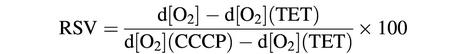Abstract
The RAS2val19 allele, which renders the cAMP–PKA pathway constitutively active and decreases the replicative life-span of yeast cells, is demonstrated to increase production of reactive oxygen species (ROS) and to elevate oxidative protein damage. Mito chondrial respiration in the mutant is locked in a non-phosphorylating mode prone to generate ROS but this phenotype is not linked to a constitutively active PKA pathway. In contrast, providing RAS2val19 cells with the mammalian uncoupling protein UCP1 restores phosphorylating respiration and reduces ROS levels, but does not correct for PKA-dependent defects. Thus, the RAS2val19 allele acts like a double-edged sword with respect to oxidation management: (i) it diminishes expression of STRE element genes required for oxidative stress defenses in a PKA-dependent fashion, and (ii) it affects endogenous ROS production and the respiratory state in a PKA-independent way. The effect of the oncogenic RAS allele on the replicative life-span is primarily asserted via the PKA-dependent pathway since Pde2p, but not UCP1, overproduction suppressed premature aging of the RAS2val19 mutant.
Keywords: PKA/RAS2/reactive oxygen species/replicative aging/respiratory state/UCP1
Introduction
The yeast Saccharomyces cerevisiae belongs to the exclusive club of microorganisms exhibiting replicative aging [expressed as the limited number of mitotic divisions that an individual cell can complete (Mortimer and Johnston, 1959)]. Most laboratory strains of S.cerevisiae can complete, on average, 20–30 divisions. During these progressive divisions, the cells undergo age-related changes, including an increased generation time (Egilmez and Jazwinski, 1989), an increase in size (Egilmez et al., 1990), a decline in mating ability (Smeal et al., 1996) and an accumulation of extrachromosomal rDNA circles (Sinclair and Guarente, 1997) and oxidatively damaged proteins (Aguilaniu et al., 2003). Most of the genes regulating yeast life-span have proven to have mammalian homologs, some of which are implicated in tumorigenesis. For example, the human homolog of the SIR2 gene, a key life-span regulator in yeast (Sinclair and Guarente, 1997), deacetylates and negatively controls the tumor suppressor p53, thereby promoting cell survival (Luo et al., 2001; Vaziri et al., 2001). On the other hand, an overactive form of p53 was recently shown to accelerate aging in mice (Tyner et al., 2002). The data implies that there may be links between oncogenesis and senescence but its detailed molecular nature remains obscure.
RAS is another example of a mammalian oncogene that is present in yeast and acts as a gerontogene (Chen et al., 1990; Sun et al., 1994; Pichova et al., 1997; Lin et al., 2000). Yeast possesses two isoforms of the Ras protein, Ras1p and Ras2p, which belong to a family of conserved polypeptides present in all eukaryotic organisms. Interestingly, Ras proteins have been shown to be functionally interchangeable between yeast and human cells (Kataoka et al., 1985; Parrini et al., 1996). They are small guanine nucleotide-binding proteins cycling between the active GTP-bound and the inactive GDP-bound states (Hall and Self, 1986). Cdc25p and Ira1p/Ira2p promote formation of the active and inactive form, respectively (Camonis and Jacquet, 1988). The Ras proteins are key regulators of the cAMP–protein kinase A (PKA) pathway, which is involved in sensing the nutritional status of the cell. The active GTP-bound form of Ras achieves this by regulating the activity of the adenylate cyclase Cyr1p (Broek et al., 1985).
Disruption of both the yeast RAS genes is lethal in a haploid genetic background, but lethality can be suppressed by overproducing the adenylate cyclase (Kataoka et al., 1985) or by deleting BCY1, encoding the regulatory subunit of the cAMP-dependent protein kinase. This is due to the fact that a bcy1 loss-of function mutation bypasses the need for cAMP; i.e. the protein kinase is constitutively active in the absence of cAMP (Toda et al., 1985). Cells with a constitutively active PKA pathway are heat sensitive, exhibit low catalase activity and fail to accumulate higher sugars such as glycogen and trehalose (Thevelein, 1994). In contrast, lack of RAS2 reduces cAMP levels about fourfold, prevents growth on non-fermentable carbon sources and upregulates genes containing a stress response element (STRE) in their promoter region (Marchler et al., 1993). Thus, RAS2 inactivation provokes elevated levels of several heat shock proteins, catalase, Cu–Zn superoxide dismutase and polyubiquitin (Martinez-Pastor et al., 1996). Consequently, the mutant is more resistant than the wild type to oxidative stresses (Longo, 1999).
Several reports have established that alterations in the activity of the Ras–cAMP–PKA pathway affect cellular replicative aging (Sun et al., 1994; Pichova et al., 1997; Jazwinski, 1999b; Lin et al., 2000). In mammalian cells, transformation of fibroblasts with the oncogenic allele of RAS (v-Ha-RAS) triggers rapid senescence (Serrano et al., 1997) in a p53-dependent way (Serrano et al., 1997; Ferbeyre et al., 2002). So far, however, the reported effects of RAS mutations on yeast life-span are not straightforward. For example, mutations causing low PKA activity have been shown to prolong the replicative life-span of cells (Pichova et al., 1997; Lin et al., 2000), whereas another report demonstrates that low PKA activity, achieved by deleting RAS2, decreases life-span (Sun et al., 1994). In addition, high PKA activity, caused by either the RAS2val19 allele (Kataoka et al., 1984; Toda et al., 1985; Pichova et al., 1997) or bcy1 loss-of-function mutations shortens life-span (Sun et al., 1994). In contrast, high PKA activity extends life-span when accomplished by overexpressing the yeast RAS2 or ectopic expression of the human v-Ha-RAS (Chen et al., 1990; Sun et al., 1994). Thus the effects of Ras/PKA activity on yeast aging remains somewhat enigmatic (Jazwinski, 1999b).
In this work, we have characterized the oncogenic RAS2val19 mutant with respect to its replicative life-span, free-radical biology and oxidative metabolism. This has revealed novel and hitherto unknown phenotypes of yeast cells expressing oncogenic RAS. These phenotypes include increased occurrence of petite mutants, increased membrane potential and endogenous production of reactive oxygen species (ROS), elevated levels of oxidatively damaged proteins, including mitochondrial proteins, and a non-phosphorylating activity of the mitochondrial electron transport chain. Furthermore, mitochondrial phosphorylating activity in the RAS2val19 mutant was not linked to a constitutively high cAMP–PKA activity but could be restored by ectopic production of UCP1, a mammalian uncoupling protein. However, reduction of cAMP levels but not UCP1 expression was found to suppress the replicative life-span defect of RAS2val19 cells.
Results
RAS2val19 cells exhibit elevated stress sensitivity, increased oxidative damage and a reduced replicative life-span
Because the effect of an overactive RAS pathway on yeast longevity has been somewhat controversial (see above for details), we analyzed whether the replicative potential of the RAS2val19 mutant is reduced also on synthetic media instead of YPD. This was the case (Figure 1A) (Pichova et al., 1997). To ensure that the RAS2val19 allele bestowed the expected phenotypes linked to a high PKA activity, we measured glycogen content by iodine–iodide staining (Figure 1B), catalase activity (Figure 1C), sensitivity to heat shock at 52°C (Figure 1D) and sensitivity to paraquat (Figure 1E). As seen in Figure 1, the results obtained are in line with the notion that RAS2val19 is an overactive allele of the RAS2 gene.
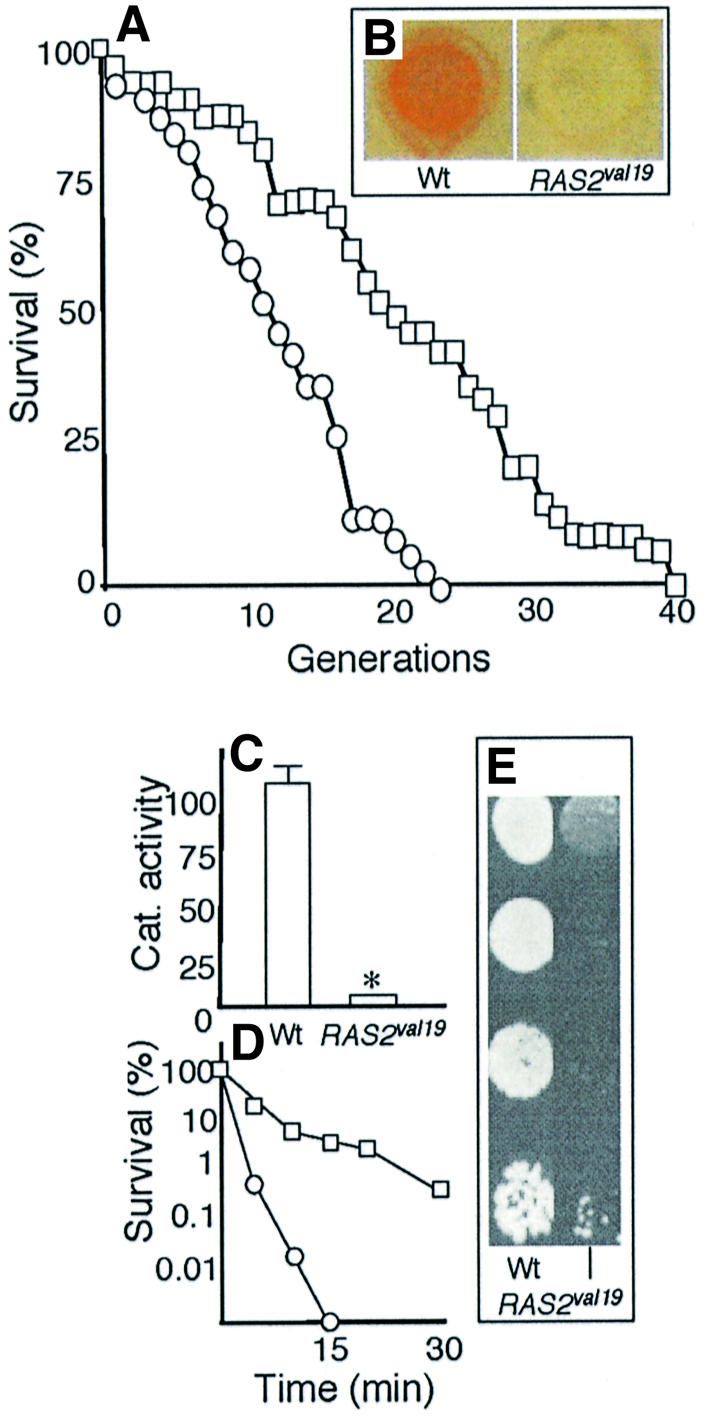
Fig. 1. Phenotypes of RAS2val19 cells. (A) Life-span analysis of wild type (open squares) and RAS2val19 (open circles) grown on SC medium as described in Materials and methods. (B) Iodine–iodide staining of wild-type and RAS2val19 colonies. Iodine–iodide stains colonies according to their glycogen content. High glycogen content is indicated by a brown appearance. (C) Catalase activities in exponentially growing wild-type and RAS2val19 cells. Wild-type levels were arbitrarily assigned a value of 100. *Note that catalase activity was often not detectable in the RAS2val19 mutant unless the assay was scaled up to include more cell material. When doing so, the values obtained corresponded to about 10% of the catalase activity of wild-type cells. (D) Heat shock sensitivity (shift from 30°C to 52°C) of exponentially growing wild-type (open squares) and RAS2val19 (open circles) cells. (E) Paraquat sensitivity of exponentially growing wild-type and RAS2val19 cells. Serial dilutions of wild-type and RAS2val19 cells taken from exponential phase (OD600 = 1) cells were dropped on YPD plates with or without (not shown) paraquat (400 µg/ml).
To explore whether the RAS2val19 mutant experiences defects in oxidative management, the frequency of the occurrence of respiratory-deficient mutants (‘petite’ mutants) was analyzed. Indeed, this frequency was elevated in the RAS2val19 mutant compared with the wild type (Figure 2A). To test whether the mutant exhibited an increased intracellular oxidative stress, we measured superoxide content by fluorescent staining with dihydroxyethidium (DHE) (Bindokas et al., 1996), and analyzed the level of protein oxidation by immunodetection of protein carbonyl groups. As depicted in Figure 2B, C and D, RAS2val19 cells contain significantly more superoxide and oxidatively damaged proteins than wild-type cells. Specifically, isolated mitochondria of RAS2val19 cells show signs of drastically elevated oxidative damage to their proteins (Figure 2C and D).
Fig. 2. Indicators of endogenous oxidative stress in wild-type and RAS2val19 cells. (A) Frequency of petite mutants in wild-type and RAS2val19 populations. (B) In situ superoxide ion concentrations detected by DHE staining. Relative values are shown and the superoxide concentration of wild-type cells was arbitrarily assigned a value of 100. (C) Protein oxidation levels of specific proteins in total protein extracts (T) and mitochondrial extracts (M) detected by western immunochemical analysis of carbonylated proteins as described previously (Dukan and Nyström, 1998; Aguilaniu et al., 2001). (D) Quantification of carbonyl levels in total protein extracts (T) and mitochondrial extracts (M) of wild-type and RAS2val19 cells as described (Dukan and Nyström, 1998). The carbonyl levels in wild-type cells were arbitrarily assign a value of 1.0. The standard deviation is shown on top of the bars.
The RAS2val19 mutation locks mitochondrial respiration in a non-phosphorylating mode
It has been reported that an in vivo respiratory state 4, a non-phosphorylating respiration prone to generate ROS (Boveris and Chance, 1973; Korshunov et al., 1997), correlates with protein oxidation in yeast G0 cells (Aguilaniu et al., 2001). Therefore, we analyzed the phosphorylating state of mitochondrial respiration in the RAS2val19 cells. Briefly, the assay used determines the contribution of ADP phosphorylation in oxygen consumption rate. In other words, it quantifies the degree of coupling between electron flux through the electron transport chain and ATP generation. This is assayed by sequentially quantifying the effect of triethyltin bromide (TET), a lipophilic inhibitor of the mitochondrial ATPase (for details, see Aguilaniu et al., 2001), and carbonyl cyanide m-chlorophenylhydrazone (CCCP), a protonophore, on the oxygen consumption rate. The CCCP treatment activates oxygen consumption by allowing protons to re-enter the mitochondrial matrix. As previously described (Aguilaniu et al., 2001), an in vivo respiratory state value (RSV) can then be calculated using the formula
Exponentially growing wild-type cells have an RSV close to a 100 (pseudostate 3) even when being glucose repressed (Aguilaniu et al., 2001). However, the RSV for the RAS2val19 was found to be essentially zero (pseudostate 4) (Figure 3A and Table I) under identical conditions. In other words, the oxygen consumption of RAS2val19 cells did not involve the ATPase under these conditions, as indicated by the fact that the inhibitor TET did not affect the oxygen consumption rate (Figure 3A). Notably, CCCP could still significantly activate respiration, confirming the existence of a membrane potential and that reducing equivalents were not limiting in RAS2val19 cells (Figure 3A). The low RSV suggests that respiration in this mutant is virtually non-phosphorylating and, as shown in a later section using the uncoupling protein UCP1, this result was not due to RAS2val19 cells being impermeable to TET. Furthermore, to ensure that the lack of inhibition was not due to an altered number of mitochondrial enzymes, we performed the experiment over large range of TET concentrations with the same results (up to 225 mM for RAS2val19 and up to 37.5 mM for wild-type cells). In addition, western blot analysis revealed that there was no significant difference in ATPase content (Figure 3B) or mitochondria content (Figure 3C) between the RAS2val119 mutant and the wild-type strain.
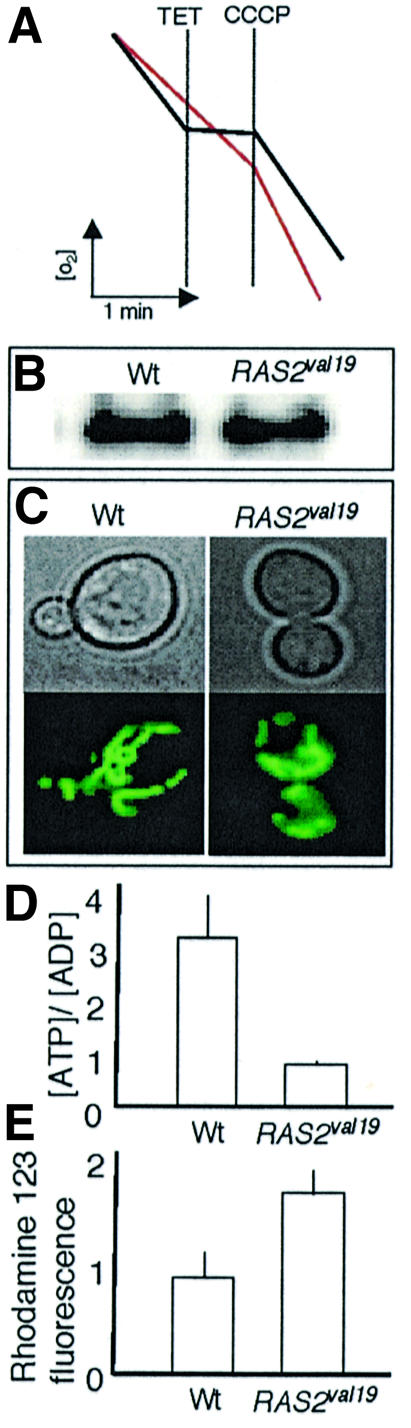
Fig. 3. Respiratory mode, mitochondrial content, membrane potential and energetics of RAS2val19 cells. (A) RSV measurements on wild-type (black line) and RAS2val19 (red line) cells growing exponentially in YPD. Cells were placed in an oxygraph chamber and oxygen consumption was followed on-line. TET and CCCP were then added to the measuring chamber and RSV was calculated as explained (Aguilaniu et al., 2001). (B) Western blot quantification of the levels of the e subunit of ATPase in wild-type and RAS2val19 cells grown exponentially in YPD. (C) Mitochondrial content of exponentially growing wild-type and RAS2val19 cells detected by staining with MitoTrackerGreen FM. (D) [ATP]/[ADP] ratios in exponentially growing wild-type and RAS2val19 cells. (E) Membrane potential of wild-type and RAS2val19 cells measured by Rhodamine 123 fluorescence. The standard deviation is shown on top of the bars.
Table I. Basal respiration rates and RSVs of different strains.
| Strains | Respiratory characteristics |
|
|---|---|---|
| Basal rate (µM O2/min) | RSV | |
| wt | 3.4 ± 0.52 | 53 ± 3.9 |
| RAS2val19 | 0.9 ± 0.15 | 0 ± 5.8 |
| wt + Yep13 | 4.2 ± 0.48 | 75 ± 2.1 |
| RAS2val19 + Yep13 | 1.2 ± 0.22 | –1 ± 8.9 |
| wt + Pde2p | 4.4 ± 0.485 | 67 ± 1.6 |
| RAS2val19 + Pde2p | 1.1 ± 0.0035 | 0 ± 0.1 |
| wt + Yepd | 4.1 ± 0.07 | 66 ± 1.9 |
| RAS2val19 + Yepd | 0.9 ± 0.1 | 0 ± 0.3 |
| wt + UCP1 | 6.2 ± 0.09 | 94 ± 3.9 |
| RAS2val19 + UCP1 | 5.3 ± 0.24 | 84 ± 2.5 |
| bcy1-13 | 2.8 ± 0.35 | 44 ± 2.1 |
Each value is calculated on 2.5 ml of a culture at OD600 nm = 1. Experiments were performed on minimal medium with glucose (2%) as a carbon source, except for experiments involving UCP1 and the corresponding empty plasmid which were performed on galactose to induce the GAL1 promoter. The wild-type basal respiration rate as well as RSV was similar for glucose- and galactose-grown cells. Yep13 is the vector plasmid used for PDE2 and Yepd is the vector for UCP1.
When grown on glucose, yeast cells produce ATP predominantly by substrate level phosphorylation but respiration proceeds concomitantly. Therefore a predict able consequence of having a non-functional ATPase is a lower [ATP]/[ADP] ratio in the cell. Indeed, the RAS2val19 strain had a significantly lower [ATP]/[ADP] than the wild type, confirming an alteration in the oxidative ATP production machinery in the mutant (Figure 3D). However, the[NAD]/[NADH] ratio was very similar in wild-type and RAS2val19 cells [5.7 and 5.2 in wild type and RAS2val19, respectively (data not shown)]. A transition to a state 4 respiration has been suggested to cause a build up of the membrane potential (e.g. Korshunov et al., 1997) and Rhodamine 123 staining indicated that this was the case in the RAS2val19 cells (Figure 3E). Thus the respiratory state of the RAS2val19 mutant appears to be locked in a non-phosphorylating mode; a mode intimately associated with an elevated membrane potential and ROS production (Boveris and Chance, 1973; Korshunov et al., 1997).
The non-phosphorylating respiration and elevated production of ROS in the RAS2val19 strain is not linked to a constitutive PKA activity
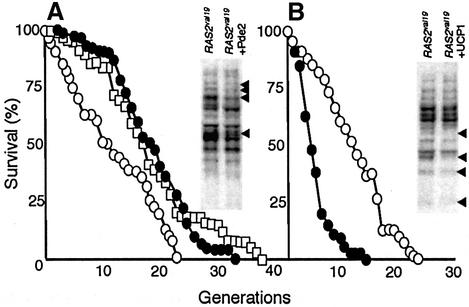
The control of ADP phosphorylation under glucose repression by the Ras pathway is a new finding. To elucidate whether this phenotype is linked to the constitutively active cAMP–PKA pathway in the RAS2val19 cells we restored normal PKA activity in the RAS2val19 background by overexpressing the high-affinity phosphodiesterase Pde2p. The Pde2p hydrolyzes cAMP, thereby lowering the fraction of cAMP bound to the regulatory subunit Bcy1p of the PKA. As expected, Pde2p overproduction restored glycogen content to wild-type levels or higher (Figure 4A). To further ascertain that the overexpression of PDE2 reduced the PKA activity to wild-type levels or lower, heat shock experiments and catalase activity measurements were performed. As shown in Figure 4B, PDE2 overexpression rendered the RAS2val19 strain even less sensitive to heat shock than the wild type, suggesting that the PDE2 plasmid conferred a phenotype closer to low PKA activity mutants (Figure 4B). This conclusion is also consistent with the results of the catalase activity measurements (Figure 4C). However, we could not detect any effect of overproducing Pde2p on either ROS levels or RSV (Figure 5A and B and Table I). This suggests that, in this background, both RSV and ROS production are [cAMP]-independent phenotypes. To confirm this view, we performed RSV analysis on cells with an inactivated allele of bcy1 (bcy1-13). Although these cells have a constitutively active PKA pathway (like RAS2val19), the RSV was similar to wild type (Figure 5B and Table I). Thus Ras2p can operate through PKA-independent routes and the non-phopshorylating respiration is achieved independently of the constitutively active PKA pathway in the RAS2val19 cells. We confirmed that the bcy1-13 mutant displayed the expected high PKA-dependent activities by analyzing glycogen content and heat sensitivity (Figure 5C).
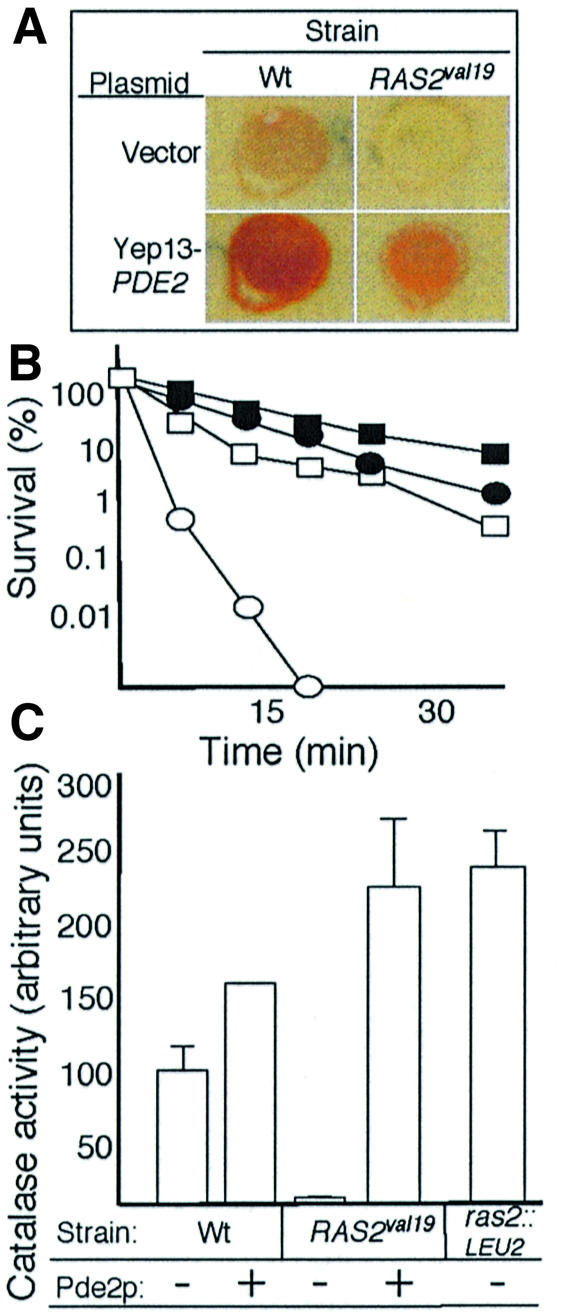
Fig. 4. Recovery of PKA activity by PDE2 overexpression in RAS2val19 (RASv) cells. (A) Iodine–iodide staining of wild-type and RAS2val19 cells carrying either the empty vector (top panels) or overexpressing PDE2 (lower panels). (B) Heat shock sensitivity (shift from 30°C to 52°C) of wild-type and RAS2val19 cells carrying either the empty vector (wt, open squares; RAS2val19, open circles) or overexpressing PDE2 (wt, filled squares; RAS2val19, filled circles). (C) Catalase activity in wild-type and RAS2val19 cells carrying the empty vector (–) and overexpressing PDE2 (+). The catalase activity of Ras2::LEU2 mutant cells carrying the vector is included for comparison.
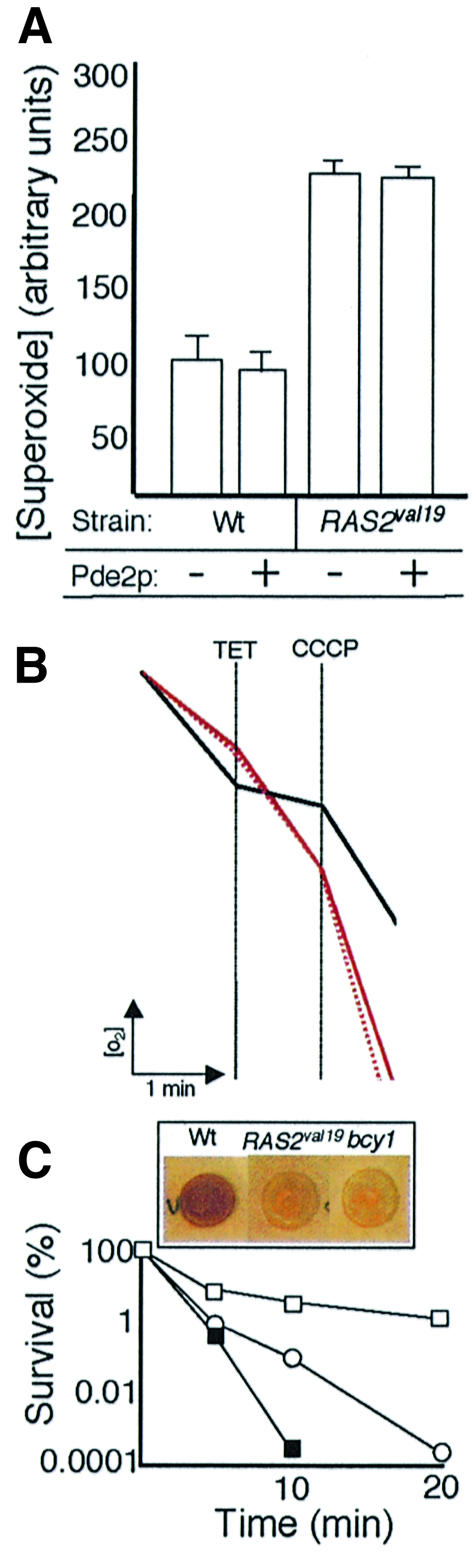
Fig. 5. Effect of PDE2 overexpression on superoxide concentration and RSV in RAS2val19 and wild-type cells. (A) In situ superoxide ion concentrations detected by DHE staining in wild-type and RAS2val19 cells with (+) and without (–) Pde2p overproduction. (B) RSV analysis of RAS2val19 cells carrying either the empty vector (solid red line) or overexpressing PDE2 (dotted red line). The RSV analysis of a bcy1 mutant is shown by the black line. See Materials and methods for details of the procedures. (C) Glycogen content (inset) and heat sensitivity (shift from 30°C to 52°C) of wild type (open squares), RAS2val19 cells (open cicrcles) and bcy1 mutants (filled squares).
The mammalian uncoupling protein UCP1 recovers phosphorylating respiration in the RAS2val19 mutant
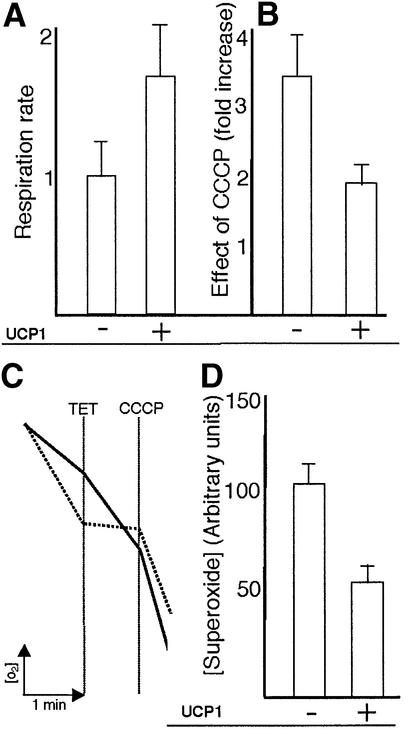
The failure of the RAS2val19 mutant to perform phosphorylating respiration under the conditions examined could be a result of membrane ATPase activity being allosterically affected and blocked, of maturation of the ATPase being altered such that no functional ATPase is made or of metabolite concentrations that are not favorable for ADP phosphorylation (e.g. a high [ATP]/[ADP] ratio). Experiments using the mammalian uncoupling protein UCP1 serendipitously helped us to discriminate between these possibilities. UCP1 is exclusively expressed in brown adipose tissues (BAT) and its action is similar to that of a regulated uncoupler in yeast (Stuart et al., 2001). By allowing the re-entry of protons in the mitochondrial matrix, it dissipates ΔΨ to heat and thereby lowers ATP/O (ATP produced per oxygen molecule consumed). However, the exact details of the uncoupling mechanism are under debate (e.g. Garlid et al., 1998; Klingenberg and Huang, 1999). It has been argued that its function is mainly connected to thermogenesis based on the fact that UCP1 knockout mice are incapable of adapting to cold exposure (Enerbäck et al., 1997). However, it was more recently suggested that the UCP1, UCP2 and UCP3 proteins could also prevent the production of superoxides (Skulachev, 1998). Support for this latter view was recently provided by work showing that all three isoforms of UCP are regulated by superoxide levels (Echtay et al., 2002). We argued that an uncoupling protein might be able to increase oxygen consumption in the RAS2val19 strain by providing an alternative route for protons in the absence of ATPase activity. Indeed, transformation of RAS2val19 with pYEPD-UCP1 using a GAL1/10 promoter to express UCP1 (see Materials and methods) elevated respiration almost 2-fold in the mutant (Figure 6A). Moreover cells expressing UCP1 were partly uncoupled, as confirmed by a less pronounced effect of CCCP on the respiratory rate [1.9-fold increase in RAS2val19+pYEPD-UCP1 against a nearly 4-fold increase in RAS2val19 + empty vector (Figure 6B)]. We also measured the RSV of RAS2val19 cells expressing UCP1 and found that TET sensitivity was restored by UCP1 (Figure 6C and Table I). In other words, UCP1 appears to re-establish phopshorylating respiration via the ATPase in the RAS2val19 strain. It is unlikely that the results observed are due to TET directly inhibiting UCP1 instead of the ATPase. If this was true, one would expect TET-treated UCP1 transformants to exhibit a respiration rate similar to that of the untreated controls. This was not the case and TET is described as a specific ATPase inhibitor at the concentrations used in this work (Cain and Griffiths, 1977). In addition, the increased superoxide production of RAS2val19 cells was counteracted by ectopic UCP1 production (Figure 6D) consistent with a resumption of a state 3 type respiration. Taken together, our results suggest that in the RAS2val19 mutant the ATPase is produced, and is potentially functional, but inactive. The UCP1-expressing strain did not show signs of a reduced PKA activity [the glycogen content was similar to that of the RAS2val19 (data not shown)].
Fig. 6. Effect of the uncoupling protein UCP1 on respiration in RAS2val19 cells. (A) Rate of respiration in RAS2val19 cells with and without ectopic production of UCP1 as indicated on the graph. (B) Effect of the protonophore CCCP on the respiration rate of RAS2val19 cells carrying the empty vector or producing UCP1. (C) RSV analysis of RAS2val19 cells with (broken lines) and without (solid lines) ectopic production of UCP1. (D) Superoxide ion concentration in RAS2val19 cells with (+) and without (–) ectopic expression of UCP1.
The reduced replicative life-span of RAS2val19 cells is a cAMP–PKA-dependent phenotype
The possibility of uncoupling the PKA-dependent phenotypes, such as catalase deficiency, heat sensitivity and glycogen storage defects, from the PKA-independent ones, including low RSV and ROS accumulation, in the RAS2val19 mutant allowed us to elucidate the relative importance of these two pathways in cellular aging. We performed life-span analysis and found that overexpressing PDE2 counteracted the accelerated aging of RAS2val19 cells (Figure 7A). Since the low RSV was not suppressed by Pde2p overproduction, this suggests that the non-phosphorylating respiration and accompanying elevated ROS production is not limiting the life-span of the RAS2val19 mutant. In addition, cells expressing UCP1 (restoring normal RSV and ROS production but not PKA-dependent phenotypes) had a drastically reduced life-span (Figure 7B). Both Pde2p overproduction and ectopic UCP1 production modestly reduced carbonylation in the RAS2val19 cells (Figure 7A and B). This reduction was not general but affected specific proteins (Figure 7A and B). The different pattern of protein carbonylation in the RAS2val19 cells of Figure 7A and B is due to growth on different carbon sources (glucose and galactose). Taken together, the data demonstrate that the restricted replicative potential of RAS2val19 cells is linked to their overactive PKA pathway rather than their mode of respiration.
Fig. 7. Effect of Pde2p and UCP1 on life-span and protein oxidation. (A) Life-span analysis of wild type (open squares) and RASval19 cells carrying empty vector (Yep13) (open circles) or overproducing PDE2 (filled circles). The inset shows western blot analysis of carbonylated proteins in RAS2val19 cells with and without Pde2p overproduction as indicated. The black arrows indicate polypeptides with reduced carbonylation as a result of Pde2p overproduction. (B) Life-span analysis of RASval19 cells without (open circles) and with (filled circles) UCP1. These experiments were conducted on SC medium with appropriate selection as described in Materials and methods. The inset shows western blot analysis of carbonylated proteins in RAS2val19 cells with and without ectopic production of UCP1 as indicated. The black arrows indicate polypeptides with reduced carbonylation as a result of UCP1 production.
Discussion
Cells of S.cerevisiae expressing the oncogenic RAS2val19 are demonstrated to exhibit a severe oxidative load when grown in the glucose-rich medium YPD. The mutant exhibits significantly higher concentrations of ROS and oxidatively damaged proteins, especially in the mitochondria. In addition, the emergence of petite mutants is more common in an RAS2val19 than a wild-type population. The RAS2val19 mutant’s problem in oxidation management can be linked to two independent pathways: (i) the constitutively active cAMP–PKA system which effectively reduces expression of STRE element genes, including those dealing with oxidative damage protection (Hasan et al., 2002), and (ii) a PKA-independent pathway locking the respiratory state in a non-phosphorylating mode (this work). The sequential use of TET and CCCP allows the determination of in vivo respiratory pseudostates (Aguilaniu et al., 2001). The use of these drugs demonstrated that the electron flow through the electron transport chain of the RAS2val19 mutant created a membrane potential that was not coupled to ATP synthesis by the ATP synthase complex. This state of respiration was previously demonstrated to occur in wild-type cells entering a G0 growth arrested state (Aguilaniu et al., 2001).
It is known that non-phosphorylating respiration is prone to generating ROS (Boveris and Chance, 1973; Korshunov et al., 1997). The theoretical framework proposed to explain this phenomenon is that the redox state of the quinone pool within the inner mitochondrial membrane determines the production rate of the superoxide ion (Skulachev, 1996). A state 3 respiration (phosphorylating) occurs when isolated mitochondria are incubated in the presence of respiratory substrate (typically NADH) and ADP while state 4 (non-phosphorylating) is observed after ADP is depleted. When this occurs, the rate of respiration decreases (Nicholls and Ferguson, 1992), the membrane potential increases (Fitton et al., 1994) and the residual respiration measured is mainly linked to the membrane conductance to protons since proton translocation no longer occurs via the ATPase. An increase of the electrical component of the membrane potential (ΔΨ) was proposed to alter the redox state of the quinones mechanistically and it was postulated that the life-span of the semiquinones was extended with an increased ΔΨ (Skulachev, 1996). The probability of semiquinones reacting with molecular oxygen to generate superoxide would then increase (Skulachev, 1996).
Thus the respiratory mode of RAS2val19 mutants grown under glucose-repressing conditions is locked in a state that is prone to generate ROS. This poses the question of why the mitochondrial ATPase is not operating in RAS2val19 mutants. A link between Ras pathways and mitochondrial function has been suggested previously. For example, it has been shown that the Ras2p pathway is part of a regulatory circuit controlling biogenesis of the oxidative phosphorylation complexes in yeast cells grown on lactate (Dejean et al., 2002). Moreover, the Ras2p pathway appears to modulate the retrograde response (Kirchman et al., 1999), which is an intracellular signaling pathway triggered by a reduction in mitochondrial functionality. Also, human or mouse lymphocytes expressing activated v-Ha-RAS undergo apoptosis upon protein kinase C downregulation, but this apoptotic process can be blocked by overexpressing bcl-2 (Denis et al., 2003). It has been suggested that bcl-2 might prevent this RAS-dependent apoptosis through its effects on the permeability of the mitochondrial membrane (Denis et al., 2003). At present, it is not clear exactly how the oncogenic RAS2val19 allele affects mitochondrial phosphorylating activity, but possible explanations include the following: (i) the mutant fails to produce a mature and active ATPase, (ii) the ATPase is allosterically inactivated or (iii) metabolite concentrations are unfavorable for ATPase function (i.e. a high [ATP]/[ADP] ratio). The fact that the mammalian uncoupling protein UCP1 restored phosphorylating respiration in the mutant excludes the first possibility and demonstrates that the ATPase is produced in a potentially active form. In addition, the [ATP]/[ADP] ratio was found to be lower rather than higher in the RAS2val19 cells. This suggests that the altered metabolite pool is a consequence rather than a cause of the block in ATPase activity. However, it should be noted that it is technically unattainable to assay the metabolite concentration within the mitochondria. Therefore we cannot exclude the possibility that the mitochondrial metabolite pools in the mutant are adversely affecting ATPase activity.
It is formally possible that UCP1 interacts directly with the ATPase to affect its activity. Of course, such an interaction would not be relevant in yeast cells, which lacks UCP-type uncoupling proteins, but would be highly significant in mammals. We suggest that exploring this possibility in a mammalian system may be warranted. The effect of UCP1 on yeast respiration shown herein is consistent with the idea that UCPs may act to prevent production of superoxides (Skulachev, 1998). However, our observations expand the possibilities because UCP1 may reduce ΔΨ both by acting as an uncoupling protein (e.g, Klingenberg and Huang, 1999) and by switching respiration from a type 4 to a type 3 mode. It would be interesting to determine whether overproducing UCP1 mimics the effects of overexpressing bcl-2 on apoptosis of v-Ha-RAS lymphocytes.
The non-phosphorylating mode of respiration in the RAS2val19 strain could not be suppressed by Pde2p overproduction or mimicked by a bcy1 mutation, demonstrating that this phenotype of the oncogenic RAS cells is not linked to a constitutive cAMP–PKA pathway. It has been proposed previously that Ras2 may affect cell physiology through a PKA-independent pathway (Sun et al., 1994). This suggestion stems from the observation that RAS2 overexpression and a bcy1 deletion (both conferring high cAMP–PKA activity) had opposite effects on life-span, as did ras1 and ras2 mutations despite the fact that they act similarly on the PKA pathway (Sun et al., 1994). It was proposed that Ras2p might act via the retrograde pathway in a PKA-independent fashion (Jazwinski, 1999a; Kirchman et al., 1999). The proposition that Ras2p may work, in part, independently of PKA is in line with the data presented here, but the effect of an overactive Ras2p pathway differ between earlier results (Sun et al., 1994) and this work. Possibly, the two different means of increasing Ras2p activity (overproduction versus overactivation) yield different effects on cell physiology. In addition, different yeast genetic backgrounds were used in this work (JC 482) and the work of Sun and colleagues (PS1), which may explain the apparent discrepancy. Our life-span analyses are consistent with the suggestion that low PKA activity confers long life-span whereas high PKA activity accelerates aging (Lin et al., 2000).
Similar to yeast, the oncogenic Ras of human fibroblasts shortens cellular replicative life-span. In fibroblasts, the effect on life-span appears to be mediated by an elevated ROS concentration in the oncogenic cells (Serrano et al., 1997; Lee et al., 1999). In this work, we demonstrate that the oncogenic homolog in yeast affects oxidative metabolism through a, hitherto unknown PKA-independent pathway acting on mitochondrial respiratory activity. This, together with the fact that RAS2val19 cells exhibit low expression levels of STRE element genes, further highlights that these cells experience severe problems in oxidative stress management and, most likely, homeostatic control in general. However, even though the levels of oxidation stress defense genes, oxidation damage and ROS production correlate well with life-span in different ras2 mutants exhibiting different PKA activity, there is no direct evidence for oxidation stress being the bottleneck in the life-span of these cells. It should also be noted that, in Escherichia coli, ROS levels and oxidative damage are sometimes uncoupled and no obligatory correlation exists between expression of oxidation stress defense genes and oxidative carbonylation of proteins (Dukan and Nystrom, 1999; Dukan et al., 2000; Ballesteros et al., 2001). This also calls for care in interpreting the data of the RAS2val19 strain. In addition, both Pde2p overexpression and ectopic production of UCP1 caused a modest reduction in protein carbonylation, but only UCP1 production reduced ROS levels in the RAS2val19 cells and only Pde2p overexpression prolonged life-span. Nevertheless, it is safe to conclude that the defects in oxidative management of the RAS2val19 cells demonstrated here and elsewhere hardly benefit homeostatic control in these cells. In addition, it is possible that the PKA-independent effect on respiratory activity, rather than affecting life-span, may be linked to the oncogenic nature of the RAS2val19 homologs in mammals. This possibility may deserve future experimental scrutiny.
Materials and methods
Yeast strains and plasmids
All strains were derived from wild-type yeast strain JC 482 (MATa, ura3-52, leu2, RAS2, his4-539). The construction of the RAS mutants was described elsewhere (Pichova et al., 1997). To exclude artifacts due to spontaneous suppressor mutations during experiments with cells carrying the RAS2val19 allele, we systematically stained colonies for glycogen content (Chester, 1968; Toda et al., 1985) after each experiment. The bcy1 mutant strain was isolated as sra1-13 (suppressor of RAS2) as described (Cannon et al., 1986) and transferred by mating to the wild-type JC 482 strain (kindly provided by M.Breitenbach, University of Salzburg). The multicopy plasmid Yep13 (LEU2 marker, A.Pichova’s library) was used when overexpressing PDE2. The pYEPD plasmid was used to express the human UCP1 gene behind the GAL1/10 promoter (kindly provided by M. Rigoulet, University of Bordeaux II).
Growth conditions
Cells were propagated in either YPD [1% (w/v) yeast extract, 2% (w/v) peptone, 2% (w/v) glucose, pH 6.5) or SC medium [2% (w/v) glucose, 0.17% (w/v) yeast nitrogen base, 0.5% ammonium sulphate, 0.077% (w/v) amino acids) with appropriate amino acid and nucleic acid content to impose a selection when needed (strains carrying plasmids). Cultures were grown aerobically by batch cultivation (200 r.p.m. at 28°C) with a liquid-to-air ratio of 0.2. For induction of the GAL1/10 promoter, the same media as described above were used but 2% galactose and 1% raffinose were used as the sole carbon sources. Although mutants expressing a constitutively active PKA pathway are generally described as incapable of growth on galactose, a ‘weakly fermentable carbon source’ (Thevelein, 1994), the RAS2val19 strain was able to grow on this carbon source with both the empty pYEPD plasmid and the UCP1-carrying vector. As stated above, we confirmed that the RAS2val19 strain exhibited the expected phenotypes relating to a constitutively active PKA pathway and that a bcy1 mutant strain could not grow on galactose (Cannon and Tatchell, 1987). Thus it appears as if the activity of the PKA pathway differs between the RAS2val19 strain and the bcy1 mutant strain. Regardless of the reason for this difference, it allowed us to use the GAL1/10 promoter in experiments with the RAS2val19 strain.
Stress exposure and life-span analysis
Cells were grown to exponential phase (OD600 = 1) at 28°C and small aliquots of culture were subsequently transferred to tubes held at 52°C. Cells were then withdrawn at different times, diluted and plated onto YPD plate to measure colony-forming units. To score for oxidative sensitivity, serial dilutions of wild-type and RAS mutant cells were taken from exponential phase (OD600 = 1) cultures and dropped on YPD plates with or without paraquat (400 µg/ml). Life-span was determined as previously described (Pichova et al., 1997; Park et al., 2002). Cohorts of at least 40 randomly chosen cells were examined. Cells that never budded were excluded from the analysis. All measurements of life-span were performed on SC medium.
In situ staining of ROS and mitochondria
Free intracellular radicals or strongly oxidizing molecules (ROS) were detected with DHE (Fluka). Cells were taken from the exponential phase (OD600 = 1), spun down and resuspended in HEPES buffer with 2% (w/v) glucose. DHE was added to a final concentration of 5 µg/ml and cells were incubated for 15 min before applying fluorescence microscopy using excitation and emission settings of 512 nm and 565 nm, respectively. The mitochondria of exponential phase cells (OD600 = 1) were stained with either MitoTrackerGreen FM or DiOC6 (Molecular Probes) as described by the manufacturer. Cells were then analyzed using fluorescence microscopy at excitation and emission settings of 484–490 nm and 501–516 nm, respectively. Identical exposure times were used to compare samples for all imaging experiments. Quantitative analysis of light intensity (on average 250 cells were analyzed) was performed by densitometry using the Scion Image software (Scion Image Beta 4.02 Win). This software is based on the Macintosh NIH Image Software.
Mitochondrial membrane potential
Mitochondrial membrane potential was determined by Rhodamine 123 (Molecular Probes) staining. Exponentially growing cells (OD600 = 1) were harvested and washed twice in ice-cold distilled water. Cells were resuspended in water and Rhodamine 123 was added to a final concentration of 1.25 µM. After incubation for 10 min in the dark at room temperature, cells were analyzed with fluorescent microscopy using excitation and emission settings at 500 nm and 525 nm, respectively. Quantification of light intensity was performed as described for ROS and mitochondria staining (see previous section).
Respirometry and measurement of RSV
The respirometric measurements were performed with a Cyclobios oxygraph as described (Aguilaniu et al., 2001). Briefly, samples (2.5 ml) were taken from exponentially growing cultures (glucose-repressed condition) at OD600 = 1 and oxygen consumption was followed in the respirometric chamber. TET (Aldrich) (from 37.5 mM for wild type to 225 mM for RAS2val19) and CCCP (Sigma) (∼10 mM for all tested strains) were added to the cuvette directly and the decrease in oxygen concentration was subsequently followed on-line.
Western blot analysis
Protein extracts were prepared by glass-bead disruption in a Protein Extraction Buffer (20 mM Tris–HCl pH 7.9, 10 mM MgCl2, 5% glycerol, and tablets of the protease inhibitor mix from Roche). Protein concentrations were determined using the BCA Protein Assay Reagent (Pierce) before they were loaded on SDS–polyacrylamide gels as described (Laemmli, 1970). Separated proteins on gels were transferred by semidry electroblotting onto a PVDF membrane (Millipore). Filters were then treated with the relevant primary and subsequently secondary (horseradish peroxidase conjugate) antibodies dissolved in PBS–Tween. Detection was accomplished using the ECL Plus blotting kit (Amersham). The e subunit of ATP synthesase was detected with an antibody directed against Tim11p (kindly provided by D.Rapaport, University of Munich). Quantitative analysis of light intensity was performed by densitometry using the Scion Image software (Scion Image Beta 4.02 Win).
Carbonylation assays
Detection of carbonylated proteins was performed using the OxyBlot™ Oxidized Protein Detection Kit (Intergen,USA) as described previously (Aguilaniu et al., 2001).
Catalase activity measurements
Measurement of catalase activity was performed as described (Jakubowski et al., 2000). Briefly, 4 ml of exponentially growing cultures (OD600 = 1) were washed three times with 2 ml of 50 mM Na phosphate buffer (pH 7.4). Cells were then resuspended in 0.5 ml of buffer and broken with glass beads (10 min at 4°C). Crude protein extracts were mixed with an aqueous H2O2 solution (54 mM) and Na phosphate buffer (pH 7) and the decrease in absorbance at 240 nm was measured. One unit of enzyme activity corresponds to the decomposition of 1 µmol substrate during 1 min. Reference curves were obtained using catalase C30 from bovine liver (Sigma). As a control, cell size was compared and none of the mutants used differed in that respect.
Measurement of intracellular metabolites
ATP and ADP were measured in TCA extracts (as described by Gustafsson et al., 1979) from exponentially growing cells. ATP was measured by monitoring the luciferase-catalyzed reaction (using the ATP bioluminescence assay kit from Roche) with a luminometer. ADP was detected after enzymatic conversion to ATP. Acidic extraction (TCA) was used for NAD (as described by Gustafsson et al., 1979), while basic extraction was used for NADH (as described by Bergmeyer, 1974). Intracellular NAD/NADH concentrations were determined enzymatically by measuring the oxidation or reduction of NADH or NAD, respectively, at 340 nm.
Quantification of petite (respiratory-deficient) mutant formation
Cells from an exponentially growing culture (OD600 = 1) were diluted and plated on YP + glucose 1% (w/v) and glycerol 2% (w/v) plates. Colonies were overlaid by 43°C agar containing triphenyltertazoliumchloride as described (Ogur et al., 1957). After incubation for30 min at 37°C, respiration-deficient cells were counted as white small colonies whereas respiration-sufficient cells were colored red.
Reproducibility
All experiments presented in this paper have been repeated at least three times to confirm reproducibility. Representative results are shown in the figures.
Acknowledgments
Acknowledgements
This work was funded by the Swedish Natural Science Research Council (T.N.), the European Union (L.H.), a Swedish Royal Academy grant (T.N. and L.H.), a Chalmers University of Technology Bioscience Initiative grant (H.A.) and the Grant Agency of the Czech Republic (GACR 301/03/0289)(A.P.).
References
- Aguilaniu H., Gustafsson,L., Rigoulet,M. and Nystrom,T. (2003) Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science, 299, 1751–1753. [DOI] [PubMed] [Google Scholar]
- Aguilaniu H., Gustafsson,L., Rigoulet,M. and Nystrom,T. (2001) Protein oxidation in G0 cells of Saccharomyces cerevisiae depends on the state rather than rate of respiration and is enhanced in pos9 but not yap1 mutants. J. Biol. Chem., 28, 28. [DOI] [PubMed] [Google Scholar]
- Ballesteros M., Fredriksson,A., Henriksson,J. and Nystrom,T. (2001) Bacterial senescence: protein oxidation in non-proliferating cells is dictated by the accuracy of the ribosomes. EMBO J., 20, 5280–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeyer H. (1974) Methods in Enzymatic Analysis. Academic Press, New York, NY. [Google Scholar]
- Bindokas V.P., Jordan,J., Lee,C.C. and Miller,R.J. (1996) Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J. Neurosci., 16, 1324–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A. and Chance,B. (1973) The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J., 134, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek D., Samiy,N., Fasano,O., Fujiyama,A., Tamanoi,F., Northup,J. and Wigler,M. (1985) Differential activation of yeast adenylate cyclase by wild-type and mutant RAS proteins. Cell, 41, 763–769. [DOI] [PubMed] [Google Scholar]
- Cain K. and Griffiths,D.E. (1977) Studies of energy-linked reactions. Localization of the site of action of trialkyltin in yeast mitochondria. Biochem. J., 162, 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camonis J.H. and Jacquet,M. (1988) A new RAS mutation that suppresses the CDC25 gene requirement for growth of Saccharomyces cerevisiae. Mol. Cell. Biol., 8, 2980–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J.F. and Tatchell,K. (1987) Characterization of Saccharomyces cerevisiae genes encoding subunits of cyclic AMP-dependent protein kinase. Mol. Cell. Biol., 7, 2653–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J.F., Gibbs,J.B. and Tatchell,K. (1986) Suppressors of the ras2 mutation of Saccharomyces cerevisiae. Genetics, 113, 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.B., Sun,J. and Jazwinski,S.M. (1990) Prolongation of the yeast life span by the v-Ha-RAS oncogene. Mol. Microbiol., 4, 2081–2086. [DOI] [PubMed] [Google Scholar]
- Chester V.E. (1968) Heritable glycogen-storage deficiency in yeast and its induction by ultra-violet light. J. Gen. Microbiol., 51, 49–56. [DOI] [PubMed] [Google Scholar]
- Dejean L., Beauvoit,B., Bunoust,O., Guerin,B. and Rigoulet,M. (2002) Activation of Ras cascade increases the mitochondrial enzyme content of respiratory competent yeast. Biochem. Biophys. Res. Commun., 293, 1383–1388. [DOI] [PubMed] [Google Scholar]
- Denis G.V., Yu,Q., Ma,P., Deeds,L., Faller,D.V. and Chen,C.Y. (2003) Bcl-2, via its BH4 domain, blocks apoptotic signaling mediated by mitochondrial Ras. J. Biol. Chem., 278, 5775–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukan S. and Nystrom,T. (1998) Bacterial senescence: stasis results in increased and differential oxidation of cytoplasmic proteins leading to developmental induction of the heat shock regulon. Genes Dev., 12, 3431–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukan S. and Nystrom,T. (1999) Oxidative stress defense and deterioration of growth-arrested Escherichia coli cells. J. Biol. Chem., 274, 26027–26032. [DOI] [PubMed] [Google Scholar]
- Dukan S., Farewell,A., Ballesteros,M., Taddei,F., Radman,M. and Nystrom,T. (2000) Protein oxidation in response to increased transcriptional or translational errors. Proc. Natl Acad. Sci. USA, 97, 5746–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtay K.S., et al. (2002) Superoxide activates mitochondrial uncoupling proteins. Nature, 415, 96–99. [DOI] [PubMed] [Google Scholar]
- Egilmez N.K. and Jazwinski,S.M. (1989) Evidence for the involvement of a cytoplasmic factor in the aging of the yeast Saccharomyces cerevisiae. J. Bacteriol., 171, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egilmez N.K., Chen,J.B. and Jazwinski,S.M. (1990) Preparation and partial characterization of old yeast cells. J. Gerontol., 45, B9–B17. [DOI] [PubMed] [Google Scholar]
- Enerbäck S., Jacobsson,A., Simpson,E.M., Guerra,C., Yamashita,H., Harper,M.E. and Kozak,L.P. (1997) Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature, 387, 90–94. [DOI] [PubMed] [Google Scholar]
- Ferbeyre G., de Stanchina,E., Lin,A.W., Querido,E., McCurrach,M.E., Hannon,G.J. and Lowe,S.W. (2002) Oncogenic ras and p53 cooperate to induce cellular senescence. Mol. Cell. Biol., 22, 3497–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitton V., Rigoulet,M., Ouhabi,R. and Guerin,B. (1994) Mechanistic stoichiometry of yeast mitochondrial oxidative phosphorylation. Biochemistry, 33, 9692–9698. [DOI] [PubMed] [Google Scholar]
- Garlid K.D., Jaburek,M. and Jezek,P. (1998) The mechanism of proton transport mediated by mitochondrial uncoupling proteins. FEBS Lett., 438, 10–14. [DOI] [PubMed] [Google Scholar]
- Gustafsson L. (1979) The ATP pool in relation to the production of glycerol and heat during growth of the halotolerant yeast Debaryomyces hansenii. Arch. Microbiol., 120, 15–23. [Google Scholar]
- Hall A. and Self,A.J. (1986) The effect of Mg2+ on the guanine nucleotide exchange rate of p21N-ras. J. Biol. Chem., 261, 10963–10965. [PubMed] [Google Scholar]
- Hasan R., Leroy,C., Isnard,A.D., Labarre,J., Boy-Marcotte,E. and Toledano,M.B. (2002) The control of the yeast H2O2 response by the Msn2/4 transcription factors. Mol. Microbiol., 45, 233–241. [DOI] [PubMed] [Google Scholar]
- Jakubowski W., Bilinski,T. and Bartosz,G. (2000) Oxidative stress during aging of stationary cultures of the yeast Saccharomyces cerevisiae. Free Radic. Biol. Med., 28, 659–664. [DOI] [PubMed] [Google Scholar]
- Jazwinski S.M. (1999a) Molecular mechanisms of yeast longevity. Trends Microbiol., 7, 247–252. [DOI] [PubMed] [Google Scholar]
- Jazwinski S.M. (1999b) The RAS genes: a homeostatic device in Saccharomyces cerevisiae longevity. Neurobiol. Aging, 20, 471–478. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers,S., McGill,C., Fasano,O., Strathern,J., Broach,J. and Wigler,M. (1984) Genetic analysis of yeast RAS1 and RAS2 genes. Cell, 37, 437–445. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers,S., Cameron,S., Fasano,O., Goldfarb,M., Broach,J. and Wigler,M. (1985) Functional homology of mammalian and yeast RAS genes. Cell, 40, 19–26. [DOI] [PubMed] [Google Scholar]
- Kirchman P.A., Kim,S., Lai,C.Y. and Jazwinski,S.M. (1999) Interorganelle signaling is a determinant of longevity in Saccharomyces cerevisiae. Genetics, 152, 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. and Huang,S.G. (1999) Structure and function of the uncoupling protein from brown adipose tissue. Biochim. Biophys. Acta, 1415, 271–296. [DOI] [PubMed] [Google Scholar]
- Korshunov S.S., Skulachev,V.P. and Starkov,A.A. (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett., 416, 15–18. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lee A.C., et al. (1999) Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem., 274, 7936–7940. [DOI] [PubMed] [Google Scholar]
- Lin S.J., Defossez,P.A. and Guarente,L. (2000) Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science, 289, 2126–2128. [DOI] [PubMed] [Google Scholar]
- Longo V.D. (1999) Mutations in signal transduction proteins increase stress resistance and longevity in yeast, nematodes, fruit flies and mammalian neuronal cells. Neurobiol. Aging, 20, 479–486. [DOI] [PubMed] [Google Scholar]
- Luo J., Nikolaev,A.Y., Imai,S., Chen,D., Su,F., Shiloh,A., Guarente,L. and Gu,W. (2001) Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell, 107, 137–148. [DOI] [PubMed] [Google Scholar]
- Marchler G., Schuller,C., Adam,G. and Ruis,H. (1993) A Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J., 12, 1997–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pastor M.T., Marchler,G., Schuller,C., Marchler-Bauer,A., Ruis,H. and Estruch,F. (1996) The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE). EMBO J., 15, 2227–2235. [PMC free article] [PubMed] [Google Scholar]
- Mortimer R.K. and Johnston,J.R. (1959) Life span of individual yeast cells. Nature, 183, 1751–1752. [DOI] [PubMed] [Google Scholar]
- Nicholls D.G. and Ferguson,S.J. (1992) Bioenergetics 2. Academic Press, London, UK. [Google Scholar]
- Ogur M., St John,M. and Nagai,S. (1957) Tetrazolium overlay technique for population studies of respiration deficiency in yeast. Science, 125, 982–992. [DOI] [PubMed] [Google Scholar]
- Park P.U., McVey,M. and Guarente,L. (2002) Separation of mother and daughter cells. Methods Enzymol., 351, 468–477. [DOI] [PubMed] [Google Scholar]
- Parrini M.C., Bernardi,A. and Parmeggiani,A. (1996) Determinants of Ras proteins specifying the sensitivity to yeast Ira2p and human p120-GAP. EMBO J., 15, 1107–1111. [PMC free article] [PubMed] [Google Scholar]
- Pichova A., Vondrakova,D. and Breitenbach,M. (1997) Mutants in the Saccharomyces cerevisiae RAS2 gene influence life span, cytoskeleton and regulation of mitosis. Can. J. Microbiol., 43, 774–781. [DOI] [PubMed] [Google Scholar]
- Serrano M., Lin,A.W., McCurrach,M.E., Beach,D. and Lowe,S.W. (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell, 88, 593–602. [DOI] [PubMed] [Google Scholar]
- Sinclair D.A. and Guarente,L. (1997) Extrachromosomal rDNA circles—a cause of aging in yeast. Cell, 91, 1033–1042. [DOI] [PubMed] [Google Scholar]
- Skulachev V.P. (1996) Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q. Rev. Biophys., 29, 169–202. [DOI] [PubMed] [Google Scholar]
- Skulachev V.P. (1998) Uncoupling: new approaches to an old problem of bioenergetics. Biochim. Biophys. Acta, 1363, 100–124. [DOI] [PubMed] [Google Scholar]
- Smeal T., Claus,J., Kennedy,B., Cole,F. and Guarente,L. (1996) Loss of transcriptional silencing causes sterility in old mother cells of S.cerevisiae. Cell, 84, 633–642. [DOI] [PubMed] [Google Scholar]
- Stuart J.A., Harper,J.A., Brindle,K.M., Jekabsons,M.B. and Brand,M.D. (2001) A mitochondrial uncoupling artifact can be caused by expression of uncoupling protein 1 in yeast. Biochem. J., 356, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Kale,S.P., Childress,A.M., Pinswasdi,C. and Jazwinski,S.M. (1994) Divergent roles of RAS1 and RAS2 in yeast longevity. J. Biol. Chem., 269, 18638–18645. [PubMed] [Google Scholar]
- Thevelein J.M. (1994) Signal transduction in yeast. Yeast, 10, 1753–1790. [DOI] [PubMed] [Google Scholar]
- Toda T., et al. (1985) In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell, 40, 27–36. [DOI] [PubMed] [Google Scholar]
- Tyner S.D., et al. (2002) p53 mutant mice that display early ageing-associated phenotypes. Nature, 415, 45–53. [DOI] [PubMed] [Google Scholar]
- Vaziri H., Dessain,S.K., Ng Eaton,E., Imai,S.I., Frye,R.A., Pandita,T.K., Guarente,L. and Weinberg,R.A. (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell, 107, 149–159. [DOI] [PubMed] [Google Scholar]