Abstract
Here we describe a new signaling cross-talk between the Vav/Rac1 and Ras pathways that is established through the stimulation of RasGRP1, an exchange factor for Ras subfamily GTPases. This interaction is crucial for Ras activation in lymphoid cells, since this GTPase cannot become activated in the absence of Vav proteins. The activation of RasGRP1 requires both the generation of diacylglycerol via phospho lipase C-γ and the induction of actin polymerization, two responses induced by Vav and Rac1 that facilitate the translocation of RasGRP1 to juxtamembrane areas of the cell. Consistent with this, the cross-talk can be activated by tyrosine-phosphorylated wild-type Vav, oncogenic Vav and constitutively active Rac1. Conversely, Ras activation can be blocked in lymphocytes and ectopic systems using inhibitors affecting either phospholipase C-γ or F-actin polymerization. These results indicate that a relay mechanism exists in lymphoid and other cells helping in the generation of robust signaling responses by the Rac/Rho and Ras pathways upon receptor engagement.
Keywords: actin/Rac1/Ras/RasGRP1/Vav
Introduction
Vav proteins (Vav, Vav2 and Vav3) work as guanosine nucleotide exchange factors (GEFs) for specific members of the Rho/Rac family (Bustelo, 2000). This enzyme activity is positively regulated by direct tyrosine phosphorylation (Crespo et al., 1997; Bustelo, 2000). Recent structural studies have indicated that this activation step correlates with the relaxation of an autoinhibitory loop established by the interaction of the Vav N-terminal domains with other regions of Vav proteins (Aghazadeh et al., 2000; Bustelo, 2002; Zugaza et al., 2002). The tight regulation of the Vav molecules is lost when its N-terminal domains are either eliminated or inactivated by point mutations, leading to the generation of highly oncogenic proteins whose biochemical activities are independent of tyrosine phosphorylation (Bustelo, 2000, 2002). In agreement with their catalytic role, most of the biological and pathological responses induced by Vav proteins have been linked to the stimulation of specific members of the Rho subfamily (Bustelo, 2000). However, recent results suggest that the Vav family also utilizes signaling elements outside its own cascade, especially those belonging to the Ras pathway. For instance, it has been shown that the overexpression of either Ras or Raf dominant-negative mutants leads to the inhibition of certain Vav-dependent responses such as cell transformation and NF-AT activation (Bustelo et al., 1994; Wu et al., 1995). Conversely, the elimination of Vav in T cells results in the attenuation of Ras-dependent effects, such as Erk stimulation (Costello et al., 1999). It has also been demonstrated that the overexpression of Vav in Jurkat T cells results in the activation of Ras-dependent responses, such as the enhancement of the kinase activity of Erk and the expression of the surface marker CD69 (Villalba et al., 2000). Finally, the coexpression of Vav and Ras leads to synergistic responses in terms of both oncogenesis and NF-AT activation (Bustelo et al., 1994; Wu et al., 1995), further implying that these two signaling routes are intertwined at some level(s). One possible explanation for this signaling interaction is that the Vav pathway enhances the activation of Ras downstream elements during cellular responses. Thus it has been shown that Rac1 can influence the activation of both Raf and MEK1 through one of its effectors, p21-associated kinase (PAK) (Bar-Sagi and Hall, 2000). Likewise, Rho proteins can antagonize the induction of p21WAF by Ras, resulting in more robust signaling responses (Bar-Sagi and Hall, 2000). However, experiments conducted in our laboratory indicate that the effect of Vav on the Ras pathway cannot be replicated by overexpressing the constitutive form of PAK. Moreover, biochip analyses have shown that Vav upregulates p21WAF, indicating that the synergism between Vav and Ras cannot be established at the level of this cell cycle inhibitor (F.Núñez and X.R.Bustelo, unpublished data). Such negative results suggested to us that other cross-talk mechanisms between the Vav and Ras pathways were at work.
Recent reports with knockout cells and animals have indicated that the disruption of vav, vav3 and rac2 genes negatively affects phospholipase C-γ (PLC-γ) activation in hematopoietic cells (Costello et al., 1999; Manetz et al., 2001; Yu et al., 2001; Inabe et al., 2002). These observations prompted us to investigate whether Vav could influence the status of activation of the Ras pathway via the stimulation of Ras guanosine nucleotide releasing proteins (RasGRPs). These proteins, also known as calcium- and diacylglycerol-dependent (CalDAG) GEFs, contain a catalytic domain for promoting GDP/GTP exchange on Ras and/or Rap proteins, a calcium-binding domain and a zinc finger (ZF) capable of binding to diacylglycerol (DAG), one of the second messengers generated by the activity of PLC-γ (Quilliam et al., 2002). Most RasGRPs become activated by DAG and molecular analogs such as phorbol esters and bryostatin, thus offering an obvious link between receptor activation, PLC-γ stimulation and Ras/Rap function during cell signaling (Quilliam et al., 2002). In this article, we show that Vav and one of its substrates, Rac1, promote changes in the activation state of RasGRP1. This effect requires the convergence of two separate Vav/Rac1-dependent steps, stimulation of PLC-γ activity and F-actin polymerization. We also provide evidence demonstrating that this Vav/RasGRP signaling interaction is evolutionarily conserved and crucial for the activation of the Ras pathway in lymphoid cells.
Results
The Vav pathway is required for proper Ras activation in lymphoid cells
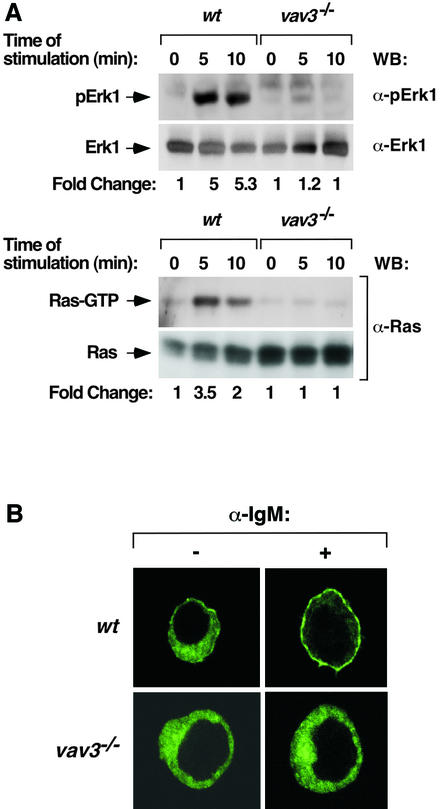
We used a genetic approach to ascertain the possible functional interaction between the Vav and Ras pathways. We speculated that if this interaction plays an important role in cell signaling, the elimination of the Vav pathway should have a direct impact on the activation of the Ras cascade in vivo. To date, this type of experiment has been difficult to approach in cells derived from knockout animals owing to the coexpression of the three Vav family members in most hematopoietic cells. To surmount this problem, we decided to use a recently described chicken B-cell line (DT40 cells) in which the vav3 gene has been eliminated by homologous recombination (Inabe et al., 2002). Since these cells express primarily this Vav family member, we expected that the possible contribution of Vav proteins to the stimulation of the Ras pathway would not be masked by the redundant function of Vav or Vav2. The biochemical phenotype of these cells is reminiscent of that previously described for mouse vav–/– T cells (Costello et al., 1999), including decreased activity levels of PLC-γ and phosphatidylinositol-3 kinase (PI3K) as well as inefficient generation of Ca2+ fluxes during B-cell receptor (BCR) signaling (Inabe et al., 2002). To monitor the status of the Ras pathway in this system, we first analyzed the levels of activated Erk1 during BCR stimulation using immunoblot analysis with phosphospecific antibodies to this kinase. As shown in Figure 1A (upper two panels), Erk1 activation is severely affected in vav3–/– DT40 cells even in the presence of optimal amounts of antibodies to the IgM receptor. In contrast, wild-type DT40 cells respond to BCR stimulation with a rapid activation of Erk1 upon anti-IgM treatment (Figure 1A, upper two panels). To discriminate whether the defective activation of Erk1 was due to lack of Ras activation or to an indirect effect of the Vav3/Rac1 pathway on Ras downstream elements, we measured the levels of GTP–Ras during the stimulation of DT40 cells using pulldown experiments with the Ras binding domain (RBD) of c-Raf1. As shown in Figure 1A (lower two panels), vav3–/– cells experience no detectable Ras activation after BCR stimulation under conditions in which their wild-type counterparts do show a strong activation of the GTPase. These results indicate that the defect in Erk1 activation found in vav3–/– DT40 cells is mostly derived from the improper stimulation of Ras during BCR signaling.
Fig. 1. (A) Status of the Ras pathway in vav3–/– DT40 cells. Wild-type (wt) or vav3–/– DT40 cells were stimulated with mouse anti-chicken IgM (10 µg/ml) for the indicated periods of time. Cell lysates were then obtained and analyzed to detect activated Erk1 (using anti-phospho-Erk1 immunoblots, first panel from top), Erk1 (using anti-Erk1 immunoblots, second panel from top), the GTP levels of endogenous Ras (using GST–Raf RBD pulldown experiments, third panel from top) and Ras protein (using anti-Ras immunoblots, bottom panel). WB, western blot. The fold change of activation for each protein was estimated by densitometric analysis of filters. The values (given as arbitrary units) were normalized taking into consideration the protein levels of each sample as determined by immunoblot analysis. This criterion was followed in subsequent figures. (B) Translocation of RasGRP1 to the plasma membrane in DT40 cells. A plasmid encoding an EGFP– RasGRP1 fusion protein was transfected in wild-type (upper panels) and vav3–/– (lower panels) DT40 cells. After transfection, cells were either non-stimulated (–) or stimulated (+) as indicated above, fixed and subjected to microscopic analysis.
Given the important role of RasGRP1 in the signaling pathways of lymphoid cells (Dower et al., 2000), we next investigated the functional status of this Ras GEF in both wild-type and Vav3-deficient DT40 cells. To this end, we expressed in those cells a version of RasGRP1 fused to the enhanced green fluorescent protein (EGFP). After expression, the subcellular localization of this chimeric protein was monitored by fluorescence microscopy in naive and BCR-stimulated cells. In non-stimulated cells, RasGRP1 is found uniformly distributed in the cytoplasm independently of the presence of Vav3 in cells (Figure 1B, left panels). Upon BCR stimulation, RasGRP1 translocates efficiently to the plasma membrane in wild-type cells (Figure 1B, upper right panel). However, such translocation does not occur in stimulated cells lacking Vav3 (Figure 1B, lower right panel). Taken together, these observations suggest that the absence of a normal signaling output from Vav-related pathways in DT40 cells leads to abnormal activation of RasGRP1 and the Ras cascade.
Activation of RasGRP1 is mediated by the direct action of Vav proteins
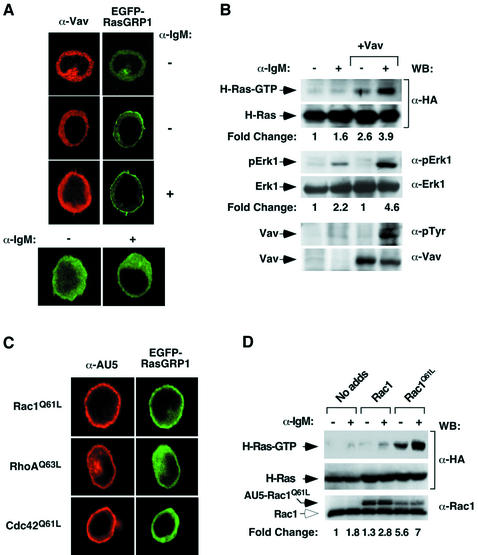
We performed reconstitution experiments in vav3–/– DT40 cells to understand better the role of the Vav pathway on the stimulation of both RasGRP1 and the Ras route. First, we coexpressed wild-type Vav and EGFP–RasGRP1 in vav3–/– DT40 cells to investigate the influence of Vav proteins in the subcellular localization of this Ras exchange factor. As shown in Figure 2A (upper two panels), we found that the coexpression of wild-type Vav with EGFP–RasGRP1 in the absence of cell stimulation induces the translocation of RasGRP1 to the plasma membrane in only a minority (∼10%) of the transfected vav3–/– cells. However, this percentage increases to 95% of the transfected cells upon stimulation of the BCR with anti-IgM antibodies. The differential effect of Vav in non-stimulated and stimulated cells is probably due to the increased phosphorylation of Vav on tyrosine residues upon BCR engagement (see below, Figure 2B). As expected (see Figure 1B), we found no translocation of EGFP–RasGRP1 in Vav3-deficient cells in the absence of Vav expression (Figure 2C, lower panels).
Fig. 2. (A) Vav rescues the defective translocation of RasGRP1 to the plasma membrane in vav3–/– DT40 cells. Cells were transfected with plasmids encoding Vav and EGFP–RasGRP1 and then were either left non-stimulated (–) or stimulated with anti-IgM antibodies (10 µg/ml) for 10 min. Cells were then fixed, stained with rabbit anti-Vav antibodies and Cy3-labeled antibodies to rabbit IgGs and visualized by microscopy. The two top panels show an isolated example of the majority (first panel from top) and minority (second panel from top) of cells coexpressing Vav and EGFP–RasGRP1 (see text for details). Images show the distribution of Vav and EGFP–RasGRP1 in red and green, respectively. (B) Vav rescues the defective activation of the Ras cascade in vav3–/– DT40 cells. Cells were transfected with a plasmid encoding H-Ras (HA-tagged) either alone or in combination with a vector encoding Vav. After transfection, cells were stimulated and the levels of activation of Ras and Erk1 determined by GST–RBD pulldown experiments and anti-phosphoErk1 immunoblot, respectively. In addition, the levels of expression of each protein were determined by western blotting using the indicated antibodies. The mobility of each protein under analysis is indicated by an arrow on the left. (C) Rac1Q61L rescues the defective translocation of RasGRP1 to the plasma membrane in vav3–/– DT40 cells. Cells were transfected with plasmids encoding EGFP–RasGRP1 and the indicated AU5-tagged GTPases. After transfection, cells were fixed, stained with mouse anti-AU5 antibodies and Cy3-labeled antibodies to mouse IgGs and visualized in a fluorescence microscope. Images show the distribution of the GTPases and EGFP–RasGRP1 in red and green, respectively. (D) Rac1Q61L rescues the defective activation of the Ras cascade in vav3–/– DT40 cells. Cells were transfected with a plasmid encoding HA-tagged H-Ras either alone or in combination with vectors encoding the indicated AU5-tagged Rac1 proteins. After transfection, the levels of activation of Ras were determined by GST–RBD pulldown experiments. The levels of expression of Rac1 and Ras were determined by immunoblotting using the indicated antibodies. The mobility of each protein under analysis is indicated by an arrow on the left. The open arrow (bottom panel) signals endogenous Rac1.
We also investigated whether the expression of wild-type Vav could re-establish the defective stimulation of the Ras pathway in vav3–/– DT40 cells. As shown in Figure 2B (first and third panels from top), wild-type Vav can trigger the effective activation of both ectopically expressed H-Ras and endogenous Erk1 in anti-IgM stimulated vav3–/– DT40 cells. Instead, Vav is not efficient in promoting H-Ras and Erk1 activation in non-stimulated cells, a result probably derived from the low levels of tyrosine phosphorylation found for this Rac1 GEF in the absence of BCR stimulation (Figure 2B, fifth panel from top). All these proteins were expressed at comparable levels in all experimental samples, as assessed by immunoblotting experiments using appropriate antibodies (Figure 2B). These loss- and gain-of-function studies indicate that the Vav pathway plays a key role in regulating RasGRP1 activity and the signaling output of the Ras pathway in DT40 B cells.
The effect of Vav on RasGRP1 is mediated by Rac1-dependent pathways
Depending on the signaling context, Vav proteins can work as exchange factors for Rho/Rac proteins and/or as adaptor proteins for other molecules (Bustelo, 2001). To elucidate the Vav-dependent pathway that regulates RasGRP1, we tested whether the constitutively active versions of Rac1 (Q61L mutant), RhoA (Q63L mutant) and Cdc42 (Q61L mutant) could functionally complement the absence of Vav3 in DT40 cells. Immunofluorescence experiments indicated that Rac1Q61L induces the effective translocation of EGFP–RasGRP1 in vav3–/– DT40 cells. Owing to its constitutive loading with GTP, the action of Rac1Q61L on the RasGRP1 pathway is independent of the stimulation status of DT40 cells (Figure 2C, top right panel). This effect is specific for Rac1, because no detectable translocation of EGFP–RasGRP1 is observed when coexpressed with either RhoAQ63L or Cdc42Q61L (Figure 2C, second and third panels on right). These three GTPases show normal expression and membrane localization in DT40 cells (Figure 2C, left panels). Pulldown experiments with the GST–RBD fusion protein also demonstrated that Rac1Q61L, but not its wild-type counterpart, rescues the defective activation of H-Ras when transfected into vav3–/– DT40 cells (Figure 2D, top panel). These results indicate that the Vav/RasGRP1 cross-talk is mediated by the main Vav family substrate and not by GTPase-independent routes. Notably, we found that this cross-talk could be reconstructed in non-hematopoietic cells provided that we coexpressed in them the main elements of this pathway (RasGRP1 and either Vav or Rac1). Using COS1 cells, we found that the constitutively active version of Vav could promote the translocation of RasGRP1 and other family members (RasGRP2, RasGRP3) to the plasma membrane (Supplementary figure 1A and B, available at The EMBO Journal Online). Likewise, RacQ61L and oncogenic Vav could enhance the activation of Erk1 mediated by RasGRP1 in this cell type (Supplementary figure 2). Interestingly, RasGRP1 is not expressed endogenously in COS1 cells, suggesting that the signal transduction element(s) of the Vav/Rac pathway that regulates this Ras exchange factor is conserved in different cell types regardless of the expression in them of RasGRP family members.
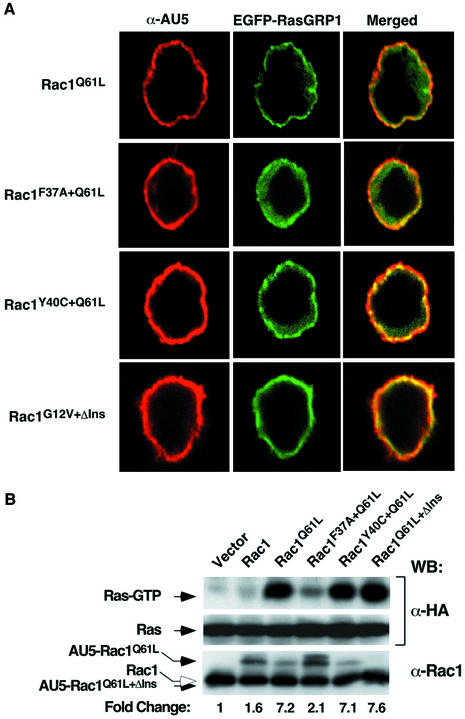
Since Rac1 can activate several signaling pathways simultaneously (Van Aelst and D’Souza-Schorey, 1997), we next utilized a collection of Rac1 effector mutants (Rac1Q61L+F37A, Rac1Q61L+Y40C and Rac1G12V+ΔIns), partially impaired in their signaling capacity, to identify the specific route(s) involved in RasGRP1 activation. Rac1Q61L+F37A activates PAK and JNK but cannot trigger transformation or cytoskeletal rearrangements. Rac1Q61L+Y40C can induce cytoskeletal changes and tumorigenesis without detectable activation of JNK or PAK kinases. Finally, Rac1G12V+ΔIns (a mutant protein lacking the α3′ insert region) can activate JNK and PAK and induce cytoskeletal changes, but cannot trigger superoxide production in cells (Joneson et al., 1996; Joneson and Bar-Sagi, 1998). Immunofluorescence and GST–RBD pulldown experiments indicated that both Rac1Q61L+Y40C and Rac1G12V+ΔIns can induce the optimal translocation of EGFP–RasGRP1 (Figure 3A) and the activation of H-Ras (Figure 3B) in vav3–/– DT40 cells. The activity of these mutants is comparable to that displayed by Rac1Q61L (Figure 3A and B). In contrast, Rac1Q61L+F37A was significantly less efficient in both responses (Figure 3A and B). Similar results were obtained with Jurkat T cells, indicating that the functional interaction between the Vav/Rac and Ras pathway is also at work in T lymphocytes (J.L.Zugaza, M.J.Caloca and X.R.Bustelo, unpublished observations). These observations suggest that a signaling route independent of PAK, JNK and superoxide production is responsible for the activation of RasGRP1 by Vav/Rac1 in these cells.
Fig. 3. (A) Effect of Rac1 effector mutants on RasGRP1 translocation in vav3–/– DT40 cells. Cells were transfected with plasmids encoding EGFP–RasGRP1 and the indicated mutants of Rac1 (AU5-tagged). After transfection, cells were fixed, stained with mouse anti-AU5 antibodies and Cy3-labeled antibodies to mouse IgGs and visualized in a fluorescence microscope. Images show the distribution of the GTPases and EGFP–RasGRP1 in red and green, respectively. The areas of colocalization of those proteins are shown in yellow. (B) Activation of Ras by Rac1 effector mutants in vav3–/– DT40 cells. Cells were transfected with a plasmid encoding HA-H-Ras either alone or in combination with vectors encoding the indicated Rac1 mutant. The levels of activation of Ras were then determined by GST–RBD pulldown experiments. The levels of expression of Rac1 and Ras were determined by immunoblotting using the indicated antibodies (right side of the panels). The mobility of each protein under analysis is indicated by an arrow on the left. The open arrow (bottom panel) signals the endogenous Rac1.
The stimulation of RasGRP1 by Vav/Rac1 requires phospholipase C-γ activity
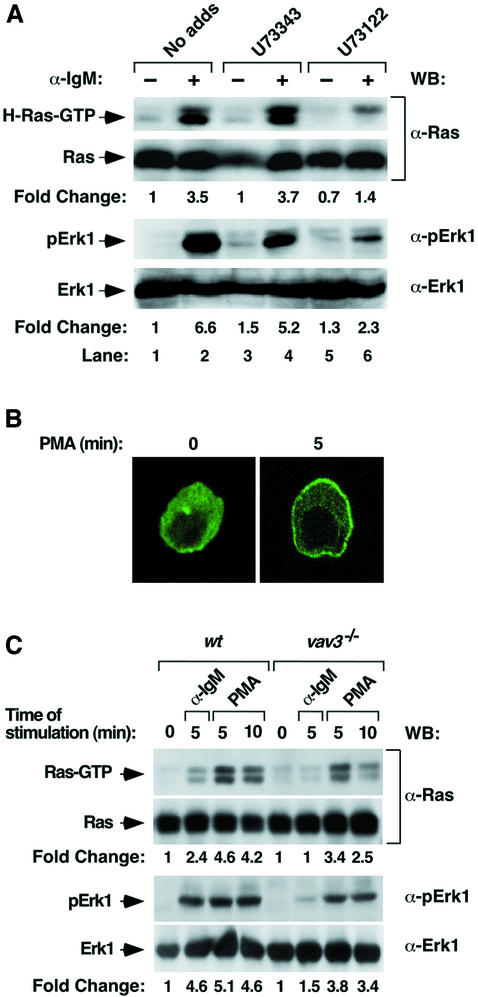
It has previously been shown that vav3–/– DT40 cells show deficient activation of PLC-γ (Inabe et al., 2002), the enzyme presumably involved in the activation of RasGRP1 via the generation of DAG. To assess the importance of PLC-γ on Ras activation in those cells, we first utilized a drug (U73122) that targets specifically the catalytic activity of this enzyme. The incubation of wild-type DT40 cells with this compound leads to a marked inhibition of both Ras and Erk1 activation during the stimulation of the BCR (Figure 4A, compare lanes 2 and 6). As negative control, a structurally similar compound incapable of inhibiting PLC-γ (U73343) does not have any effect on this signaling response (Figure 4A, compare lanes 2 and 4). Western blot experiments confirmed that U73122 and U73343 do not affect the expression levels of Erk1 and Ras in DT40 cells (Figure 4A). As additional evidence for the implication of PLC-γ in the Vav-dependent activation of RasGRP1, we investigated whether phorbol 12-myristate 13-acetate (PMA) could rescue the defective activation of RasGRP1 and Ras in Vav3-deficient DT40 cells. As shown in Figure 4B, PMA does induce the relocalization of RasGRP1 protein from the cytosol to cellular membranes. However, this translocation is not identical to that induced by either BCR engagement or Vav/RacQ61L expression, since RasGRP1 moves towards the plasma and nuclear membranes in the presence of PMA (Figure 4B). Despite this differential effect on the subcellular localization of RasGRP1, PMA promotes an efficient rescue of both Ras and Erk1 activation in Vav3-deficient DT40 cells (Figure 4C). Taken together, these results suggest that PLC-γ is part of the signaling machinery involved in the activation of the Ras by the Vav3 pathway in DT40 cells.
Fig. 4. (A) Effect of PLC-γ inhibitors on the Ras pathway in DT40 cells. Wild-type DT40 cells were either non-stimulated (–) or stimulated (+) with anti-IgM antibodies in the absence or presence of U73343 and U73122 (10 µM each) (Alexis Biochemicals). Cells were then lysed and subjected to either GST–RBD pulldown experiments (top panel) or anti-phosphoErk1 immunoblots (third panel from top) to detect the levels of activity of Ras and Erk1, respectively. Parallel samples were analyzed by western blotting using anti-Ras (second panel from top) and anti-Erk1 (lower panel) antibodies to visualize the levels of these proteins in each condition. (B) PMA induces the translocation of RasGRP1 in DT40 cells. vav3–/– DT40 cells expressing an EGFP–RasGRP1 fusion protein were treated for the indicated periods of time with PMA (100 nM), fixed and subjected to microscope analysis. (C) Rescue of Ras pathway activation in vav3–/– DT40 cells by phorbol esters. Wild-type and vav3–/– DT40 cells were stimulated for the indicated periods of time with either anti-IgM (10 µg/ml) or PMA (1 µM). The levels of activated Ras (upper panel) and phospho-Erk1 (third panel from top) were then determined as indicated in Materials and methods. Parallel samples were immunoblotted with specific antibodies to detect the levels of Ras (second panel from top) and Erk1 (bottom panel) in each condition.
The activation of PLC-γ by Vav proteins is not an exclusive property of lymphoid cells. Using COS1 cells as experimental system, we found that the Vav oncoprotein can induce high levels of PLC activity in this cell type (see Supplementary figure 3A). Moreover, we observed that the U73122 inhibitor provokes a 3-fold reduction in the activation of Erk1 derived from the coexpression of the Vav oncoprotein and RasGRP1 (see Supplementary figure 3B). The importance of these results is 2-fold. First, they indicate that the pathway regulated by Vav family proteins has an active role in the direct activation of PLC-γ. Secondly, they suggest that this pathway is conserved in most cell types regardless of whether or not it is used for RasGRP1 regulation.
F-actin contributes to the stimulation of RasGRPs by the Vav/Rac1 pathway
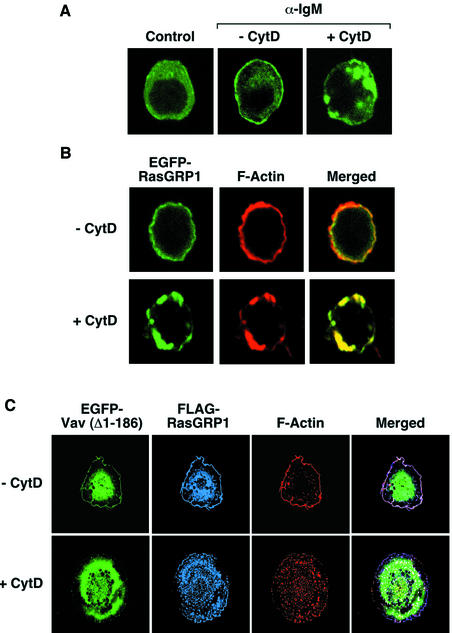
In addition to PLC-γ activity, some observations suggested to us that there could be other signaling components involved in the activation of Ras mediated by Vav. For instance, we observed that the translocation of RasGRP1 induced by the Vav oncoprotein in COS1 cells could occur even with RasGRP1 proteins lacking the ZF domain, the region involved in DAG binding (see Supplementary figure 1C). Since RasGRP1 molecules colocalize in COS1 cells with F-actin in the presence of Vav oncoproteins (see Supplementary figure 1B), we decided to evaluate the possibility that the translocation of RasGRPs could be aided by the interaction with cytoskeletal components. To this end, we first investigated the effect of an F-actin disrupting agent (cytochalasin D) in the subcellular localization of RasGRP1 in DT40 and COS1 cells. The incubation of both cell types with this drug induces the initial collapse of the peripheral F-actin structures into cytoplasmic clusters of F-actin (Figure 5A, B and C). Under these conditions RasGRP1 shows a behavior similar to that of F-actin in both DT40 cells (Figure 5A and B) and COS1 cells (Figure 5C), an indication of an interaction between RasGRP1 and polymerized actin. Identical results are obtained in COS1 cells when RasGRP2 is used in these experiments (data not shown). This biological property is specific for RasGRP molecules, because Vav does not show colocalization with F-actin under the same experimental conditions (Figure 5C).
Fig. 5. (A and B) Effect of cytochalasin D in the subcellular localization of RasGRP1 in wild-type DT40 cells. Cells expressing EGFP–RasGRP1 were incubated with cytochalasin D (10 µM) for 2 h and either left non-stimulated (A, first panel) or stimulated (A and B) with anti-IgM antibodies (10 µg/ml) for 5 min. Cells were then fixed, stained with rhodamine-phalloidin (B) and subjected to microscope analysis. Images show the areas of localization of EGFP–RasGRP1 and F-actin in green (A and B) and red (B), respectively. The areas of overlap between those two proteins are shown in yellow (B). (C) Effect of cytochalasin D in the subcellular localization of RasGRP1 in COS1 cells. Cells expressing FLAG-RasGRP1 and EGFP-Vav (Δ1–186) were either left untreated (upper panels) or were treated with cytochalasin D (2 µM) for 30 min (lower panels). After this step, cells were fixed, incubated with rabbit anti-FLAG antibodies followed by Cy5-labeled antibodies to rabbit IgGs, stained with rhodamine-phalloidin and analyzed by immunofluorescence analysis. Images show the localization of EGFP-Vav (Δ1–186), FLAG-RasGRP1 and F-actin in green, blue and red, respectively. The areas of overlap between RasGRP1 and F-actin are shown in purple (right panels).
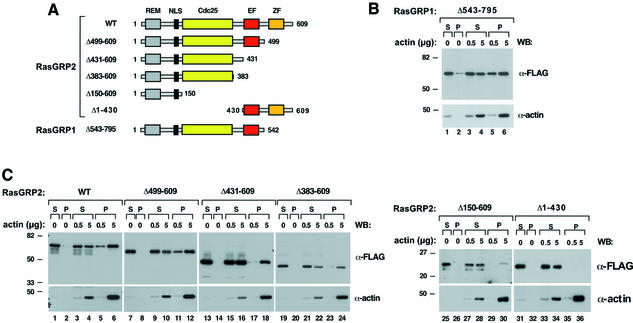
We next reasoned that if RasGRPs bind F-actin, they should be able to coprecipitate with polymerized actin upon ultracentrifugation. To test this possibility, we incubated COS1 cell lysates containing RasGRP1 (Δ543–795 mutant, Figure 6A) with purified in vitro polymerized F-actin and evaluated the binding between these proteins by ultracentrifugation (Scita et al., 2001). The full-length version of RasGRP1 could not be used in these experiments due to its insolubility in the extraction buffer, a property probably derived from its subcellular localization in endomembranes (see Supplementary figure 1A). In agreement with our hypothesis, RasGRP1 (Δ543–795) is detected in the post-ultracentrifugation pellets of F-actin (Figure 6B, upper panel, lanes 5–6). Instead, only residual precipitation of RasGRP1 is observed when the centrifugation experiments are done in the absence of F-actin (Figure 6B, upper panel, lane 2). Identical results were obtained with cell lysates containing full-length RasGRP2 (Figure 6C, first panel, compare lanes 5–6 with lane 2), indicating that this biological property is shared by different RasGRP family members.
Fig. 6. (A) Schematic representation of RasGRP mutants used in this study: REM, Ras exchange motif; NLS, putative nuclear localization signal; Cdc25, GDP/GTP exchange domain for Ras/Rap GTPases; EF, E–F hands; ZF, zinc finger. (B and C) RasGRP molecules associate with F-actin in cosedimentation assays. Total cell lysates from COS1 cells expressing the indicated FLAG-tagged versions of (B) RasGRP1 or (C) RasGRP2 were incubated with the indicated amounts of F-actin. Samples were then ultracentrifuged and the resulting supernatants (S) and pellets (P) were analyzed by anti-FLAG (upper panels) or anti-actin (lower panels) immunoblots after electrophoretic separation. The migration of molecular weight markers (in kDa) is shown on the left. The antibodies used in the immunoblots are indicated on the right.
To define further the region of interaction of RasGRPs with F-actin, we performed similar ultracentrifugation experiments using a collection of truncated versions of RasGRP2 already available in the laboratory (Figure 6A). As shown in Figure 6C, all proteins containing the N-terminal region of this GEF can associate and precipitate with F-actin (Figure 6C, upper panels, lanes 11–12, 17–18, 23–24, 29–30). These proteins do not sediment by ultracentrifugation in the absence of F-actin (Figure 6C, upper panels, lanes 2, 8, 14, 20, 26), demonstrating that the protein–protein interaction is specific. As an additional negative control, a RasGRP2 fragment encompassing its C-terminal region (residues 430–609, see Figure 6A) does not associate with F-actin under the same experimental conditions used for the other RasGRP1 and RasGRP2 fragments (Figure 6C, upper panel, lanes 35–36). Taken together, our results indicate that F-actin, via interaction with the N-terminal region of RasGRP family members, contributes to the translocation of these exchange factors to the plasma membrane.
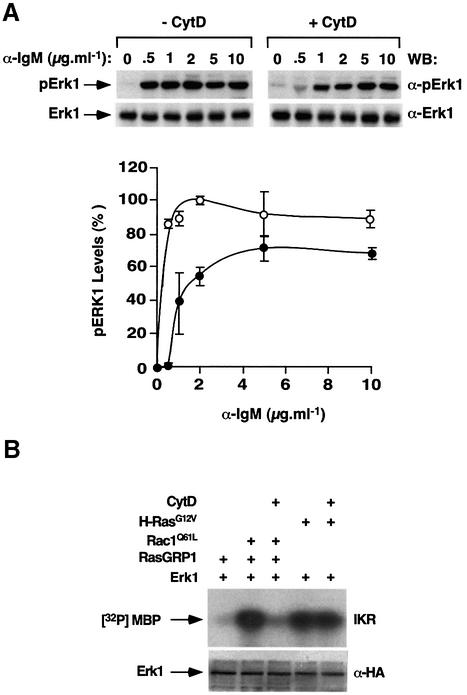
To evaluate the importance of F-actin in the regulation of RasGRP1, we studied the influence of cytochalasin D on the activation of the Ras pathway in vivo. When tested in DT40 cells, cytochalasin D severely affects the activation of Erk1 induced by low levels of antigenic stimulation (0.5–2 µg/ml of anti-IgM, Figure 7A). This effect is partially abrogated when saturating concentrations of anti-IgM antibody are used (5–10 µg/ml, Figure 7A). This is probably due to the presence of residual RasGRP1 molecules at the plasma membrane under those stimulation conditions (see Figure 6A and B). Cytochalasin D has no effect on Erk1 protein levels, as determined by immunoblot analysis using anti-Erk1 antibodies (Figure 7A, top panels). These results, together with the observation that PMA alone can induce the activation of the Ras pathway in DT40 cells, suggest that F-actin contributes to the optimal activation of the Ras pathway under physiological conditions of stimuli when limiting amounts of DAG are present. When the effect of cytochalasin D was tested in COS1 cells, this drug showed dramatic inhibitory effects on the activation of Erk1 derived from the coexpression of RasGRP1 with Rac1Q61L (Figure 7B). This effect is specific for the Rac1 pathway, because cytochalasin D does not interfere with the activation of Erk1 induced by the H-ras oncogene product (Figure 7B). Taken together, our results demonstrate that F-actin polymerization plays an important role in the activation of RasGRP molecules by the Vav/Rac1 pathway.
Fig. 7. (A) Effect of cytochalasin D on the activation of the Ras cascade in DT40 cells. Exponentially growing cells were cultured for 2 h in the absence (–CytD) or presence (+CytD) of cytochalasin D (10 µM) and then stimulated for 10 min with the indicated amounts of anti-IgM antibodies. Cells were then lysed and the resulting extracts analyzed by immunoblot analysis using either anti-phosphoErk1 (top panels) or anti-Erk1 (bottom panels) antibodies. The graph shows the mean and SD values of Erk1 activation obtained in three independent experiments (100% is the maximal value of activation of Erk1). (B) Effect of cytochalasin D on the Vav/Rac–RasGRP1 interaction in COS1 cells. COS1 cells expressing HA-Erk1 in the presence of the indicated proteins were incubated with cytochalasin D for 1 h before evaluating the kinase activity of Erk1 using in vitro kinase reactions (upper panel). Cell lysates from the same experiments were analyzed in parallel by anti-HA immunoblots to evaluate the expression levels of Erk1 under each experimental condition (lower panel).
Discussion
In this article, we demonstrate a new type of functional interaction between the Vav/Rac1 and Ras pathways that is based on the activation of the Ras/Rap-specific RasGRPs by signals emanated from Vav/Rac proteins. The analysis of this new regulatory step has revealed unique features not usually found in other cross-talk (Bar-Sagi and Hall, 2000). Thus, to our knowledge, this is the first case involving the direct activation of a Ras-specific GEF by an independent signaling pathway. Consequently, this mechanism establishes a signaling hierarchy in which Rho/Rac GTPases are acting upstream of Ras, a total inversion of the usual relationships existing between these two GTPase subfamilies. In addition, the reported cross-talk is probably cell type restricted because, unlike other signaling elements of the Ras pathway, the expression profile of RasGRPs are highly specific.
The point of activation of the Vav/Rac1 interaction is simple, since it involves the activation of Vav and the subsequent loading of GTP into Rac1. However, the dissemination of this signal from Vav/Rac proteins down to RasGRPs is more complex, requiring the participation of two cooperating routes. One of the steps important for such activation is the enhancement of PLC-γ activity by Vav/Rac proteins. This activation leads to the production of DAG, a second messenger that modulates the activation levels of RasGRP1 in vivo. The implication of PLC-γ in the Vav/Rac and RasGRP cross-talk has been demonstrated by the use of chemical inhibitors as well as by gain- and loss-of-function experiments using both vav3–/– DT40 and COS1 cells. Thus, we have demonstrated that Vav regulates the stimulation of PLC-γ activity. Moreover, we have shown that PMA can restore the signaling deficiency in the RasGRP/Ras pathway observed in vav3–/– DT40 cells. Finally, we have seen that the inhibition of PLC-γ activity using a specific chemical antagonist blocks the effect of the Vav/Rac1 pathway on RasGRP1 in DT40 and COS1 cells. The mechanism by which Vav/Rac1 activates PLC-γ activity remains to be elucidated. In mouse vav–/– T cells, it has been shown that the deficiency of PLC-γ activity is probably due to low levels of activation of Itk, a protein tyrosine kinase whose activity is essential for the optimal activation of this phospholipase. In addition, vav–/– cells show poor association of PLC-γ1 with the adaptor molecule Slp76 (Reynolds et al., 2002). The reason for the lack of proper PLC-γ activation in mouse vav2–/– B cells has not been fully determined, although it seems that it follows different schemes to those described for T cells. While these effects are probably restricted to lymphocytes, the fact that Vav can also trigger the activation of PLC-γ in non-hematopoietic cells points toward additional cell type-specific regulatory mechanisms. In this respect, our preliminary results indicate that the activation of PLC-γ induced by Vav in COS1 cells is not due to either autocrine signaling or higher levels of PLC-γ tyrosine phosphorylation. In contrast, we have observed that PLC-γ is preferentially located in focal adhesions in Vav-transformed cells, suggesting that other cytoskeletal regulators such as the focal adhesion kinase could be involved (M.J.Caloca and X.R.Bustelo, unpublished observations). In any case, our results showing that Vav can regulate PLC-γ1 activation in vivo indicate that the lack of activation of this enzyme in vav–/– cells is a direct consequence of the lack of Vav activity rather than an indirect effect of the cytoskeletal defects found in Vav-deficient cells.
Unexpectedly, we have also observed that the activation of this new signaling cross-talk requires the convergence of a second signal involving the translocation of the Ras/Rap GEFs to subcellular localizations enriched in F-actin. Using ultracentrifugation experiments, we have demonstrated that such translocation is mediated by the physical interaction between the most N-terminal domains of RasGRP molecules and F-actin. This second step is essential for RasGRP1 activation in both DT40 and COS1 cells, as determined by the inhibitory experiments using the F-actin interfering agent cytochalasin D. Further evidence indicates that this step is independent of the activation of PLC-γ because the levels of activity of PLC-γ are maintained in the presence of cytochalasin D (data not shown). Moreover, PLC-γ inhibitors do not affect the polymerization of F-actin induced by activated versions of Vav and Rac1 (data not shown), further supporting the independence of the two events. The specific contribution of DAG and F-actin to the activation of the RasGRP1 pathways seems to be different in DT40 and COS1 cells. In B cells, DAG seems to be the main driving force for the translocation of RasGRP1, since PLC-γ1 inhibitors are more potent than cytochalasin D in blocking Ras activation when saturating concentrations of anti-IgM antibodies are used to stimulate DT40 cells. In addition, cytochalasin D cannot abolish totally the translocation of RasGRP1 to the plasma membrane of DT40 cells despite the fact that the majority of this protein remains in the cytosol associated with actin clusters. In contrast with this, the role of F-actin seems crucial for the translocation of RasGRP to juxtamembrane areas of COS1 cells. For instance, we have observed that RasGRP mutants non-responsive to DAG still translocate to membrane ruffles when cotransfected with Vav oncoproteins or Rac1Q61L. Moreover, cytochalasin D has major inhibitory effects on the activation of the Ras pathway promoted by the coexpression of RasGRP1 with Rac1Q61L.
The biological reason for the requirement of two independent routes for the activation of RasGRP-dependent pathways is as yet unknown. One possibility is that DAG and F-actin are required to ensure an effective translocation and anchoring of RasGRPs to juxtamembrane areas under physiological conditions of stimulation. This idea is supported by our results indicating that the cytochalasin D-dependent inhibition of Ras signaling in DT40 cells is more intense when low concentrations of extracellular stimuli are used. The effect of saturating amounts of PMA in RasGRP1 translocation and Ras pathway activation independently of actin polymerization is also in agreement with this possibility. Another plausible explanation is that this dual mechanism may help the compartmentalization of the signal output of RasGRP molecules in specific areas of the cell. This is an interesting possibility, since recent results indicate that RasGRP1 may be involved not only in the stimulation of Ras at the plasma membrane but also in the Golgi apparatus (Bivona and Philips, 2003). In this context, it is worth noting that we have observed a basal stimulation of H-Ras in the Golgi apparatus when RasGRP1 is expressed in the absence of Vav (Δ1–186) or Rac1Q61L, as determined by the translocation of an EGFP–RBD protein to this cellular organelle in exponentially growing COS1 cells (M.J.Caloca, J.L.Zugaza and X.R.Bustelo, manuscript in preparation). This property seems to be cell-type specific, because we have observed RasGRP1 in the Golgi of COS1, NIH3T3 and Jurkat cells but not in DT40 cells (this work and data not shown). If the activation of Ras by RasGRP1 in the Golgi is a physiologically relevant event, it is apparent that F-actin may contribute to the concentration of RasGRP1 signaling in peripheral areas of the cell during the initial steps of the signal transduction.
Another important question that remains to be answered is how widespread is this Vav/Rac-Ras cross-talk in the different tissues of the organism. We have observed a similar activity of both Vav and Rac1 in the Jurkat T cell line (J.L.Zugaza and X.R.Bustelo, unpublished observations), suggesting that this regulatory mechanism may have a crucial importance in different lymphocyte lineages. It is also worth noting that RasGRP (RasGRP2 and RasGRP3) and Vav family proteins (Vav2, Vav3) are coexpressed in some non-hematopoietic tissues such as brain (Schuebel et al., 1996; Kawasaki et al., 1998; Movilla and Bustelo, 1999), suggesting that the signaling cross-talk described here will be operational in tissues other than the hematopoietic system to allow the efficient activation of different GTPases of the Ras subfamily. Our observations indicating that Vav proteins can promote high levels of PLC-γ activity in non-hematopoietic cells (COS1 cells) independently of the presence of RasGRP molecules also suggest that this Vav/Rac1-mediated pathway could be oriented to other Ras-independent functional purposes. For instance, the generation of DAG may contribute to the regulation of other intracellular phorbol ester receptors such as chimerin or protein kinase C family members (Kazanietz, 2002). Further work in this area will give us a more comprehensive idea of the cell types in which this cross-talk operates and the spectrum of biological responses under its regulation.
Materials and methods
Tissue culture and DNA transfections
DT40 and COS1 cells were cultured as previously described (Crespo et al., 1996; Lopez-Lago et al., 2000; Inabe et al., 2002). DT40 cells were transfected by electroporation, as indicated elsewhere (Lopez-Lago et al., 2000). For colocalization studies, DT40 cells were transfected with EGFP–RasGRP1-encoding vectors (2.5 µg), either alone or in combination with plasmids encoding wild-type Vav (10 µg), or AU5-tagged Rho/Rac GTPases (10 µg). For biochemical experiments, DT40 cells were transfected with vectors encoding HA-H-Ras (5 µg), Vav (10 µg) and/or AU5-tagged GTPases (10 µg). For immunofluorescence assays, COS1 cells were transfected with liposomes (FuGENE-6, Roche Molecular Biochemicals) according to the manufacture’s instructions. For biochemical experiments, COS1 cells were transfected using the DEAE–dextran method. In immunofluorescence and actin cosedimentation assays, cells were transfected with 1 µg of FLAG-tagged RasGRP-encoding vectors and 0.5 µg of either Vav (EGFP-tagged or untagged) or EGFP–GTPase encoding plasmids. For in vitro kinase assays, cells were transfected with plasmids encoding HA-Erk (1 µg), FLAG-tagged RasGRP1 (2 µg), Vav (Δ1–66, 2 µg), AU5-tagged Rac1Q61L (2 µg) or AU5-tagged H-RasG12V (2 µg) in the appropriate combinations. When appropriate, plasmids encoding RasGRF1 were utilized using either 0.25 or 2 µg DNA per transfection. In all cases, transfections were supplemented with empty vectors to normalize the amount of transfected DNA. Details about the construction of expression vectors and mutants are available upon request.
Ras activation assays
DT40 cells were lysed in a buffer containing 10 mM Tris–HCl pH 7.6, 150 mM NaCl, 10 mM MgCl2, 1% nonidet P-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 µg/ml leupeptin and 10 U/ml aprotinin. Lysates were centrifuged at 14 000 g for 10 min at 4°C. The resulting supernatants were incubated for 30 min at 4°C with 50 µg of GST–RBD immobilized onto glutathione Sepharose beads (Amersham Biosciences). After incubation, the beads were collected and washed with lysis buffer three times. Bound proteins were then analyzed by immunoblotting using anti-HA antibodies (Babco).
Determination of Erk1 activity by in vitro kinase assays and phosphospecific antibodies
Activation of endogenous Erk1 was monitored in DT40 cells using phosphospecific antibodies (New England Biolabs), according to standard procedures. In the case of COS1 cells, the activation of ectopically expressed HA-Erk1 was monitored by in vitro kinase reactions (Crespo et al., 1996). To this end, cells were lysed in a buffer containing 20 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 1 mM β-glycerophosphate, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM PMSF, 10 µg/ml leupeptin and 10 U/ml aprotinin. Lysates were centrifuged at 14 000 g for 10 min at 4°C and the resulting supernatants were incubated with anti-HA antibodies (Babco) for 2 h at 4°C. Immunocomplexes were collected with GammaBind Sepharose beads (Amersham Biosciences), washed once with lysis buffer and three times with kinase buffer [25 mM Tris–HCl pH 7.5, 5 mM β-glycerophosphate, 2 mM dithiothreitol (DTT), 0.1 mM Na3VO4, 10 mM MgCl2]. Immunocomplexes were incubated with 200 µM ATP, 5 µCi [γ-32P]ATP and 5 µg of myelin basic protein as phosphate acceptor for 30 min at 30°C. Reactions were terminated by adding an equal volume of 2× SDS–PAGE sample buffer, separated electrophoretically and subjected to autoradiography. Expression of Erk1 protein was determined by anti-HA immunoblotting of total cellular lysates, according to standard procedures. Immunoblots were revealed using an enhanced chemioluminiscent method (ECL, Amersham Biosciences).
PLC activity and F-actin cosedimentation assays and immunofluorescence studies
PLC activity in COS1 was determined exactly as described (Gutkind et al., 1991). Actin cosedimentation assays were performed as reported (Scita et al., 2001) using purified actin, anti-FLAG M2 and anti-actin antibodies (Sigma). For immunofluorescence studies, DT40 and COS1 cells were processed as indicated (Movilla and Bustelo, 1999; Lopez-Lago et al., 2000). Immunological reagents used were anti-FLAG M2 (Sigma), Cy5-, Cy3- and Cy2-labeled anti-mouse or anti-rabbit IgG antibodies (Jackson Immunolabs), rhodamine phalloidin (Molecular Probes) and cytochalasin D (Sigma).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
The authors thank M.Blázquez for technical assistance, Dr T.Kurosaki for the generous gift of vav3–/– DT40 cells and Dr M.Dosil for helpful comments on the manuscript. This work was supported by grants from the US NCI (X.R.B.), the British Association for International Cancer Research (X.R.B. and P.C.) and the PGC Program of the Spanish Ministry of Science and Technology (X.R.B. and P.C.). M.J.C. and J.L.Z. are Ramón y Cajal investigators (Spanish Ministry of Science and Technology) associated with the University of Salamanca.
References
- Aghazadeh B., Lowry,W.E., Huang,X.Y. and Rosen,M.K. (2000) Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell, 102, 625–633. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D. and Hall,A. (2000) Ras and Rho GTPases: a family reunion. Cell, 103, 227–238. [DOI] [PubMed] [Google Scholar]
- Bivona T.G. and Philips,M.R. (2003) Ras pathway signaling on endomembranes. Curr. Opin. Cell Biol., 15, 136–142. [DOI] [PubMed] [Google Scholar]
- Bustelo X.R. (2000) Regulatory and signaling properties of the Vav family. Mol. Cell Biol., 20, 1461–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustelo X.R. (2001) Vav proteins, adaptors and cell signaling. Oncogene, 20, 6372–6381. [DOI] [PubMed] [Google Scholar]
- Bustelo X.R. (2002) Regulation of Vav proteins by intramolecular events. Front. Biosci., 7, D24–D30. [DOI] [PubMed] [Google Scholar]
- Bustelo X.R., Suen,K.L., Leftheris,K., Meyers,C.A. and Barbacid,M. (1994) Vav cooperates with Ras to transform rodent fibroblasts but is not a Ras GDP/GTP exchange factor. Oncogene, 9, 2405–2413. [PubMed] [Google Scholar]
- Costello P.S., Walters,A.E., Mee,P.J., Turner,M., Reynolds,L.F., Prisco,A., Sarner,N., Zamoyska,R. and Tybulewicz,V.L. (1999) The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK and NF-κB pathways. Proc. Natl Acad. Sci. USA, 96, 3035–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo P., Bustelo,X.R., Aaronson,D.S., Coso,O.A., Lopez-Barahona,M., Barbacid,M. and Gutkind,J.S. (1996) Rac-1 dependent stimulation of the JNK/SAPK signaling pathway by Vav. Oncogene, 13, 455–460. [PubMed] [Google Scholar]
- Crespo P., Schuebel,K.E., Ostrom,A.A., Gutkind,J.S. and Bustelo,X.R. (1997) Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature, 385, 169–172. [DOI] [PubMed] [Google Scholar]
- Dower N.A., Stang,S.L., Bottorff,D.A., Ebinu,J.O., Dickie,P., Ostergaard,H.L. and Stone,J.C. (2000) RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol., 1, 317–321. [DOI] [PubMed] [Google Scholar]
- Gutkind J.S., Novotny,E.A., Brann,M.R. and Robbins,K.C. (1991) Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc. Natl Acad. Sci. USA, 88, 4703–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inabe K., Ishiai,M., Scharenberg,A.M., Freshney,N., Downward,J. and Kurosaki,T. (2002) Vav3 modulates B cell receptor responses by regulating phosphoinositide 3-kinase activation. J. Exp. Med., 195, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joneson T. and Bar-Sagi,D. (1998) A Rac1 effector site controlling mitogenesis through superoxide production. J. Biol. Chem., 273, 17991–17994. [DOI] [PubMed] [Google Scholar]
- Joneson T., McDonough,M., Bar-Sagi,D. and Van Aelst,L. (1996) RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science, 274, 1374–1376. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., et al. (1998) A Rap guanine nucleotide exchange factor enriched highly in the basal ganglia. Proc. Natl Acad. Sci. USA, 95, 13278–13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanietz M.G. (2002) Novel “nonkinase” phorbol ester receptors: the C1 domain connection. Mol. Pharmacol., 61, 759–767. [DOI] [PubMed] [Google Scholar]
- Lopez-Lago M., Lee,H., Cruz,C., Movilla,N. and Bustelo,X.R. (2000) Tyrosine phosphorylation mediates both activation and downmodulation of the biological activity of Vav. Mol. Cell Biol., 20, 1678–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetz T.S., Gonzalez-Espinosa,C., Arudchandran,R., Xirasagar,S., Tybulewicz,V. and Rivera,J. (2001) Vav1 regulates phospholipase C γ activation and calcium responses in mast cells. Mol. Cell Biol., 21, 3763–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movilla N. and Bustelo,X.R. (1999) Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol. Cell Biol., 19, 7870–7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilliam L.A., Rebhun,J.F. and Castro,A.F. (2002) A growing family of guanine nucleotide exchange factors is responsible for activation of Ras-family GTPases. Prog. Nucleic Acid Res. Mol. Biol., 71, 391–444. [DOI] [PubMed] [Google Scholar]
- Reynolds L.F., Smyth,L.A., Norton,T., Freshney,N., Downward,J., Kioussis,D. and Tybulewicz,V.L. (2002) Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and -independent pathways. J. Exp. Med., 195, 1103–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuebel K.E., Bustelo,X.R., Nielsen,D.A., Song,B.J., Barbacid,M., Goldman,D. and Lee,I.J. (1996) Isolation and characterization of murine vav2, a member of the vav family of proto-oncogenes. Oncogene, 13, 363–371. [PubMed] [Google Scholar]
- Scita G. et al. (2001) An effector region in Eps8 is responsible for the activation of the Rac- specific GEF activity of Sos-1 and for the proper localization of the Rac-based actin-polymerizing machine. J. Cell Biol., 154, 1031–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L. and D’Souza-Schorey,C. (1997) Rho GTPases and signaling networks. Genes Dev., 11, 2295–2322. [DOI] [PubMed] [Google Scholar]
- Villalba M., Hernandez,J., Deckert,M., Tanaka,Y. and Altman,A. (2000) Vav modulation of the Ras/MEK/ERK signaling pathway plays a role in NFAT activation and CD69 up-regulation. Eur. J. Immunol., 30, 1587–1596. [DOI] [PubMed] [Google Scholar]
- Wu J., Katzav,S. and Weiss,A. (1995) A functional T-cell receptor signaling pathway is required for p95vav activity. Mol. Cell Biol., 15, 4337–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Leitenberg,D., Li,B. and Flavell,R.A. (2001) Deficiency of small gtpase rac2 affects T cell activation. J. Exp. Med., 194, 915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugaza J.L., Lopez-Lago,M.A., Caloca,M.J., Dosil,M., Movilla,N. and Bustelo,X.R. (2002) Structural determinants for the biological activity of vav proteins. J. Biol. Chem., 277, 45377–45392. [DOI] [PubMed] [Google Scholar]