected for the indicated time with siRNA1, and analyzed by SDS–PAGE and western blotting, using antibodies against annexin II, the transferrin receptor (TfR) and Rab5. (B) HeLa cells were transfected for 24 h with siRNA1 or siRNA2; 3 mg/ml rhodamine-dextran was then endocytosed for 5 min at 37°C and chased for 40 min. Cells were then processed for fluorescence microscopy. Stars show cells containing undetectable amounts of both annexin II and dextran. The extent of annexin II down-regulation was compatible with (A), but panels show (rare) examples of cells still expressing annexin II for comparison. Annexin II association to endosomes is not clearly visible with the fixation/permeabilization protocol used to detect endocytosed dextran—in contrast to Figure 2. Bar: 5 µm.
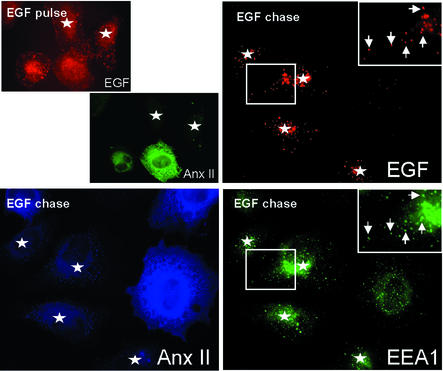
We then followed the fate of transferrin, EGF, and rhodamine-dextran after annexin II down-modulation. Both amounts and distribution of internalized transferrin did not appear to be affected in siRNA1-transfected cells, when compared with untransfected cells (Figure 8), indicating that the recycling pathway was not significantly affected by annexin II down-modulation. Similarly, both rhodamine-dextran (data not shown) and EGF (Figure 8) reached early endosomes after 5 min at 37°C in cells lacking annexin II, like in control cells. After chase, however, the rhodamine-dextran pulse failed to reach late endosomes and was no longer detected intracellularly in cells lacking annexin II (Figure 7B), much like in U18666A-treated cells (Figure 5C), while transport to perinuclear late endosomes was unaffected in untransfected cells or siRNA2-transfected cells (Figure 7B). Conversely, EGF was no longer detected in untransfected cells expressing annexin II after chase, as expected since EGF is degraded in lysosomes, but the ligand accumulated intracellularly within early endosomes containing EEA1, in the cells lacking annexin II (Figure 8). Altogether, these observations show that annexin II regulates transport from early to late endosomes.
Fig. 8. EGF-receptor down-regulation. HeLa cells were transfected for 24 h with siRNA1 (Figure 7), and incubated for a 5 min pulse at 37°C with 0.4 µg/ml biotin–EGF and phycoerythrin-streptavidin, followed or not by a 60 min chase without the marker. Cells were then processed for immunofluorescence microscopy using anti-EEA1 antibodies and FITC-labeled secondary antibodies (EGF chase), as well as the HH7 anti-annexin II antibody and AMCA-labeled secondary antibodies, and analyzed by triple-channel immunofluorescence. As in Figure 7, annexin II association to endosomes is not clearly visible with this fixation/permeabilization protocol. Stars show cells containing undetectable amounts of annexin II. Bar: 5 µm.
In vitro fusion of early endosomes
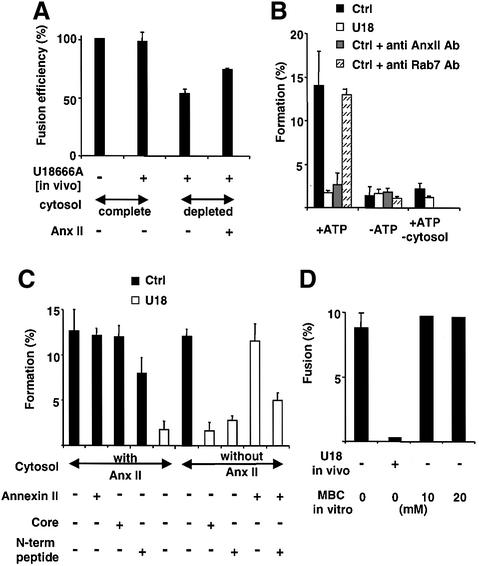
Since annexin II is present on early endosomes and required for early to late endosome transport, we reasoned that the protein might be involved in early endosome dynamics. To this end, we used a well-established in vitro fusion assay (Gruenberg et al., 1989; Gorvel et al., 1991; Aniento et al., 1993). To prepare early endosomes depleted of annexin II, we used early endosomes prepared from cells treated with U18666A treatment in vivo, since the protein is very tightly membrane associated and not easily released in vitro, unless perturbating agents are used (Figure 1; Emans et al., 1993; Harder et al., 1997). When tested in the fusion assay, endosomes from U18666A-treated cells exhibited the same fusion activity as control endosomes (Figure 9A), demonstrating that the in vivo treatment with U18666A did not affect the endosome docking/fusion machinery. Since annexin II is also present in the cytosol, annexin II-depleted cytosol was then prepared and tested in the same assay. When using both membranes and cytosol depleted of annexin II, fusion was reduced to 50% of the control, and this inhibition was specific, since fusion was partially restored by the addition of the purified annexin II heterotetramer. Annexin II thus appears to play a role, perhaps indirectly, in early endosome fusion, suggesting that it contributes to the dynamic properties of these membranes. However, this relatively minor reduction in fusion activity is unlikely to account for the dramatic inhibition of early to late endosome transport observed after annexin II down-modulation or redistribution to late endosomes.
Fig. 9. Biochemical analysis of endosomal transport. (A) The homotypic fusion of early endosomes was measured using early endosomes prepared from cells treated or not with U18666A, and cytosol depleted or not of annexin II. When indicated purified annexin II (AnxII) was added to the assay. Fusion is expressed as a percentage of the untreated control in complete cytosol. (B) Formation of ECV/MVBs was measured from donor early endosomal membranes prepared from cells treated (U18) or not (Ctrl) with U18666A, and using HRP as a marker of the endosomal content. In the assay, complete cytosol was supplemented or not with annexin II (AnxII) or antibodies (Ab) against annexin II or Rab7. Amounts of HRP sequestered within ECVs formed in vitro corresponded to ∼12% of the total early endosomal content, as expected (Aniento et al., 1993). (C) Formation of ECV/MVBs in vitro was as in (B), but the cytosol was depleted or not of annexin II and supplemented or not with purified annexin II, the core C-terminal domain of annexin II or the N-terminal peptide. (D) Donor membranes were prepared from cells treated or not with U18666A in vivo, as in (B), and then treated or not with MBCD at the indicated concentrations in vitro. After separation on gradients, the fusion capacity of these ECV/MVBS with late endosomes was measured in vitro.
Biochemical analysis of ECV/MVB biogenesis
We then used another well-established transport assay that reconstitutes the biogenesis of transport intermediates (ECV/MVBs) destined for late endosomes (Bomsel et al., 1990; Aniento et al., 1996; Gu and Gruenberg, 2000). ECV/MVBs formed in vitro were functional since they lost the capacity to fuse with early endosomes (data not shown), but acquired the capacity to fuse with late endosomes (Figure 9D), as expected (Aniento et al., 1996). When using donor early endosomes depleted of annexin II after in vivo treatment with U18666A, the formation of ECV/MVBS was abolished (Figure 9B and C), and obviously also subsequent fusion with late endosomes (Figure 9D). Inhibition was not due to a decrease in the amounts of early endosomal cholesterol, since removal of cholesterol from donor early endosomes with MBCD in vitro did not inhibit the reaction (Figure 9D), in agreement with our findings that MBCD does not release annexin II from endosomes (Figure 1).
Annexin II depletion from the cytosol alone had no effect on ECV/MVB formation from control, untreated membranes (Figure 9C), demonstrating that the membrane-associated pool of the protein is sufficient to support the reaction. In contrast, annexin II depletion from endosomes (after U18666A treatment in vivo) abolished ECV/MVB formation, even in the presence of complete cytosol (Figure 9B–D). This indicates that ECV/MVB formation is more sensitive to annexin II membrane depletion than early endosome fusion (see Figure 9A), perhaps because amounts of the protein in the cytosol are limiting in the former, but not latter situation. Indeed, addition of the purified annexin II heterotetramer (Figure 9C) or monomer (data not shown) could restore ECV/MVB formation, in the presence of complete cytosol (data not shown) or annexin II-depleted cytosol (Figure 9C). These effects of annexin II addition were specific, since the core annexin II domain, lacking the N-terminal peptide containing the endosomal targeting motive (Konig and Gerke, 2000), did not rescue ECV/MVB formation (Figure 9C), in good agreement with our in vivo experiments (Figure 6C). Moreover, the N-terminal peptide itself partially inhibited the stimulatory effects of purified annexin II on ECV/MVB formation from annexin-depleted endosomes (Figure 9C), presumably by competing with the purified protein. Similarly, the peptide also partially inhibited ECV/MVB formation from control, untreated endosomes (Figure 9C). Finally, ECV/MVB formation from control, untreated endosomes was also inhibited by antibodies against annexin II (Figure 9B), but not against the small GTPase Rab7, which is involved in the same pathway (Feng et al., 1995). Altogether, these data show that annexin II is a necessary component of the machinery required for the biogenesis of transport intermediates destined for late endosomes.
Ultrastructural analysis of ECV/MVB biogenesis
To gain some insights into the precise role of annexin II during ECV/MVB biogenesis, we analyzed the ultrastructure of endosomes in mock- and siRNA1-treated cells. (Immunofluorescence of coverslips from the same dishes showed that annexin II was undetectable in >90% siRNA1-treated cells.) After microtubule depolymerization, HRP was endocytosed for 5 min to label early endosomes, or for 5 min followed by a 45 min chase to label ECV/MVBs (Gruenberg et al., 1989). Cells were processed for plastic embedding, and semi-thick sections (250 nm) were cut parallel to the substratum to facilitate visualization of the complex organization of the endocytic compartments as in previous studies (Parton et al., 1992).
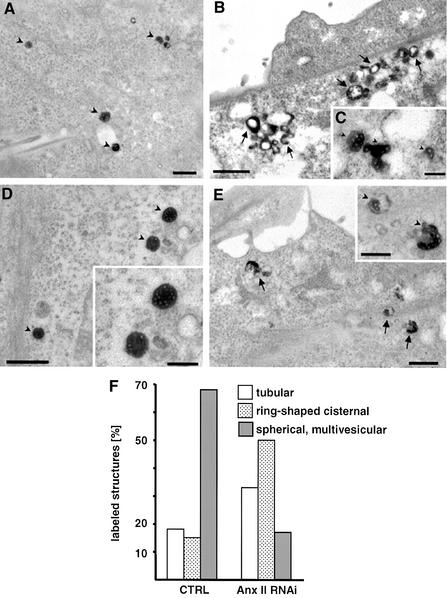
HRP internalized for 5 min gave similar labeling patterns, characteristic of early endosomes, in the mock- and siRNA-treated cells (data not shown). In contrast, clear differences were evident in control and siRNA-treated cells after HRP chase. In the mock-treated cells, HRP mainly labeled isolated vesicles with the characteristic multivesicular appearance of ECV/MVBs (Figure 10A and D, quantification in F), as previously observed (Gruenberg et al., 1989). In the siRNA-treated cells, far fewer characteristic ECVs were observed, with the bulk of the HRP reaction product within ring-shaped and tubular early endosomes (Figure 10B,C and E, quantification in F). Although labeled multivesicular regions were frequently observed, they were often associated with, or part of, the ring-shaped early endosomes (Figure 10E, inset). Hence, multivesicular regions are formed in the absence of annexin II, but ECV/MVB biogenesis is strongly inhibited. We conclude that annexin II regulates ECV/MVB budding from early endosomes, but not the membrane invagination process.
Fig. 10. Endosome ultrastructure after annexin II down-regulation. In siRNA1- or mock-treated HeLa cells, microtubules were depolymerized with 10 µM nocodazole for 2 h, and HRP endocytosed for 5 min followed by a 45 min chase. (A–E) In control cells (A and D), HRP labels predominantly ECV/MVBs (arrowheads) without associated tubules. In siRNA1-treated cells (B, C and E), HRP was mainly concentrated within ring-shaped and tubular structures with the typical morphology of early endosomes (arrows). Some multivesicular structures were labeled (C) but they lacked the uniform shape of the ECVs in the mock-treated cells (compare insets in D and E). (F) In 10 cell profiles chosen at random, HRP-labeled structures were classified according to morphology: tubular, thin tubules without associated multivesicular regions; ring-shaped cisternal, ring-shaped structures surrounding an electron-lucent space sometimes including multivesicular areas—resemble early endosomes; and spherical multivesicular, ECV/MVB. Each category is expressed as a percentage of the total number of labeled structures. Bars (A and B): 1 µm; (C and inset): 0.5µm.
Discussion
Annexin II platforms
Our observations strongly suggest that annexin II interacts physically with cholesterol. In particular, we find that annexin II protects a limited number of cholesterol molecules from extraction by cyclodextrin, presumably because these are shielded by the protein. Interactions with the bilayer may involve the hydrophobic face of a predicted amphipathic helix at the N-terminus of the protein (residues 1–24), which is separated from the conserved C-terminal core domain involved in Ca2+ binding (Gerke and Moss, 2002). Indeed, the N-terminal domain is required for endosome targeting in vivo (Rosengarth et al., 1998) and endosome or liposome binding in vitro (Harder et al., 1997; this study), as well as for endosomal transport in vivo and in vitro. In most cells, annexin II forms a heterotetrameric (annexin II)2(p11)2 complex with p11 (Johnsson et al., 1988), and the p11 binding site contains the N-terminal residues 1–14 (Rety et al., 1999). But, we find that p11 is not required for endosome or liposome binding, consistently with previous findings (Jost et al., 1997; Konig and Gerke, 2000). Moreover, the overexpression of annexin II, but not of p11, can rescue transport after accumulation of cholesterol (and annexin II) in late endosomes. Future work will be required to determine the precise regulatory function of the annexin II light chain.
Our previous data indicated that annexin II is associated with specialized domains of early endosomal membranes, visible by electron microscopy (Harder et al., 1997). Since annexin II interacts with a cholesterol-rich bilayer, and presumably with cholesterol itself, the protein may not only be associated with cholesterol-rich domains, but may contribute to organize such domains directly. However, the subcellular distribution of annexin II is unlikely to depend solely on membrane cholesterol, e.g. annexin II is absent from recycling endosomes that contain cholesterol (Gagescu et al., 2000). Hence, the stable association of annexin II with early endosomes may also depend on protein–protein interactions (Konig and Gerke, 2000). But, such interactions are unlikely to be stoichiometric, since no single endosomal protein is sufficiently abundant to act as a receptor (Harder et al., 1997). Some annexins seem to be endowed with the ability to self-organize at the membrane surface into bidimensional ordered arrays (Oling et al., 2001). This, together with our findings on the biochemical properties, distribution and function of annexin II, lead us to propose that annexin II forms cholesterol-rich platforms on early endosomes, which may be stabilized by sub-stoichiometric interactions with endosomal proteins.
Lipid storage diseases
We find that cholesterol accumulation in late endosomes, including in the genetic disorder Niemann-Pick C, is accompanied by a dramatic relocalization of annexin II from early to late endosomes, reinforcing the notion that annexin II membrane interactions depend on the membrane cholesterol content. The time-course of annexin II accumulation in late endosomes in U18666A-treated cells is much slower than that of cholesterol accumulation, and thus the protein is unlikely to be relocalized by vesicular transport. Our observations rather suggest that the cytosolic protein becomes titrated by late endosomal cholesterol, eventually causing the displacement of all annexin II pools by mass action law. Strikingly, our electron microscopy studies show that annexin II is then present, at least in part, inside the multivesicular late endosomes, presumably because it interacts with membrane invaginations and is then trapped, in a process reminiscent of microautophagy (Ohsumi, 2001; Stromhaug and Klionsky, 2001). Since the protein is no longer functional within late endosomes and since endocytic transport is then affected, one may predict that this mistargeting will contribute to the complex pathology observed in NPC patients (Pentchev et al., 1995), and in sphingolipidosis that are also accompanied by cholesterol accumulation in late endosomes (Puri et al., 1999).
Membrane transport
Evidence is accumulating that early endosomes are composed of a mosaic of structural and functional regions or molecular assemblies, which presumably reflect the need for a tightly controlled entry into the degradation pathway (Gruenberg, 2001). Protein complexes that are believed to play a role in protein sorting and/or ECV/MVB formation in mammalian cells include an endosomal COP complex and ARF1, as well as an Hrs together with ESCRT complexes and presumably a bilayered clathrin coat (Gruenberg, 2001; Katzmann et al., 2002; Lloyd et al., 2002; Raiborg et al., 2002; Sachse et al., 2002). However, the precise role of these proteins in endosome biogenesis is not known.
The formation of multivesicular endosomes involves a dramatic reorganization of early endosomal membranes. Proteins and lipids must negotiate abrupt changes in membrane curvature, for example at the neck of forming invaginations, where diffusion is presumably limited by geometrical and biophysical constraints. Such changes are likely to involve local modifications in the fluidity and asymmetry of the bilayer (Mukherjee and Maxfield, 2000; Gruenberg, 2001), which may in turn depend on lipid acyl chains and cholesterol. Indeed, lipid analogs with different alkyl tails that are expected to partition within membranes of varying fluidity or curvature, are differentially sorted in early endosomes (Mukherjee et al., 1999). In addition, recycling of internalized GPI-anchored proteins is modulated by cholesterol, which may retain these proteins in early endosomes to some extent (Mayor et al., 1998). Recent studies, in fact, show that GPI-anchored proteins are targeted either to recycling or to late endosomes, depending on the cell type, and suggest that their sorting in early endosomes is regulated by their residence time in rafts (Fivaz et al., 2002).
Our data indicate that annexin II forms cholesterol-rich platforms, perhaps akin to specialized rafts (Oliferenko et al., 1999), and is necessary for the formation of ECV/MVBs, but apparently not for membrane invagination within nascent ECV/MVBs, and that it also plays an accessory role in fusion. It thus seems logical to conclude that annexin II serves as an endosomal platform that organizes membranes at the onset of the degradation pathway—a role likely to involve interactions with the actin cytoskeleton (Harder et al., 1997; Oliferenko et al., 1999)—and thereby regulates the complex process of membrane reorganization that lead to the formation of multivesicular endosomes. It is attractive to believe that other annexins, much like annexin II and presumably annexin XIII (Lafont et al., 1998), define specific platforms on other cellular membranes, thereby controlling various cellular processes, providing a simple explanation for the numerous cellular functions that have been attributed to annexin family members (Lecat et al., 2000; Gerke and Moss, 2002).
Materials and methods
Reagents
Antibodies against rab7 were described previously (Kobayashi et al., 1998). Human serum against EEA1 was a gift from B.H.Toh (Monash Medical School, Victoria, Australia). Monoclonal antibodies against annexin II (HH7 and H28), cDNAs encoding for human GFP–annexin II, core GFP–annexin II and GFP–p11, as well as the annexin II N-terminal peptide (residues 14–27), and baculovirus expressing monomeric annexin II were gifts from V.Gerke (University of Münster, Münster, Germany). The monoclonal antibody against rab5 was a gift from R.Jahn (Göttingen, Germany). We obtained antibodies against the human transferrin receptor from Zymed Laboratories Inc. (South San Francisco, CA), fluorescently labeled secondary antibodies from Jackson ImmunoResearch Laboratories (West Grove, PA), peroxidase-conjugated secondary antibodies from BioRad Labs (Hercules, CA), rhodamine-dextran, rhodamine-transferrin, biotin-EGF and Streptavidin R-phycoerythrin conjugate from Molecular Probes, Inc. (Eugene, OR), U18666A (3-β-[2-(diethylamino)ethoxy]androst-5-en-17-one) from Biomol Research Laboratories (Plymouth Meeting, PA), phospholipids from Avanti Polar Lipids (Birmingham, AL), and [1α-,2α(n)-3H] cholesterol from Amersham Biosciences (Uppsala, Sweden).
In vivo experiments, transfection, RNA interference and microscopy
Baby Hamster kidney cells (BHK-21) (Gruenberg et al., 1989), BHK cells expressing ZnT-2 (Palmiter et al., 1996), and control or NPC cultured skin fibroblasts (Omura et al., 1989) were maintained as described previously. The analysis of HRP endocytosis and recycling was described previously, as was the internalization of rhodamine-dextran (Gu et al., 1997). BHK cells transfected 24 h after seeding with FuGene 6 from Roche Diagnostics (Rotkreuz, Switzerland) and 1 µg cDNA were incubated for 36 h. For RNAi, 2 1 nucleotide RNA duplexes with 2 nucleotide (2′-deoxy)-thymidine-3′ overhangs directed against nucleotides 65–83 (siRNA1) and 85–103 (siRNA2) of the human annexin II sequence were obtained from Xeragon Inc. (Huntsville, Al). RNAi in HeLa cells was performed as described previously (Elbashir et al., 2001), using annealed siRNA duplexes (0.1 µM) transfected with Oligofectamine (Invitrogen, Basel Switzerland). Immunofluorescence microscopy was described previously (Gu et al., 1997), but BHK cells were permeabilized with 0.1% Triton X-100 in 100 mM KCl, 2 mM MgCl2, 1 mM CaCl2 and 10 mM Hepes pH 6.9, and fixed with 3% PFA to reveal the distribution of annexin II. Electron microscopy after immunogold labeling of cryosections (Griffiths et al., 1984) or plastic embedding (Parton et al., 1992) were described previously. Quantification of HRP activity was as described previously (Gruenberg and Howell, 1989).
Subcellular fractionation and in vitro transport assays
Endosomal fractions and cytosol were prepared as described previously (Aniento et al., 1993, 1996) (see Supplementary data). Well-established assays were used to measure early endosome fusion (Gruenberg et al., 1989; Gorvel et al., 1991), as well as ECV/MVB formation from donor early-endosomal membranes and fusion with late endosomes (Aniento et al., 1996; see Supplementary data).
Annexin II binding to liposomes and endosomes
The purification of monomeric and heterotetrameric annexin II, the preparation of liposomes and [3H]-cholesterol-labeled endosomes, as well as the binding of annexin II to liposomes or endosomes and the analysis of lipids are described in the Supplementary data.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We would like to thank Marie-Hélène Beuchat for expert technical assistance. We also wish to thank Gisou van der Goot and Frank Lafont for critical reading of the manuscript, as well Chas Ferguson and Margaret Lindsay for assistance with the electron microscopy. This work was supported by grant number 31-37296.93 and 31/55325.98 from the Swiss National Science Foundation (to J.G.), by grants RG 355/94 and RG0260/19999-M from the International Human Frontier Science Program (to J.G. and R.G.P.), and by grants from the National Health and Medical Research Council of Australia (to R.G.P.). The Institute for Molecular Bioscience is a Special Research Centre of the Australian Research Council.
References
- Aniento F., Emans,N., Griffiths,G. and Gruenberg,J. (1993) Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J. Cell Biol., 123, 1373–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F., Gu,F., Parton,R.G. and Gruenberg,J. (1996) An endosomal bCOP is involed in the pH-dependent formation of transport vesicles destined for late endosomes. J. Cell Biol., 133, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiychuk E.B. and Draeger,A. (2000) Annexins in cell membrane dynamics. Ca(2+)-regulated association of lipid microdomains. J. Cell Biol., 150, 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N., Horman,A. and Woodman,P. (2002) Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein–ubiquitin conjugates. J. Cell Biol., 157, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood R.A. and Ernst,J.D. (1990) Characterization of Ca2+-dependent phospholipid binding vesicle aggregation and membrane fusion by annexins. Biochem. J., 266, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M., Parton,R., Kuznetsov,S.A., Schroer,T.A. and Gruenberg,J. (1990) Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell, 62, 719–731. [DOI] [PubMed] [Google Scholar]
- Daro E., Sheff,D., Gomez,M., Kreis,T. and Mellman,I. (1997) Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP. J. Cell Biol., 139, 1747–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir S.M., Harborth,J., Lendeckel,W., Yalcin,A., Weber,K. and Tuschl,T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411, 494–498. [DOI] [PubMed] [Google Scholar]
- Emans N., Gorvel,J.P., Walter,C., Gerke,V., Kellner,R., Griffiths,G. and Gruenberg,J. (1993) Annexin II is a major component of fusogenic endosomal vesicles. J. Cell Biol., 120, 1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Press,B. and Wandinger-Ness,A. (1995) Rab 7: an important regulator of late endocytic membrane traffic. J. Cell Biol., 131, 1435–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K., Lafont,F., Parton,R.G. and Simons,K. (1995) Annexin XIIIb: a novel epithelial specific annexin is implicated in vesicular traffic to the apical plasma membrane. J. Cell Biol., 128, 1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fivaz M., Vilbois,F., Thurnheer,S., Pasquali,C., Abrami,L., Bickel,P.E., Parton,R.G. and Van Der Goot,F.G. (2002) Differential sorting and fate of endocytosed GPI-anchored proteins. EMBO J., 21, 3989–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter C.E., Felder,S., Schlessinger,J., Ullrich,A. and Hopkins,C.R. (1993) Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J. Cell Biol., 120, 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagescu R., Demaurex,N., Parton,R.G., Hunziker,W., Huber,L. and Gruenberg,J. (2000) The recycling endosome of MDCK cells is a mildly acidic compartment rich in raft components. Mol. Biol. Cell, 11, 2775–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galve-de Rochemonteix B. et al. (2000) Interaction of anti-phospholipid antibodies with late endosomes of human endothelial cells. Arterioscler. Thromb. Vasc. Biol., 20, 563–574. [DOI] [PubMed] [Google Scholar]
- Gerke V. and Moss,S.E. (2002) Annexins: from structure to function. Physiol. Rev., 82, 331–371. [DOI] [PubMed] [Google Scholar]
- Gorvel J.P., Chavrier,P., Zerial,M. and Gruenberg,J. (1991) Rab 5 controls early endosome fusion in vitro. Cell, 64, 915–925. [DOI] [PubMed] [Google Scholar]
- Griffiths G., McDowall,A., Back,R. and Dubochet,J. (1984) On the preparation of cryosections for immunocytochemistry. J. Ultrastruct. Res., 89, 65–78. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. (2001) The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell Biol., 2, 721–730. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. and Howell,K.E. (1989) Membrane traffic in endocytosis: insights from cell-free assays. Annu. Rev. Cell. Biol., 5, 453–481. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Griffiths,G. and Howell,K.E. (1989) Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J. Cell Biol., 108, 1301–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F. and Gruenberg,J. (2000) ARF1 Regulates pH-dependent COP functions in the early endocytic pathway. J. Biol. Chem., 275, 8154–8160. [DOI] [PubMed] [Google Scholar]
- Gu F., Aniento,F., Parton,R.G. and Gruenberg,J. (1997) Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J. Cell Biol., 139, 1183–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T. and Gerke,V. (1993) The subcellular distribution of early endosomes is affected by the annexin II2p11(2) complex. J. Cell Biol., 123, 1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T., Kellner,R., Parton,R.G. and Gruenberg,J. (1997) Specific release of membrane-bound annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol. Mol. Biol. Cell, 8, 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson N., Marriott,G. and Weber,K. (1988) p36, the major cytoplasmic substrate of src tyrosine protein kinase, binds to its p11 regulatory subunit via a short amino-terminal amphiphatic helix. EMBO J., 7, 2435–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M., Zeuschner,D., Seemann,J., Weber,K. and Gerke,V. (1997) Identification and characterization of a novel type of annexin-membrane interaction: Ca2+ is not required for the association of annexin II with early endosomes. J. Cell Sci., 110, 221–228. [DOI] [PubMed] [Google Scholar]
- Katzmann D.J., Odorizzi,G. and Emr,S.D. (2002) Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol., 3, 893–905. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Stang,E., Fang,K.S., de Moerloose,P., Parton,R.G. and Gruenberg,J. (1998) A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature, 392, 193–197. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Beuchat,M., Lindsay,M., Frias,S., Palmiter,R., Sakuraba,H., Parton,R. and Gruenberg,J. (1999) Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat. Cell Biol., 1, 113–118. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Vischer,U.M., Rosnoblet,C., Lebrand,C., Lindsay,M., Parton,R.G., Kruithof,E.K. and Gruenberg,J. (2000) The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol. Biol. Cell, 11, 1829–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig J. and Gerke,V. (2000) Modes of annexin-membrane interactions analyzed by employing chimeric annexin proteins. Biochim. Biophys Acta, 1498, 174–180. [DOI] [PubMed] [Google Scholar]
- Lafont F., Lecat,S., Verkade,P. and Simons,K. (1998) Annexin XIIIb associates with lipid microdomains to function in apical delivery. J. Cell Biol., 142, 1413–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand C., Corti,M., Goodson,H., Cosson,P., Cavalli,V., Mayran,N., Faure,J. and Gruenberg,J. (2002) Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J., 21, 1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecat S., Verkade,P., Thiele,C., Fiedler,K., Simons,K. and Lafont,F. (2000) Different properties of two isoforms of annexin XIII in MDCK cells. J. Cell Sci., 113, 2607–2618. [DOI] [PubMed] [Google Scholar]
- Liscum L. and Faust,J.R. (1989) The intracellular transport of low density lipoprotein-derived cholesterol is inhibited in Chinese hamster ovary cells cultured with 3-β-[2-(diethylamino)ethoxy]androst-5-en-17-one. J. Biol. Chem., 264, 11796–11806. [PubMed] [Google Scholar]
- Liscum L. and Klansek,J.J. (1998) Niemann-Pick disease type C. Curr. Opin. Lipidol., 9, 131–135. [DOI] [PubMed] [Google Scholar]
- Lloyd T.E., Atkinson,R., Wu,M.N., Zhou,Y., Pennetta,G. and Bellen,H.J. (2002) Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell, 108, 261–269. [DOI] [PubMed] [Google Scholar]
- Mayor S., Sabharanjak,S. and Maxfield,F.R. (1998) Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J., 17, 4626–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S. and Maxfield,F.R. (2000) Role of membrane organization and membrane domains in endocytic lipid trafficking. Traffic, 1, 203–211. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Soe,T.T. and Maxfield,F.R. (1999) Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J. Cell Biol., 144, 1271–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y. (2001) Molecular dissection of autophagy: two ubiquitin-like systems. Nat. Rev. Mol. Cell Biol., 2, 211–216. [DOI] [PubMed] [Google Scholar]
- Oliferenko S., Paiha,K., Harder,T., Gerke,V., Schwarzler,C., Schwarz,H., Beug,H., Gunthert,U. and Huber,L.A. (1999) Analysis of CD44-containing lipid rafts: recruitment of annexin II and stabilization by the actin cytoskeleton. J. Cell Biol., 146, 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oling F., Bergsma-Schutter,W. and Brisson,A. (2001) Trimers, dimers of trimers, and trimers of trimers are common building blocks of annexin a5 two-dimensional crystals. J. Struct. Biol., 133, 55–63. [DOI] [PubMed] [Google Scholar]
- Omura K., Suzuki,Y., Norose,N., Sato,M., Maruyama,K. and Koeda,T. (1989) Type C Niemann-Pick disease: clinical and biochemical studies on 6 cases. Brain Dev., 11, 57–61. [DOI] [PubMed] [Google Scholar]
- Palmiter R.D., Cole,T.B. and Findley,S.D. (1996) ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J., 15, 1784–1791. [PMC free article] [PubMed] [Google Scholar]
- Parton R.G., Schrotz,P., Bucci,C. and Gruenberg,J. (1992) Plasticity of early endosomes. J. Cell Sci., 103, 335–348. [DOI] [PubMed] [Google Scholar]
- Pentchev P.G., Vanier,M.T., Suzuki,K. and Patterson,M. (1995) Niemann-Pick disease type C: a cellular cholesterol lipidosis. In C.Scriver,A.L.Beaudet,W.S.Sly and D.Valle (eds), The Metabolic and Molecular Basis of Inherited Disease, VII Edition. McGraw-Hill, Inc., New York, NY, pp. 2625–2639. [Google Scholar]
- Piguet V., Gu,F., Foti,M., Demaurex,N., Gruenberg,J., Carpentier,J.L. and Trono,D. (1999) Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of β-COP in endosomes. Cell, 97, 63–73. [DOI] [PubMed] [Google Scholar]
- Powell M.A. and Glenney,J.R. (1987) Regulation of calpactin I phospholipid binding by calpactin I light-chain binding and phosphorylation by p60v-src. Biochem. J., 247, 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri V., Watanabe,R., Dominguez,M., Sun,X., Wheatley,C.L., Marks,D.L. and Pagano,R.E. (1999) Cholesterol modulates membrane traffic along the endocytic pathway in sphingolipid-storage diseases. Nat. Cell Biol., 1, 386–388. [DOI] [PubMed] [Google Scholar]
- Raiborg C., Bache,K.G., Mehlum,A., Stang,E. and Stenmark,H. (2001) Hrs recruits clathrin to early endosomes. EMBO J., 20, 5008–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C., Bache,K.G., Gillooly,D.J., Madshus,I.H., Stang,E. and Stenmark,H. (2002) Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol., 4, 394–398. [DOI] [PubMed] [Google Scholar]
- Rety S., Sopkova,J., Renouard,M., Osterloh,D., Gerke,V., Tabaries,S., Russo-Marie,F. and Lewit-Bentley,A. (1999) The crystal structure of a complex of p11 with the annexin II N-terminal peptide. Nat. Struct. Biol., 6, 89–95. [DOI] [PubMed] [Google Scholar]
- Rosengarth A., Wintergalen,A., Galla,H.J., Hinz,H.J. and Gerke,V. (1998) Ca2+-independent interaction of annexin I with phospholipid monolayers. FEBS Lett., 438, 279–284. [DOI] [PubMed] [Google Scholar]
- Sable C.L. and Riches,D.W. (1999) Cloning and functional activity of a novel truncated form of annexin IV in mouse macrophages. Biochem. Biophys. Res. Commun., 258, 162–167. [DOI] [PubMed] [Google Scholar]
- Sachse M., Urbe,S., Oorschot,V., Strous,G.J. and Klumperman,J. (2002) Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol. Biol. Cell, 13, 1313–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian T., Pradel,L.A., Henry,J.P., Aunis,D. and Bader,M.F. (1991) The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J. Cell Biol., 114, 1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Aasland,R., Toh,B.H. and D’Arrigo,A. (1996) Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J. Biol. Chem., 271, 24048–24054. [DOI] [PubMed] [Google Scholar]
- Stromhaug P.E. and Klionsky,D.J. (2001) Approaching the molecular mechanism of autophagy. Traffic, 2, 524–531. [DOI] [PubMed] [Google Scholar]
- Turpin E., Russo-Marie,F., Dubois,T., de Paillerets,C., Alfsen,A. and Bomsel,M. (1998) In adrenocortical tissue, annexins II and VI are attached to clathrin coated vesicles in a calcium-independent manner. Biochim. Biophys Acta, 1402, 115–130. [DOI] [PubMed] [Google Scholar]
- Whitney J.A., Gomez,M., Sheff,D., Kreis,T.E. and Mellman,I. (1995) Cytoplasmic coat proteins involved in endosome function. Cell, 83, 703–713. [DOI] [PubMed] [Google Scholar]